Бактериальный вагиноз, несмотря на сотни исследований, по сегодняшний день остается одной из самых актуальных проблем в акушерстве и гинекологии, снижая качество жизни пациенток и приводя к сверхранним преждевременным родам и неблагоприятным перинатальным исходам. В этиологии и патогенезе этого заболевания все еще много белых пятен; методы диагностики и лечения совершенствуются с каждым днем, но при этом проблема рецидивирующего бактериального вагиноза заставляет искать новые нестандартные подходы в терапии.
Синдром бактериального вагиноза отражает состояние дисбаланса влагалищной микрофлоры вследствие снижения пула защитных лактобацилл и активного размножения анаэробных бактерий, в большей степени Gardnerella vaginalis и Atopobium vaginae. Кроме того, присутствие облигатных анаэробов способствует размножению порядка 300 видов других микроорганизмов, что формирует полимикробную биопленку. Хорошо известен тот факт, что рецидивирующий бактериальный вагиноз повышает риск инфекционно-воспалительных заболеваний органов малого таза, а во время беременности может быть причиной сверхранних преждевременных родов. Еще более тревожным становится наличие рецидивирующего анаэробного дисбиоза при многоплодной беременности, особенно протекающей на фоне коррекции истмико-цервикальной недостаточности и длительного присутствия во влагалище инородного тела – шва или пессария. Риск рождения недоношенных детей из многоплодной беременности вследствие преждевременного разрыва плодных оболочек на фоне рецидивирующего анаэробного дисбиоза становится серьезным страхом акушеров-гинекологов и неонатологов.
Помимо двухэтапной терапии, направленной на санацию анаэробов и восстановление здоровой микробиоты, в настоящий момент обсуждаются новые подходы к лечению бактериального вагиноза: это и трансплантация лактобактерий от здорового донора [1, 2], и культивирование собственных лактобактерий с последующим назначением их в виде вагинальных аутопробиотиков [3]. Одной из основных причин неэффективности стандартной общепринятой пробиотической терапии является чужеродность микроорганизмов, входящих в состав пробиотиков. Экспериментальные работы по коррекции дисбиозов показали, что максимального эффекта можно достичь, либо при подборе донорских штаммов, либо при культивировании собственных бактерий и использовании их в качестве аутопробиотиков [4]. Поэтому технологии приготовления аутопробиотиков, основанные на выделении лучших образцов у конкретного индивидуума, их культивировании и приготовлении пробиотиков на их основе, отвечают всем принципам персонализированной и безопасной современной медицины. Одними из пионеров технологии аутопробиотической терапии были отечественные ученые под руководством проф. А.Н. Суворова [5–7]. Широко применяется создание аутопробиотиков для нормализации кишечной микрофлоры, однако данные о применении их для коррекции вагинальных дисбиозов ограничены.
Клиническое наблюдение
В ООО «Клиника профессора Буштыревой» обратилась пациентка, в возрасте 32 года, с беременностью малого срока и третьими предстоящими родами.
Акушерско-гинекологический анамнез крайне отягощен.
Первая беременность в 2010 г. закончилась физиологическими родами в срок, масса новорожденного 3600 г, оценка по Апгар 9–10 баллов.
Вторая беременность в 2010 г. завершилась самопроизвольным абортом в сроке 7 недель, без выскабливания стенок полости матки.
Третья беременность в 2013 г. завершилась срочными родами через естественные родовые пути, масса новорожденного 4000 г, оценка по Апгар 9–10 баллов, беременность осложнилась преэклампсией тяжелой степени, роды – послеродовым гипотоническим кровотечением, интимным прикреплением плаценты с последующим ручным отделением плаценты и выделением последа. Поздний послеродовый период осложнился лохиометрой с выскабливанием стенок полости матки на 3-и сутки после родов.
Четвертая беременность в 2019 г. завершилась антенатальной гибелью плода в сроке 15 недель беременности. Прерывание беременности проводилось медикаментозным способом, однако консервативное ведение было неэффективным, в связи с чем прерывание завершилось инструментальным удалением плода и последа с последующим выскабливанием стенок полости матки. В послеоперационном периоде дважды произошло гипотоническое кровотечение, оба раза сопровождавшееся выскабливанием стенок полости матки, было проведено несколько курсов антибактериальной терапии. Пациентка получила последовательные курсы антибактериальной терапии препаратами цефалоспоринов 3 поколения, азитромицином, офлоксацином, антимикробной терапии метронидазолом.
Кроме того, в 2018 г. в результате планового скрининга (цитологический жидкостный ПАП-тест) была выявлена тяжелая дисплазия шейки матки HSIL (CIN 3), по поводу чего проводилась радиоволновая конизация шейки матки.
Настоящая беременность пятая, без прегравидарной подготовки. По данным ультразвукового исследования в сроке 8 недель беременности выявлена тройня. На ранних сроках пациентка получала стандартную витаминотерапию, препараты прогестерона. По данным мазка на флору в раннем сроке беременности 7 недель – лейкоциты 3–4 в поле зрения, эпителий 5–6 в поле зрения, флора – кокковая, «ключевые» клетки обнаружены, грибы рода Candida не обнаружены. С ранних сроков беременности по данным теста «Фемофлор-16» (Россия, ООО «НПО ДНК-Технология») отмечались признаки анаэробного дисбиоза (рис. 1), а также полное отсутствие лактобактерий. По данным бактериологического посева из цервикального канала, выполненного в рамках обследования в женской консультации, в сроке 7 недель беременности выявлены Enterococcus faecalis 10⁶, Escherichia coli 10⁵. Посев вагинального отделяемого производился только на жидкие среды накопления. С 4–5 недель беременности и до родов пациентка получала терапию синбиотиками per os (комплекс живых бактерий: Lactobacilus acidophilus, Bifidobacterium bifidum, Bifidobacterium longum, Lactobacilus bulgaricus, Streptococcus termophillus, фруктоолигосахариды), поскольку до беременности перенесла неоднократные курсы антибактериальной терапии и длительное время страдала от обстипационного синдрома и рецидивирующего анаэробного дисбиоза влагалища.
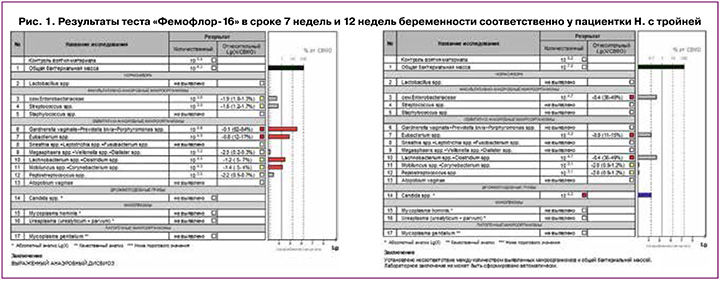
В сроке 12 недель был проведен первый скрининг, диагностирована дихориальная, триамниотическая тройня. По данным «Фемофлор-16» было выявлено полное отсутствие лактобактерий, и значения грибов рода Candida, а также Eubacterium, Lachnobacterium и Clostridium соответствовали верхней границе нормы. Однако с учетом анамнеза и факта беременности тройней, вероятность потери беременности в связи с излитием вод оставалась высокой на фоне такого состава микробиоты. Кроме того, планировалась хирургическая коррекция шейки матки. Антибактериальная терапия с учетом энтерококков только усугубила бы эту ситуацию, поэтому на данном этапе и был выбран путь длительной коррекции синбиотиками и местной терапии субклинических концентраций анаэробов и грибов рода Candida. В связи с этим, после 12 недель принято решение провести курс местной терапии вагинальными суппозиториями с содержанием метронидазола и миконазола. Позднее, в сроке 13 недель и 4 дня, с учетом ультразвуковых признаков укорочения шейки матки (длина шейки матки 24 мм), а также данных анамнеза (конизация шейки матки по поводу CIN3) и беременности тройней, была проведена хирургическая коррекция шейки матки путем наложения кругового подслизистого шва с мерсиленовой нитью. Далее выполнялся обязательный контроль рН-метрии отделяемого влагалища еженедельно и контроль за состоянием микробиоты влагалища один раз в 3–4 недели.
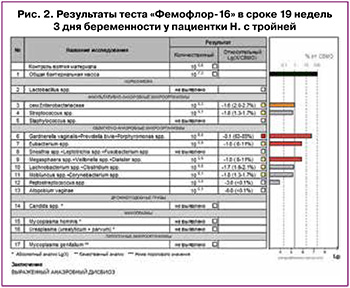
Помимо высоких рисков фето-фетального трансфузионного синдрома, рисков преждевременных родов при беременности тройней на фоне скомпрометированной конизацией шейки матки, вызывал опасение высокий риск преждевременного разрыва плодных оболочек в связи с рецидивирующим анаэробным дисбиозом и низким процентным содержанием лактобактерий на фоне инородного тела во влагалище – мерсиленовой нити шва. В сроке 19 недель и 3 дня по данным теста «Фемофлор-16» снова выявлен выраженный анаэробный дисбиоз (рис. 2), полное отсутствие лактобактерий. Проведена санация влагалища вагинальными суппозиториями с содержанием клиндамицина на 7 дней, назначен второй этап терапии суппозиториями с лактобактериями (лиофилизированная культура лактобацилл L. casei rhamnosus Doderleini – 1×108 КОЕ жизнеспособных лактобактерий) на 14 дней.
Помимо исследования микробиоты влагалища беременной с помощью полимеразной цепной реакции, в сроке 20 недель, до начала терапии анаэробного дисбиоза, мы провели анализ бактериального разнообразия с помощью секвенирования 16s рРНК для выделения видового состава микробиоты влагалища (исследование микробиома урогенитального тракта, ООО «Сербалаб», Санкт-Петербург). Суммарную ДНК выделяли с использованием набора «Рибо-преп» (ФБУН ЦНИИ Эпидемиологии Роспотребнадзора, Москва, Россия) по протоколу производителя. Библиотеки фрагментов гена 16S рРНК готовили по протоколу компании «Иллюмина» «16S Metagenomic Sequencing Library Preparation» (Part №15044223 Rev. B). 5 нг суммарной ДНК амплифицировали в течение 25 циклов с использованием рекомендованных в протоколе праймеров к участкам V3 и V4 гена 16S рРНК бактерий и готовой смеси для ПЦР KAPA HiFi HotStart ReadyMix (2X) (Roche Diagnostics, Швейцария). Полученные фрагменты ДНК очищали с использованием парамагнитных частиц AMPure XP beads (Beckman Coulter, США). Для проведения индексирующей ПЦР 5 нг ДНК после первого раунда амплификации подвергали восьми циклам амплификации с использованием биркодированных праймеров из набора Nextera XT Index Kit (Illumina, США) и готовой смеси для ПЦР KAPA HiFi HotStart ReadyMix (2X) (Roche Diagnostics, Швейцария). Полученные библиотеки очищали с помощью парамагнитных частиц, пулировали в эквимолярном соотношении и секвенировали на приборе MiSeq System (Illumina, США) в режиме парноконцевого секвенирования 2*151. Результаты представлены на рисунке 3.

По результатам секвенирования доминирующими видами бактерий оказались Prevotella bivia (относительная представленность 34,8%), Bifidobacterium breve (31,9%), Streptococcus anginosus (5,5%), Alloscardovia omniocolens (3,9%), Atopobium parvulum (3,7%). Lactobacillus rhamnosus – единственный вид лактобактерий, обнаруженный у пациентки, регистрировались в низком процентном соотношении, всего 3,6% от общей бактериальной массы. Очевидно, что данные «Фемофлор-16» и секвенирования отличались, что можно, с одной стороны, объяснить тем фактом, что «Фемофлор-16» тест не разделяет Gardnerella vaginalis, Prevotella bivia и Porphyromonas spp, присваивая этим трем видам суммарный вклад в микробиоту влагалища – 85%. Все же, по сравнению с секвенированием, «Фемофлор-16» можно считать скрининговым тестом. По данным секвенирования, как более точного метода диагностики, Prevotella bivia выделена отдельно, составляя 34,8% микробиоты влагалища. Bifidobacterium breve и вовсе не учитываются в «Фемофлор-16» тесте. Streptococcus диагностированы по данным обоих тестов примерно в одинаковых концентрациях. Кроме того, более 12% неклассифицируемых микроорганизмов выявлено по данным секвенирования; то есть в имеющейся библиотеке микроорганизмов лаборатории отсутствовали данные об обнаруженных микроорганизмах. В целом картина секвенирования подтверждала картину «Фемофлор-16» теста: сниженное количество лактобактерий и доминирование анаэробов.
Образец отделяемого влагалища для исследования микробиома был взят в сроке 20 недель после проведенной санации влагалища в сроке 12 недель свечами с миконазолом и метронидазолом. Курс повторной терапии клиндамицином и вагинальными суппозиториями с культурой Lactobacillus rhamnosus был назначен уже после забора материала для секвенирования. В связи с этим, можно исключить возможное влияние вагинальных суппозиториев с лактобактериями на результаты анализа микробиоты влагалища. Кроме того, синбиотики, которые получала пациентка перорально в своем составе также не содержали Lactobacillus rhamnosus.
В сроке 28 недель при очередном плановом ультразвуковом исследовании выявлены признаки несостоятельности шва на шейке матки. При осмотре в зеркалах визуализировались «провисающие» нити шва, а также пролабирование плодного пузыря до уровня наружного зева шейки матки. Был проведен консилиум и принято решение об удалении старого шва и проведении повторного серкляжа выше места наложения первого шва. После повторного наложения шва, учитывая рецидивирующий анаэробный дисбиоз, неоднократные курсы проводимой терапии анаэробного дисбиоза без эффекта, сложную акушерскую ситуацию, пристальное внимание должно было уделяться именно состоянию микробиоты влагалища в связи с высоким риском преждевременного разрыва плодных оболочек. Решено было воспользоваться альтернативным способом терапии, а именно созданием аутопробиотиков на основании собственных лактобактерий пациентки. По решению врачебной комиссии клиники метод терапии вагинальными аутопробиотиками был признан перспективным и безопасным в конкретной клинической ситуации.
Технология приготовления аутопробиотиков включала в себя: забор биоматериала в специальную транспортную среду, культивирование бактерий на питательных средах, отбор индивидуальных клонов бактерий из биоматериала, тестирование штаммов на предмет возможного присутствия генов патогенности, антибиотикоустойчивости; затем приготовление капсул для вагинального применения в желатиновой оболочке (ООО «Микробиом», Санкт-Петербург). Забор материала осуществлялся с задней и боковой стенок влагалища методом соскоба эпителиальных клеток, материал помещался в пробирку с транспортной средой. Идентификация лактобактерий проводилась методом масс-спектрометрии по спектру их рибосомальных белков, штамм лактобацилл выращивался при температуре 37°C на MRS-бульоне, на бифидум-среде, на молоке в условиях с пониженным содержанием кислорода. После культивирования бактерий, оценки их безопасности, лиофилизации бактерии были расфасованы в желатиновые капсулы и с соблюдением термического режима отправлены пациентке.
Лактобациллы и бифидобактерии признаны ВОЗ «generally recognized as safe» (GRAS) [8–10] и, согласно международным нормам, не требуют дополнительных доказательств их безопасности, кроме определения вида и источника выделения исключительно от человека. Согласно Методическим указаниям МУ 2.3.2.2789-10 «Методические указания по санитарно-эпидемиологической оценке безопасности и функционального потенциала пробиотических микроорганизмов, используемых для производства пищевых продуктов» для штаммов должен быть установлен профиль антибиотикоустойчивости. L. rhamnosus имеет устойчивость к ванкомицину, которая кодируется генами, расположенными на хромосоме; она не индуцибельна и не может передаваться горизонтально.
Данные теста «Фемофлор-16» до начала терапии аутопробиотиками представлены на рисунке 4. Отмечалось крайне низкое количество лактобактерий (103, менее 0,1% от общей бактериальной массы), высокая концентрация анаэробных микроорганизмов (Gardnerella vaginalis – 105,5, Megasphaera spp, Veilonella spp, Dialister spp – 106,7, Mobiluncus spp, Corynebacterium spp – 106, грибы рода Candida – 103,7).
Пациентка получала терапию вагинальными аутопробиотиками с 30 недель беременности и до 37 недель (срок родоразрешения) – ежедневно самостоятельно вводила в задний свод влагалища капсулу с аутопробиотиком. Через неделю после начала применения терапии показатели рН-метрии составили 4,5 (тест-полоска «рН-Баланс»).
Учитывая отсутствие фето-фетального трансфузионного синдрома, фетоплацентарной недостаточности, дискордантного роста плодов, удовлетворительное состояние каждого из трех плодов, пациентка была родоразрешена в сроке беременности 37 недель путем операции кесарева сечения. Родились живые доношенные новорожденные: девочка с массой тела 2250 г, оценкой по Апгар 8–9 баллов, мальчики с массой тела 2050 г (8–9 баллов) и 2360 г (7–8 баллов). Накануне планового кесарева сечения произведен забор материала из влагалища для теста «Фемофлор-16» (рис. 5).

Забор материала произведен на фоне применения вагинального аутопробиотика; отменить его заблаговременно, чтобы получить истинную картину состава микробиоты влагалища, не позволяла клиническая ситуация. Целью терапии являлось достижение доношенного срока беременности, именно поэтому забор материала для исследования был осуществлен в день оперативного родоразрешения.
Впервые за всю беременность по данным теста «Фемофлор-16» был выявлен абсолютный нормоценоз с абсолютным доминированием лактобактерий – 106,8 (81–100% от общей бактериальной массы); грибы рода Candida отсутствовали совсем, анаэробная микрофлора регистрировалась в субклинических значениях. Сложно утверждать, лактобактерии аутопробиотика принадлежали к естественному штамму лактобактерий влагалища пациентки, к лактобактериям синбиотика, который она длительное время применяла или к лактобактериям вагинального пробиотика. Эта тема однозначно перспективна и требует дальнейшего изучения.
По техническим причинам не производился забор влагалищного отделяемого для секвенирования; кроме того, не произведен и забор для бактериологического посева с целью выделения конкретного вида лактобактерий в составе влагалищного микробиома, что является ограничением данного исследования. Однако достигнутое состояние абсолютного нормоценоза к сроку родоразрешения, а также факт доношенного срока беременности у пациентки с тройней и крайне отягощенным акушерско-гинекологическим анамнезом, с высочайшим риском преждевременного разрыва плодных оболочек, может служить косвенным подтверждением эффективности вагинальных аутопробиотиков, которое требует дальнейших исследований.
Обсуждение
Рассмотренное клиническое наблюдение демонстрирует применение экспериментального метода восстановления влагалищной микробиоты, который в данной конкретной клинической ситуации был применен в связи с неэффективностью всех традиционных методик лечения. Преждевременные роды на фоне анатомически неполноценной шейки матки после конизации, беременности тройней, которая сама по себе является значимым фактором риска преждевременных родов, при дважды проведенной хирургической коррекции шейки матки в данной ситуации были практически неизбежны, а рецидивирующий анаэробный дисбиоз, не поддающийся стандартной терапии, прописанной в клинических рекомендациях, многократно увеличивал риски преждевременного разрыва плодных оболочек, запуская неотвратимость завершения беременности.
Терапия аутопробиотиками, по нашему мнению, является наиболее физиологичным и безопасным методом терапии дисбиозов, особенно при беременности.
Если рассматривать бактериальное сообщество какого бы то ни было локуса тела каждого индивидуума, как отдельный орган, обладающий особой иммунологической, метаболической активностью, становится очевидной, как минимум, инертность пробиотических бактерий во влиянии на состав микробиоты хозяина, а как максимум – вероятная реакция отторжения промышленно произведенных готовых фармакологических штаммов бактерий, используемых в качестве пробиотиков, будь то пробиотики для кишечника или для влагалищного применения. Вероятнее всего, эти промышленные штаммы функционируют только в момент их активного применения, а при прекращении курса терапии элиминируются из организма полностью. Касательно вагинальных пробиотиков: механизм их действия заключается в локальном закислении среды и смещении рН влагалищного отделяемого в кислую сторону, что потенциально способно создавать благоприятные условия для роста собственных лактобактерий, если они имеются там в достаточном количестве. Дополнительной причиной временной эффективности коммерческих пробиотиков является способность большей части пробиотических штаммов лактобацилл вырабатывать антимикробные пептиды, приводящие к гибели патогенных бактерий [11]. В дальнейшем чужеродные лактобациллы постепенно элиминируются из организма в силу особенностей индивидуального иммунитета хозяина, который сформировался еще в детском возрасте.
У пациентки в вышеописанном клиническом наблюдении лактобактерии в биотопе влагалища присутствовали в крайне низкой концентрации – по данным секвенирования 16s рРНК всего 3,6% (рис. 3). Применение аутопробиотиков в этом клиническом примере – собственных лактобацилл пациентки – снижало вероятность их отторжения до минимума, поскольку входящие в состав капсул лактобактерии не вступали в какие-либо конфликтные иммунологические отношения с организмом хозяина; их генотип был привычен и не распознавался как чужеродно агрессивный.
Несмотря на это, у нашего исследования имеется ряд серьезных ограничений, которые требуют дальнейшего изучения этой проблемы. Первое сомнение, которое возникло, касалось результатов секвенирования, а именно конкретного вида лактобактерий – Lactobacillus rhamnosus (рис. 3), который не является характерным для вагинального биотопа. Исследования микробиоты влагалища с помощью секвенирования нового поколения позволяют, по данным литературы, выделить 5 типов вагинального микробиома, основываясь на доминировании тех или иных видов лактобактерий: CST I типа (Lactobacillus crispatus), CST II типа (Lactobacillus gasseri), CST III типа (Lactobacillus iners), CST V типа (Lactobacillus jensenii) и CST IV типа с доминированием облигатных и факультативных анаэробов без доминирования лактобацилл [12]. Кроме того, существует обновленная классификация вагинальных типов VALENCIA (VAginaL community state type Nearest CentroId clAssifier), которая делит вышеуказанные типы на подтипы [13]. Как видно, по общепринятым представлениям Lactobacillus rhamnosus не является доминирующим ни в одном из подтипов вагинальной микробиоты. Можно было предположить, что этот вид лактобактерий оказался в вагинальном биотопе пациентки из вагинальных свечей с лактобактериями, однако пациентка начала получать их уже после забора материала для исследования, или из синбиотика, который беременная принимала длительное время для восстановления микробиоты кишечника после антибактериальной терапии, но в составе конкретного синбиотика нет указания на этот вид лактобактерий. При изучении литературы, удалось найти немало публикаций, свидетельствующих о том, что Lactobacillus rhamnosus является нормальным обитателем вагинального биотопа [14–17]. Поскольку знания о микробном составе влагалища совершенствуются год от года, нельзя исключить, что не только 4 основных вида лактобактерий являются нормальными обитателями влагалища, а их гораздо больше. Тем не менее, независимо от доминирующего вида лактобактерий, все они обладают различной степени выраженности способностью к синтезу молочной кислоты и перекиси водорода, обладающих бактерицидным эффектом. Нельзя исключить тот факт, что для конкретной пациентки после многократных курсов антибактериальной терапии именно Lactobacillus rhamnosus смогла занять пустующую «эконишу» и играть антимикробную роль вместо наиболее часто встречающихся видов лактобактерий.
Второе ограничение исследования заключалось в том, что накануне родоразрешения не проведено повторное секвенирование влагалищного отделяемого и бактериологический посев на питательную среду с целью идентификации вида лактобактерий и сопоставления его с видом в аутопробиотике. Остается неясным, выявленные по данным «Фемофлор-16» теста лактобактерии являются собственными бактериями пациентки, выросшими в благоприятных условиях на фоне применения аутопробиотика, или же это идентичные аутопробиотику лактобактерии, или же это бактерии, которые входили в состав синбиотика, назначенного перорально. При планировании следующих исследований важно идентифицировать вид лактобактерий на этапе контроля лечения.
В то же время, нельзя не согласиться с тем фактом, что клинический благополучный итог обсуждаемой беременности – а именно, рождение тройни в доношенном сроке гестации, был достигнут, в том числе, благодаря разнонаправленной терапии биопрепаратами – как синбиотиками, так и местными вагинальными аутопробиотиками.
Аутопробиотикотерапия – достаточно новый подход к коррекции дисбиотических нарушений, который основан на использовании аутопробиотиков, живых индигенных облигатных представителей микробиоты (лактобацилл, бифидобактерий и энтерококков) [3, 5, 11, 18–20]. Что особо примечательно, разработчики данной методики – отечественные ученые, выступающие за персонализированный подход к выбору пробиотиков [5, 6, 18–21], предполагающий тщательный отбор, тестирование и хранение аутопробиотических бактерий для различных локусов тела в момент физиологического здоровья человека – будущего реципиента, с периодической заменой их в биобанке.
В настоящий момент в нашей стране активно развивается идеология биобанкирования, создания банка собственных индивидуальных «полезных» бактерий, которые берутся у конкретного человека, культивируются и консервируются по специальной методике, хранятся длительное время и могут быть в любой момент извлечены из биобанка, культивированы на специальных питательных средах снова и расфасованы в различные лекарственные формы (капсулы, сыворотки) для дальнейшего применения с целью восстановления эубиотического состояния. Поводом для такой терапии может стать и рутинная антибактериальная терапия, и химиотерапия, и различные метаболические состояния (сахарный диабет, ожирение, метаболический синдром), и системные заболевания. Этот подход соответствует концепции современной персонализированной медицины, в первую очередь безопасной и эффективной для каждого конкретного пациента.
Заключение
Терапия вагинальными аутопробиотиками при беременности может стать безопасным и персонализированным ресурсом для восстановления микрофлоры влагалища и снижения риска акушерских осложнений, в первую очередь – преждевременных родов, ассоциированных с преждевременным разрывом плодных оболочек.
Дальнейшие исследования требуются для популяризации этой методики в акушерстве и гинекологии и даже возможного включения в действующие клинические рекомендации.



