The role of vascular and angiogenic factors in the development of selective fetal growth restriction in monochorionic multiple pregnancy
Background: Selective fetal growth restriction (sFGR) affects 10–25% of all monochorionic twin pregnancies. If not detected and treated, sFGR is associated with high perinatal mortality and a wide range of neonatal complications. However, currently, due to the introduction of the dynamic fetal monitoring and intrauterine treatments, including fetoscopic laser coagulation of placental vascular anastomoses and the determination of optimal delivery time, it is possible to achieve high survival rates for both fetuses. The main etiological factor of sFGR is placental dysfunction, including morphological and functional changes associated with reduced placental volume, impaired development of arterio-arterial anastomoses, and vascular and angiogenic factors during the formation of an early monochorionic pregnancy.Gladkova K.A., Sakalo V.A., Vtorushina V.V., Piskulina A.A., Frolova E.R., Kostyukov K.V., Khodzhaeva Z.S.
Objective: To investigate the role of vascular endothelial factors in the development of sFGR in monochorionic twin pregnancy.
Materials and methods: The study included 51 patients with monochorionic diamniotic twins. Group 1 comprised patients with sFGR (n=31) and Group 2 consisted of patients with uncomplicated monochorionic twin pregnancies (n=20). The study retrospectively analyzed the role of maternal vascular and angiogenic factors at 18–26 weeks’ gestation in the development of sFGR. The analysis also included the evaluation of perinatal outcomes, the course of the early neonatal period, stay in the intensive care unit, and length of hospital stay at the second stage of nursing.
Results: The mean age of participants was 31.6 (5.13) years. There were no statistically significant differences in concentrations of vascular and angiogenic factors, pregnancy and early neonatal outcomes. However, high concentrations of maternal VEGF-R1, VEGF-C, and HIF-1a were associated with neonatal mortality, and a high concentration of HIF-1a was associated with stillbirth. These findings may be used in clinical practice to predict antenatal and early neonatal loss. Pregnant women with sVEGF-R1 greater than 0.787 ng/mL and Ang-2 greater than 8255.91 ng/mL were found to have an increased risk of low-birthweight babies.
Conclusion: Comparative assessment of vascular and angiogenic factors in sFGR pregnancies offers the opportunity to prevent the progression of this condition by timely fetoscopic intervention, improve perinatal outcomes, and minimize the risks of severe antenatal and neonatal complications.
Authors’ contributions: Gladkova K.A., Sakalo V.A. – conception and design of the study, statistical analysis, manuscript drafting; Piskulina A.A., Frolova E.R., Kostyukov K.V. – material collection; Vtorushina V.V. – data and statistical analysis;
Khodzhaeva Z.S. – conception and design of the study, manuscript drafting and editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was supported by the Russian Foundation for Basic Research within the framework of Research Project № 121040600434-3.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Gladkova K.A., Sakalo V.A., Vtorushina V.V., Piskulina A.A., Frolova E.R., Kostyukov K.V., Khodzhaeva Z.S. The role of vascular and angiogenic factors in the development of selective fetal growth restriction in monochorionic multiple pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; 1: 48-54 (in Russian)
https://dx.doi.org/10.18565/aig.2022.278
Keywords
One of the most frequent (10–25%) complications of monochorionic twin pregnancy is selective fetal growth restriction (sFGR). With delayed diagnosis and inadequate obstetric management sFGR is associated with increased risk of adverse pregnancy outcomes, peri- and neonatal morbidity and mortality. The presumed cause of sFGR is the unequal placental territories shared by the fetuses [1]. At the same time, advances in fetal surgery with favorable pregnancy outcomes and better twin survival are achieved in sFGR in the case of timely diagnosis, dynamic ultrasound, and Doppler monitoring, allowing for determination of the optimal timing of intrauterine intervention and subsequent delivery.
The 2018 Delphi consensus on the definition and essential reporting parameters of sFGR in twin pregnancies established diagnostic criteria for sFGR, but no consensus was reached on the optimal timing of treatment and delivery [2]. Currently, therapeutic options include expectant management and emergency delivery in cases of high risk of antenatal fetal death. Surgical intrauterine intervention includes selective elimination of the growth restricted fetus or fetoscopic laser coagulation of placental anastomoses (FLCA). Selective termination of pregnancy contributes to a more predictable favorable outcome for the second twin, while FLCA can ensure the survival of both twins. However, the risk for antenatal death of the smaller twin and neurological complications for the larger one is quite high [3–5].
In this context, the study of vascular and angiogenic factors that are not only etiological, but can also be used to monitor the severity of sFGR and predicting fetal decompensation.
According to many studies, the volume of the placenta directly affects the mass and growth indicators of the newborn [6–8]. The main prerequisites for the development of fetal-placental dysfunction (FPD) are established early in pregnancy and are largely due to the features of vascularity and angiogenesis in both the uteroplacental and fetal-placental beds.
The monochorionic pregnancy complicated by sFGR is characterized by inhibition of angiogenesis. However, the available literature lacks research evidence on vascular and angiogenic factors in sFGR [8–10]. According to studies, the proteins of the vascular endothelial growth factor (VEGF) family, placental growth factor (PlGF), angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), a soluble form of the vascular endothelial growth factor receptor (sVEGF-R) have a significant influence on the development of this pathological process. Increased expression of soluble fms-like tyrosine kinase-1(sFlt-1) leads to an associated decrease in PlGF synthesis and changes in VEGF synthesis, which is one of the clearest signs of endothelial growth factor production imbalance [9, 10]. It should be noted that the effect of angiopoietin-2 (Ang-2) on angiogenesis depends on the concentration of VEGF-A. In the absence of VEGF-A, ANGPT-2 causes apoptosis and promotes vascular regression, while when the concentration of VEGFA increases, it stimulates angiogenesis [11, 12]. Lyall et al. reported that VEGF is secreted in significantly higher concentrations during the development of maternal blood hypoxia, which suggests significant hypoxemia when its levels are high [13].
In this regard, it is relevant to analyze the course of multiple twin pregnancy complicated by sFGR.
This study aimed to investigate the role of vascular endothelial factors in the development of sFGR in monochorionic twin pregnancy.
Materials and methods
This retrospective study was conducted at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology between January 2020 and December 2021. The study analyzed the course of 51 monochorionic diamniotic twin pregnancies and vascular and angiogenic factors in relation to the development of specific complications. Group 1 (n=31) consisted of patients with selective fetal growth restriction; Group 2 (n=20) consisted of patients with uncomplicated monochorionic twins.
Inclusion criteria: monochorionic diamniotic twins.
Exclusion criteria: monochorionic monoamniotic twins, dichorionic diamniotic twins, higher-order multiple pregnancies (triplets, quadruplets).
The diagnosis of sFGR was based on ultrasound findings that included the estimated fetal weight of a twin under the 10th percentile, estimated weight discordance greater than 25%, and impaired blood flow in the umbilical artery and the fetal ductus venosus in growth-restricted fetus.
The content of the following markers in peripheral blood plasma was determined by solid-phase enzyme immunoassay using the following test systems: Angiopoietin 2 (Ang-2) (RayBiotech), hypoxia-inducible growth factor 1α (HIF1α) (RayBiotech), the vascular endothelial growth factor-C (VEGF-C) (Invitrogen), soluble vascular endothelial growth factor receptor (sVEGF-R1) (Invitrogen), transforming growth factor β1 (TGF-β1) (Invitrogen). The results were recorded on an Infinite F50 Tablet Spectrophotometer (TECAN).
Concentrations of maternal plasma vascular and angiogenic factors were compared at 18 to 26 weeks of gestation to determine which factor was most important in the diagnosis of sFGR and the prediction of adverse pregnancy and neonatal outcomes.
Statistical analysis
Statistical analysis was performed using Microsoft Excel and IBM SPSS Statistics Standard Edition 23.0 statistical software. The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. The results were presented as mean and standard deviation M (SD) for data with a normal distribution. The Mann–Whitney test was used to compare two independent non-parametric samples. Medians (Me) and upper and lower quartiles (Q1; Q3) were assessed to describe quantitative data with non-normal distribution. The results were considered statistically significant at p<0.05.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Results
The age of the patients ranged from 19 to 45 years, averaging 31.6 (5.13) years. The ages of the pregnant women in Group 1 ranged from 21 to 45 years, while in Group 2 they were 22 to 44 years. Patients with sFGR were significantly younger than those in the comparison group (p<0.001).
Analysis of anthropometric parameters did not show differences between the groups. The BMI was significantly higher in patients with normal monochorionic pregnancy and was 27.46 (2.998) kg/m2, while in the sFGR group it was 25.76 (3.49) kg/m2 (p=0.019).
Comparison of gestational age at delivery showed that it was 36.2 [35.85; 36.5] weeks in patients with uncomplicated pregnancy (Group 2), whereas in Group 1 it was 32.4 [31.45; 34.8] weeks (p<0.001).
Stillbirth occurred in 2/57 (3.5%) in Group 1; no cases of antenatal death were diagnosed in Group 2, p=0.249. The early neonatal mortality was 7.02% (4/57) in Group 1 and 2.5% (1/40) in Group 2, p=0.272. Among the surviving neonates, 59/90 (65.56%) had normal birth weight and 31/90 (34.44%) were below the 3rd percentile.
We performed a comparative analysis of maternal plasma concentrations of vascular and angiogenic factors at 18–26 weeks of gestation to determine which was most significant in the diagnosis of sFGR and the prediction of adverse pregnancy and neonatal outcomes (Table 1).
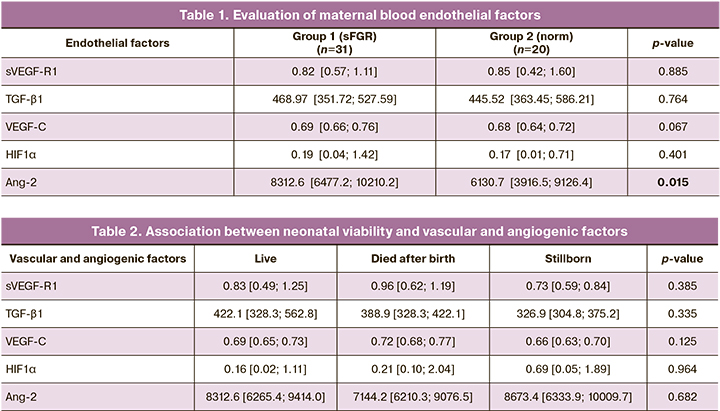
As shown in Table 1, there were no significant differences between both groups in plasma concentrations of the factors except for angiopoietin-2 (Ang-2), which was significantly higher in the sFGR group (8312.6 [6477.2; 10210.2] versus 6130.7 [3916.5; 9126.4], p=0.015). It is possible that excessive secretion of angiopoietin-2 by endothelial cells leads to impaired fetal blood vessel formation and mediates the development of selective fetal growth restriction.
A comparative analysis of the associations between vascular/angiogenic factors and pregnancy and early neonatal outcomes did not show significant statistical differences (Table 2). However, early neonatal losses were characterized by increased maternal sVEGF-R1, VEGF-C, and HIF1α. Stillbirths were associated with high concentration of HIF1α.
The high maternal plasma HIF1α concentration is a consequence of oxidative stress and endothelial dysfunction. Given the pronounced placental dysfunction, a severe neonatal condition and high neonatal mortality are associated with elevated HIF1α.
Analysis of the impact of vascular and angiogenic factors on neonatal viability did not show diagnostically significant differences (Table 2).
There were statistically significant differences in birthweight and body length parameters, which was due to earlier delivery in Group 1 patients. Apgar scores for newborns were also significantly lower in Group 1, which was associated with a higher degree of prematurity and high morbidity (Table 3).
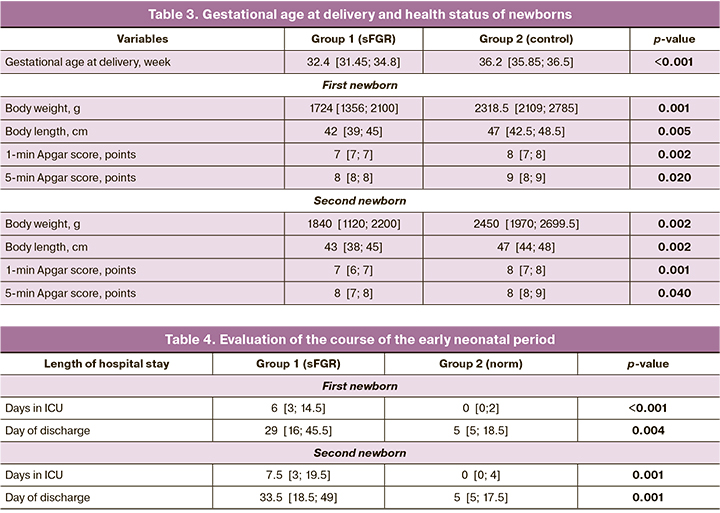
The evaluation of the course of the early neonatal period, stay in the intensive care unit (ICU), and the duration of hospitalization at the second stage of nursing is presented in Table 4.
Healthy infants were observed significantly more frequently in the comparison group, due to the timing of their delivery and the absence of selective fetal growth restriction syndrome.
Analysis of the early neonatal period showed a significant difference in the length of stay in the NICU of newborns from Group 1, p<0.0001, due to the degree of prematurity and high morbidity.
Discussion
In the scientific world, special attention is paid to the search for molecular factors to predict complications of monochorionic multiple pregnancies. Researchers have observed elevated maternal plasma TGF-β1 concentrations in selective fetal growth restriction. According to the literature, high levels of TGF-β1 in decidual tissue suppress the activation of specific subsets of DNA.
This contributes to uteroplacental dysfunction and leads to the development of preeclampsia [14, 15]. Impaired expression of this factor can lead to pregnancy loss [16]. Probably, the genesis of selective fetal growth restriction syndrome in monochorionic multiple pregnancies is also associated with uteroplacental dysfunction. However, in our study, TGF-β1 did not affect sFGR development and fetal viability, probably due to a small sample size.
The results of our study revealed elevated levels of angiogenic Ang-2 and antiangiogenic sVEGF-R1 factors in the plasma of patients with selective fetal growth restriction in the second trimester of pregnancy. Our findings are consistent with the results of previous studies and provide insight into the possible pathogenesis of sFGR development [17–21]. It is known that sVEGF-R1 is a negative regulator of angiogenesis that inhibits excessive blood vessel formation. Ang-2 prevents vascular stabilization, causes apoptosis, and promotes vascular regression [11]. Due to the imbalance of these factors, the process of placental angiogenesis is altered, leading to the formation of selective fetal growth.
The angiogenic VEGF-C factor contributes to the formation of fetal anastomoses [22]. Large placental arterio-arterial anastomoses are known to be the most common in selective fetal growth restriction. However, in our study, we did not find a significant effect of VEGF-C on the development of sFGR.
Our results in studying vascular and angiogenic factors confirmed the multidirectional nature of the identified secretion patterns in sFGR in monochorionic twin pregnancies.
Conclusion
Analysis of the effects of vascular and angiogenic factors on fetal viability showed no diagnostically significant differences. However, high concentrations of maternal VEGF-R1, VEGF-C, and HIF-1a in the second trimester of pregnancy were associated with neonatal mortality, and a high concentration of HIF-1a was associated with stillbirth. These findings may be used in clinical practice to predict antenatal and early neonatal loss.
More studies on a larger cohort of patients with expanded systemic analysis of the course of multiple pregnancies complicated by sFGR will optimize the obstetric management strategy, thus improving perinatal outcomes in monochorionic twin pregnancies and reducing the risk of severe perinatal and neonatal complications.
References
- Mackie F.L., Morris R.K., Kilby M.D. The prediction, diagnosis and management of complications in monochorionic twin pregnancies: The OMMIT (Optimal Management of Monochorionic Twins) study. BMC Pregnancy Childbirth. 2017; 17(1): 153. https://dx.doi.org/10.1186/s12884-017-1335-3.
- Khalil A., Beune I., Hecher K., Wynia K., Ganzevoort W., Reed K. et al. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: a Delphi procedure. Ultrasound Obstet. Gynecol. 2019; 53(1): 47-54. https://dx.doi.org/10.1002/uog.19013.
- Parra-Cordero M., Bennasar M., Martínez J.M., Eixarch E., Torres X., Gratacós E. Cord occlusion in monochorionic twins with early selective intrauterine growth restriction and abnormal umbilical artery Doppler: a consecutive series of 90 cases. Fetal Diagn. Ther. 2016; 39(3): 186-91.https://dx.doi.org/10.1159/000439023.
- Chalouhi G.E., Marangoni M.A., Quibel T., Deloison B., Benzina N., Essaoui M. et al. Active management of selective intrauterine growth restriction with abnormal Doppler in monochorionic diamniotic twin pregnancies diagnosed in the second trimester of pregnancy. Prenat. Diagn. 2013; 33(2): 109-15. https://dx.doi.org/10.1002/pd.4031
- Townsend R., D'Antonio F., Sileo F.G., Kumbay H., Thilaganathan B., Khalil A. Perinatal outcome of monochorionic twin pregnancy complicated by selective fetal growth restriction according to management: systematic review and meta‐analysis. Ultrasound Obstet. Gynecol. 2019; 53(1): 36-46. https://dx.doi.org/10.1002/uog.20114.
- Wang X., Li L., Yuan P., Zhao Y., Wei Y. Placental characteristics in different types of selective fetal growth restriction in monochorionic diamniotic twins. Acta Obstet. Gynecol. Scand. 2021; 100(9): 1688-93. https://dx.doi.org/10.1111/aogs.14204.
- Valsky D.V., Eixarch E., Martinez J.M., Crispi F., Gratacós E. Selective intrauterine growth restriction in monochorionic twins: pathophysiology, diagnostic approach and management dilemmas. Semin. Fetal Neonatal Med. 2010; 15(6): 342-8. https://dx.doi.org/10.1016/j.siny.2010.07.002.
- Wu J., He Z., Gao Y., Zhang G., Huang X., Fang Q. Placental NFE2L2 is discordantly activated in monochorionic twins with selective intrauterine growth restriction and possibly regulated by hypoxia. Free Radic. Res. 2017; 51(4): 351-9. https://dx.doi.org/10.1080/10715762.2017.1315113.
- Kaufmann P., Mayhew T.M., Charnock-Jones D.S. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004; 25(2-3): 114-26. https//dx.doi.org/10.1016/j.placenta.2003.10.009.
- Lyall F., Greer I.A., Boswell F., Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br. J. Obstet. Gynaecol. 1997; 104(2): 223-8. https://dx.doi.org/10.1111/j.1471-0528.1997.tb11050.x.
- Kappou D., Sifakis S., Konstantinidou A., Papantoniou N., Spandidos D.A. Role of the angiopoietin/Tie system in pregnancy (Review). Exp. Ther. Med. 2015; 9(4): 1091-6. https://dx.doi.org/10.3892/etm.2015.2280.
- Barut F., Barut A., Gun B.D., Kandemir N.O., Harma M.I., Harma M. et al. Intrauterine growth restriction and placental angiogenesis. Diagn. Pathol. 2010; 5: 24. https://dx.doi.org/10.1186/1746-1596-5-24.
- Hunter A., Aitkenhead M., Caldwell C., McCracken G., Wilson D., McClure N. Serum levels of vascular endothelial growth factor in preeclamptic and normotensive pregnancy. Hypertension. 2000; 36(6): 965-9. https://dx.doi.org/10.1161/01.hyp.36.6.965.
- Zhang J., Dunk C.E., Shynlova O., Caniggia I., Lye S.J. TGFb1 suppresses the activation of distinct dNK subpopulations in preeclampsia. EBioMedicine. 2019; 39: 531-9. https://dx.doi.org/10.1016/j.ebiom.2018.12.015.
- Basavaraja R., Drum J.N., Sapuleni J., Bibi L., Friedlander G., Kumar S. et al. Downregulated luteolytic pathways in the transcriptome of early pregnancy bovine corpus luteum are mimicked by interferon-tau in vitro. BMC Genomics. 2021; 22(1): 452. https://dx.doi.org/10.1186/s12864-021-07747-3.
- Mert I., Oruc A.S., Yuksel S., Cakar E.S., Buyukkagnici U., Karaer A., Danisman N. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012; 38(4): 658-64. https://dx.doi.org/10.1111/j.1447-0756.2011.01771.x.
- Gunatillake T., Yong H.E., Dunk C.E., Keogh R.J., Borg A.J., Cartwright J.E. et al. Homeobox gene TGIF-1 is increased in placental endothelial cells of human fetal growth restriction. Reproduction. 2016; 152(5): 457-65. https://dx.doi.org/10.1530/REP-16-0068.
- Ivanov D., Mazzoccoli G., Anderson G., Linkova N., Dyatlova A., Mironova E. et al. Melatonin, its beneficial effects on embryogenesis from mitigating oxidative stress to regulating gene expression. Int. J. Mol. Sci. 2021;22(11): 5885. https://dx.doi.org/10.3390/ijms22115885.
- Anh N.D., Thuong P.H., Sim N.T., Thao T.T.P., Anh L.T.L., Canh T.T.T. et al. Maternal vascular endothelial growth factor receptor and interleukin levels in pregnant women with twin-twin transfusion syndrome. Int. J. Med. Sci. 2021; 18(14): 3206-13. https://dx.doi.org/10.7150/ijms.61014.
- Olaya-C.M., Garrido M., Hernandez-Losa J., Sesé M., Ayala-Ramirez P., Somoza R. et al. The umbilical cord, preeclampsia and the VEGF family. Int. J. Womens Health. 2018; 10: 783-95. https://dx.doi.org/10.2147/IJWH.S174734.
- He Y., Smith S.K., Day K.A., Clark D.E., Licence D.R., Charnock-Jones D.S. Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol. Endocrinol. 1999; 13(4): 537-45. https://dx.doi.org/10.1210/mend.13.4.0265.
- Tammela T., Zarkada G., Nurmi H., Jakobsson L., Heinolainen K., Tvorogov D. et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat. Cell Biol. 2011; 13(10): 1202-13. https://dx.doi.org/10.1038/ncb2331.
Received 17.11.2022
Accepted 22.12.2022
About the Authors
Kristina A. Gladkova, Ph.D., Senior Researcher at the Fetal Medicine Unit, Institute of Obstetrics, Head of the 1st Obstetric Department of Pregnancy Pathology,Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-07-88, k_gladkova@oparina4.ru, https://orcid.org/0000-0001-8131-4682,
117997, Russia Moscow, Ac. Oparina str. 4.
Viktoriya A. Sakalo, Ph.D., Junior Researcher at the Department of Pregnancy Pathology, Institute of Obstetrics, doctor at the 1st Obstetric Department of Pregnancy Pathology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-07-88, v_sakalo@oparina4.ru, https://orcid.org/0000-0002-5870-4655, 117997, Russia Moscow, Ac. Oparina str. 4.
Valentina V. Vtorushina, Ph.D., doctor of laboratory and clinical diagnostics at the Laboratory of Clinical Immunology, Academician V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, +7(495)438-11-83, v_vtorushina@oparina4.ru, 117997, Russia Moscow, Ac. Oparina str. 4.
Aleksandra A. Piskulina, clinical resident, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-07-88, a_piskylina@oparina4.ru,
117997, Russia Moscow, Ac. Oparina str. 4.
Ekaterina R. Frolova, graduate student at the High Risk Pregnancy Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-07-88, e_frolova@oparina4.ru, https://orcid.org/0000-0003-2817-3504, 117997, Russia Moscow, Ac. Oparina str. 4.
Kirill V. Kostyukov, Dr. Med. Sci, Senior Researcher at the Fetal Medicine Unit, Institute of Obstetrics, Head of the Department of the Ultrasound and Functional Diagnosis, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-25-29, k_kostyukov@oparina4.ru, https://orcid.org/0000-0003-3094-4013,
117997, Russia Moscow, Ac. Oparina str. 4.
Zulfiya S. Khodzhaeva, Dr. Med. Sci., Professor, Deputy Director of Obstetrics Institute, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-07-88, z_khodzhaeva@oparina4.ru, https://orcid.org/0000-0001-8159-3714, 117997, Russia Moscow, Ac. Oparina str. 4.



