The role of vasculogenic and angiogenic factors in perinatal outcomes, neonatal morbidity, and mortality in complicated monochorionic multiple pregnancies
Gladkova K.A., Khodzhaeva Z.S., Sakalo V.A., Frolova E.R., Shakaya M.N., Kirtbaya A.R.
Relevance: Selective fetal growth restriction (sFGR) and twin-to-twin transfusion syndrome (TTTS) are the most common complications of monochorionic multiple pregnancies (10–35%) and are characterized by high perinatal mortality and a wide range of neonatal complications. The main etiological factor in the development of TTTS and sFGR is placental dysfunction, which is primarily associated with vasculogenic and angiogenic factors during monochorionic pregnancy.
Objective: This study aimed to investigate the role of vascular endothelial growth factors in perinatal outcomes, neonatal morbidity, and mortality in complicated monochorionic twin pregnancies.
Materials and methods: This was a prospective study with comparative analysis of the relationship between vasculogenic and angiogenic factors and perinatal outcomes, neonatal morbidity, and mortality in monochorionic multiple pregnancies complicated by sFGR and TTTS. The study included 84 pregnant women with monochorionic diamniotic twins and 152 newborns. An analysis of the course of pregnancy and childbirth, perinatal outcomes, and neonatal morbidity was also performed. The study also analyzed vasculogenic and angiogenic factors in the second trimester of pregnancy depending on the development of specific complications.
Results: Antenatal losses occurred in 6.45% (n=2) of patients in the TTTS group (p=0.249). Early neonatal mortality was observed in 14.3% (n=4) of the TTTS group and in 5% (n=1) of the sFGR group (p=0.272). The gestational age at delivery was significantly different in the complicated pregnancy groups (32.2–32.4 weeks) compared to the control group (36.2 weeks); (p<0.001). A study of vasculogenic and angiogenic factors in the second trimester of pregnancy showed an increase in maternal blood VEGF-C to 0.66 ng/ml and HIF-1a to 0.69 ng/ml, a decrease in VEGF-R1 to 0.73 ng/ml with neonatal mortality. In case of stillbirth, a high concentration of HIF-1a was observed (0.69 ng/ml). Changes in maternal blood concentrations of VEGF-R1 (0.787 ng/ml) and ANGPT2 (8255.91 pg/ml) were associated with the risk of fetal growth restriction. Analysis of the early neonatal period showed high respiratory, cardiovascular, and gastrointestinal morbidity in neonates from the TTTS and sFGR groups associated with prematurity and intrauterine deficits.
Conclusion: An association was found between changes in vasculogenic and angiogenic factors in the blood of pregnant women and high neonatal morbidity and mortality in complicated monochorionic twins. Specifically, the concentrations of VEGF-C and HIF-1a increased while VEGF-R1 decreased. The data obtained make it possible to predict antenatal and neonatal risks, improve perinatal outcomes, and minimize the risk of severe neonatal complications.
Authors' contributions: Gladkova K.A., Sakalo V.A. – conception and design of the study, statistical analysis, drafting of the manuscript; Khodzhaeva Z.S. – concept and design of the study, editing, structuring and finalizing of the manuscript; Frolova E.R., Shakaya M.N., Kirtbaya A.R. – collection and analysis of material, statistical analysis.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the State Assignment of the Ministry of Health of the Russian Federation No. 121040600434-3 “Molecular biological determinants of the formation of complications of multiple pregnancy: pathogenetic approaches to prevention and treatment.”
Ethical approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Gladkova K.A., Khodzhaeva Z.S., Sakalo V.A., Frolova E.R., Shakaya M.N., Kirtbaya A.R. The role of vasculogenic and angiogenic factors in perinatal outcomes, neonatal morbidity, and mortality in complicated monochorionic multiple pregnancies.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (12): 95-103 (in Russian)
https://dx.doi.org/10.18565/aig.2023.243
Keywords
Monochorionic multiple pregnancy is a high-risk condition owing to the increased incidence of maternal and fetal complications of varying severity, adversely impacting obstetric and perinatal outcomes [1].
Selective fetal growth restriction (sFGR) is the most common complication, occurring in up to 25% of cases [2]. Its primary etiological factor is the uneven division of the placental site between twins and the presence of vascular anastomoses within the placenta. The second most common complication, twin-to-twin transfusion syndrome (TTTS), occurs in 8–15% of cases and develops due to an imbalanced shunting of intertwin blood flow through placental anastomoses [3].
Several studies indicate a direct correlation between the volume of the placental area and the weight and height parameters of newborns [4–6]. Vascular anastomoses present a substrate for specific complications in monochorionic multiple pregnancies [7–9]. Disturbances in the fetus-placenta system are primarily established during early pregnancy, specifically during trophoblast invasion, and are influenced by vascular and angiogenic characteristics in the uteroplacental and feto-placental beds.
Adverse perinatal outcomes in complicated monochorionic pregnancies have driven the search for specific predictors of obstetric failure. Understanding the vasculogenic and angiogenic factors in sFGR and TTTS is crucial, serving as a unique tool for monitoring fetal conditions and forming the basis for intrauterine intervention or early delivery. Notably, both sFGR and TTTS inhibit angiogenesis. However, the literature lacks data on vasculogenic features in monochorionic placentation.
Studies have indicated that the anti-angiogenic factor soluble fms-like tyrosine kinase 1 (sFlt-1) reduces cytotrophoblast invasion rates. Elevated sFlt-1 expression correlates with decreased placental growth factor (PlGF) synthesis and altered vascular endothelial growth factor (VEGF) synthesis, marking an imbalance in endothelial growth factor production [7]. VEGF primarily stabilizes vascular endothelial cells, which are crucial for kidney, liver, and brain endothelial functions. sFlt-1, a variant of the membrane-bound VEGF receptor, possesses antiangiogenic properties and is mainly secreted by syncytiotrophoblasts, impeding VEGF and PlGF interactions with their respective receptors [8].
Angiogenic and anti-angiogenic factors are released into the maternal bloodstream, exhibiting quantitative and qualitative variability based on gestational age. Lyall F. et al. (1997) documented increased VEGF secretion in mothers experiencing hypoxia, suggesting a possible association between severe hypoxemia and high VEGF concentrations [9].
Another aspect of significance is the diagnostic value of sVEGFR-1 in the umbilical cord blood. Kaufmann P. et al. (2004) demonstrated increased sVEGFR-1 levels in both the early and late second trimesters of normal multiple monochorionic pregnancies [10]. In pregnancies diagnosed with sFGR, significant increases in sVEGFR-1 levels were observed only in the late second trimester of pregnancy. PlGF levels were notably lower in both the early and late second trimesters in women with sFGR, correlating with lower birth weight fetuses. Apart from VEGF, transforming growth factor β1 (TGF-β1) plays a crucial role in placentation regulation; its impaired expression potentially leading to pregnancy loss [11, 12]. TGF-β1 is involved in angiogenesis, cytotrophoblast implantation and differentiation, altering L-arginine metabolism, which disrupts nitric oxide generation and initiates structural changes in the vascular wall.
Wang X.-H. et al.'s study exhibits the link between hypoxia-inducible factor (HIF) and preeclampsia and fetal growth restriction [13]. Oxygen levels critically regulate trophoblast invasion and proliferation, directing trophoblast stem cell differentiation through HIF signaling pathways under low-oxygen conditions.
Several studies have reported fetal growth restriction resulting from an imbalance in the concentrations of angiopoietin 1 and 2. Gunatillake T. et al. described premature maturation of terminal villi capillaries due to increased angiopoietin 1 (ANGPT-1) concentration and decreased angiopoietin 2 (ANGPT-2) concentration, leading to preeclampsia and fetal growth restriction [14].
Comparison of pro- and anti-angiogenic factors in complicated multiple pregnancies will aid in refining the examination and management algorithms for pregnant women with multiple pregnancies, ultimately improving perinatal outcomes and reducing severe perinatal and neonatal complications.
This study aimed to investigate the prognostic role of vascular endothelial growth factors in perinatal outcomes, neonatal morbidity, and mortality in monochorionic multiple pregnancies complicated with TTTS and sFGR.
Materials and methods
This prospective study was conducted at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation from January 2020 to December 2021. The study analyzed the course of pregnancy in 84 women with monochorionic diamniotic twins and 152 newborns born alive, and vasculogenic and angiogenic factors in relation to the development of specific complications. Group 1 consisted of patients with TTTS (n=33), Group 2 included patients with sFGR (n=31), and Group 3 included patients with uncomplicated monochorionic twins (n=20).
The inclusion criteria were monochorionic diamniotic twins and the presence of two living fetuses.
The non-inclusion criteria were monochorionic monoamniotic twins, dichorionic diamniotic twins, and higher-order multiple pregnancies (triplet or greater).
TTTS diagnosis was based on the following echographic criteria: detection of oligohydramnios in the donor fetus (maximum vertical pocket (MVP) less than 2 cm) and polyhydramnios in the recipient fetus (MVP > 8–10 cm). The severity of TTTS was assessed according to the classification proposed by Quintero et al. (1999) [15].
The diagnosis of sFGR was based on ultrasound data, including the estimated weight of one of the fetuses being less than the 10th percentile, the difference in the estimated weight of the fetuses being more than 25%, impaired blood flow in the umbilical cord artery, and ductus venosus of the fetus with growth restriction.
The levels of the following markers in the peripheral blood plasma were determined by enzyme-linked immunosorbent assay using the following test systems: angiopoietin 2 (ANPGT2) (RayBiotech), hypoxia inducible factor 1α (HIF1α) (RayBiotech), vascular endothelial growth factor C (VEGF-C) (Invitrogen), soluble vascular endothelial growth factor receptor (sVEGF-R1) (Invitrogen), and transforming growth factor β1 (TGF-β1) (Invitrogen). The results were recorded using an Infinite F50 tablet spectrophotometer (TECAN).
Statistical analysis
Statistical analysis was performed using Microsoft Excel and IBM SPSS Statistics Standard Edition 23.0 statistical software. Quantitative variables showing normal distribution were expressed as means and standard deviations and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Normality of distribution was assessed using the Kolmogorov–Smirnov test. Differences between the groups for continuous variables were assessed using Mann–Whitney nonparametric test. Categorical data are described as counts and percentages. The Kruskal–Wallis test was used to compare numerical data with non-normal distributions between three or more groups. Categorical variables were compared using the χ2 test (chi-square, contingency table analysis) and the Wilcoxon signed-rank test. Results were considered significant at p<0.05.
The predictive diagnostic potential of growth factors for the probability of birth of newborns with normal birth weight was assessed using ROC analysis (allowing evaluation of the diagnostic effectiveness of the prognostic model), determination of the area under the curve (AUC), and determination of its specificity and sensitivity.
The study was reviewed and approved by the Research Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation/
Results
Gestational age at delivery was later in uncomplicated pregnancies (Group 3) - 36.2 weeks (35.85; 36.5), compared to Group 1, 32.2 (28.4; 34.1) and Group 2, 32.4 (31.45; 34.8) weeks (p<0.001) (Table 1).
Analysis of the impact of vasculogenic and angiogenic factors on newborn viability did not show any significant differences (Table 2). However, neonatal mortality was characterized by an increase in the levels of VEGF-R1, VEGF-C, and HIF-1a. In the case of stillbirth, there were high concentrations of HIF-1a.
Of the surviving newborns, 86/140 (61.4%) had a normal birth weight and 54/140 (38.6%) were growth restricted below the 3rd percentile.
When assessing the probability of low-birth-weight delivery in relation to the levels of TGFb1 – 468.92 pg/mL, VEGF-C – 0.69 ng/mL and HIF-1a – 0.19 ng/mL in maternal blood plasma during pregnancy using ROC-curves. analysis did not produce statistically significant models (p=0.084, p=0.549, and p=0.758, respectively).
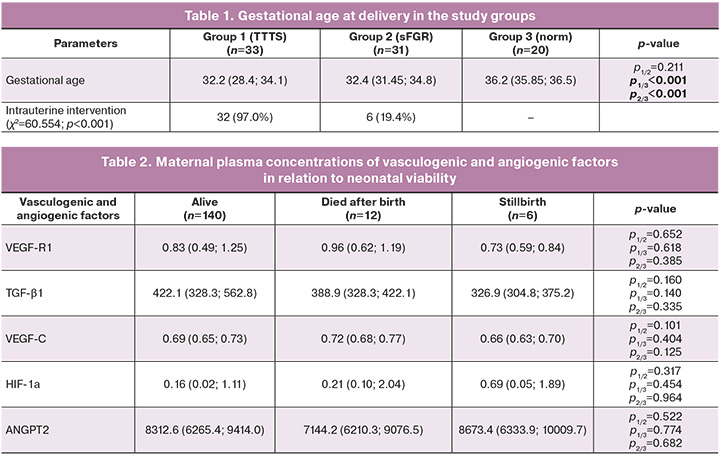
When assessing the probability of low-birth-weight delivery in relation to the level of VEGF-R1 in the maternal blood plasma during pregnancy using ROC analysis, the following results were obtained (Fig. 1).
The area under the ROC curve was 0.602±0.051 with 95% CI: 0.502–0.702). The predictive model was statistically significant (p=0.042).
The threshold value of VEGF-R at the cutoff point was 0.787. Patients with VEGF-R levels > 0.787 ng/ml were expected to have a high likelihood of low-birth-weight delivery. The sensitivity and specificity of this method were 66.7% and 51.2%, respectively.
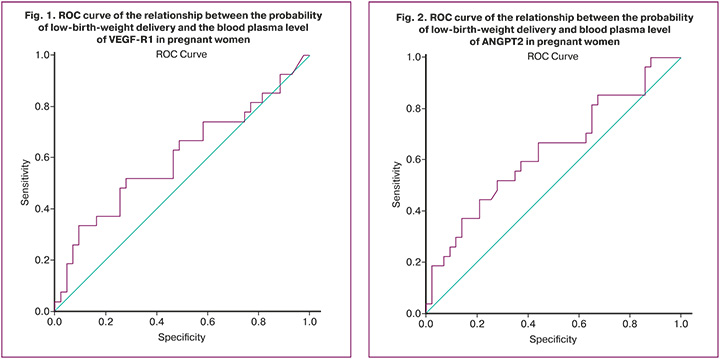
When assessing the probability of low-birth-weight delivery in relation to the level of ANPGT2 in maternal blood plasma during pregnancy using ROC analysis, the following results were obtained (Fig. 2).
The area under the ROC curve was 0.632±0.049 with 95% CI: 0.535–0.728. The predictive model was statistically significant (p=0.009).
The ANPGT2 threshold value at the cut-off point was 8255.91. Patients with ANPGT2 greater than 8255.91 ng/ml were expected to have a high likelihood of low-birth-weight delivery. The sensitivity and specificity of this method were 66.7% and 55.8%, respectively.
Table 3 presents the health status of newborns in the study groups. When studying weight and height indicators, statistically significant differences were revealed, which were due to the earlier gestational age at delivery in patients in groups 1 and 2. Apgar scores were also significantly lower in infants from these groups, which is associated with higher rates of prematurity and higher morbidity.
The number of boys and girls born did not differ significantly between groups (Table 4).
The incidence of newborns with monochorionic multiple pregnancy is presented in Table 5. Healthy children were observed significantly more often in the comparison group owing to the timing of their delivery. It is noteworthy the high incidence of diseases of the bronchopulmonary system in the TTTS and sFGR groups, which is mainly associated with the degree of prematurity. Respiratory distress syndrome, congenital pneumonia, and bronchopulmonary dysplasia were significantly more common in groups 1 and 2, and transient tachypnea in newborns in Group 3.
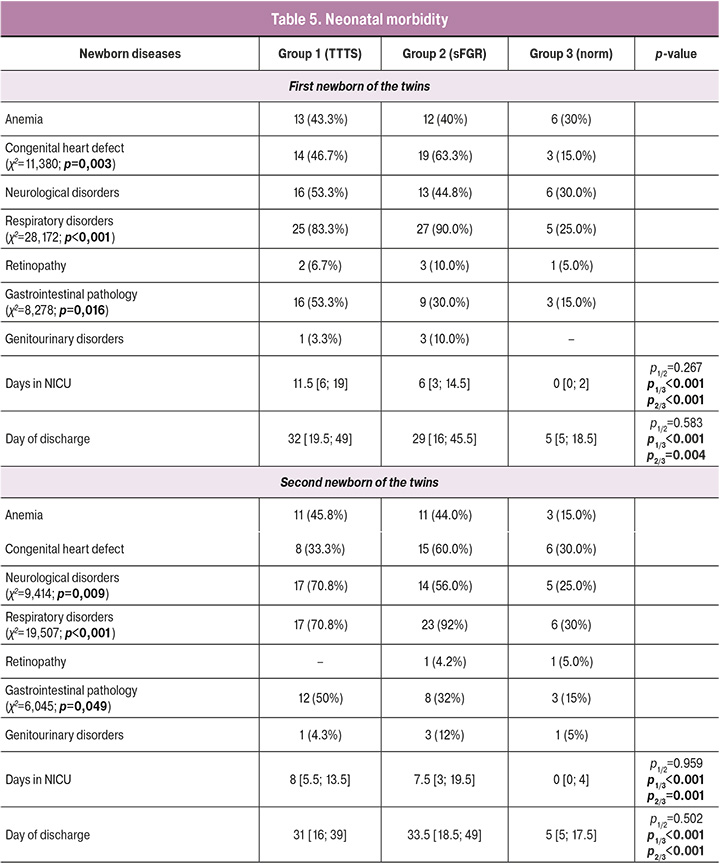
When assessing perinatal lesions of the central nervous system (CNS), intraventricular hemorrhage of grades I–II was noted in the first fetus (recipient fetus in the TTTS group, a fetus with normal weight in the sFGR group) in all groups (in Group 1 (53.3%), Group 2 (44.8%), Group 3 (30%), p=0.0105), as well as manifestations of cerebral ischemia, which were significantly more often diagnosed in groups 1 and 2 (16.67% and 3.57%, p<0.0001). Lesions of the central nervous system of the second fetus (donor fetus in the TTTS group, a fetus with a lower body weight in the sFGR group) were significantly more often recorded in groups 1 and 2 (in Group 1 – 0.8%, in Group 2 – 56%, Group 3 – 25%, p=0.009). Severe CNS lesions, such as grade III–IV intraventricular hemorrhage and periventricular leukomalacia, were observed in newborns only in the TTTS group.
An analysis of the cardiovascular system of newborns showed that congenital heart defects of the first fetus (recipient fetus in the TTTS group, a fetus with normal weight in the sFGR group) were diagnosed significantly more often than uncomplicated monochrionic twins (46.7%, 63.3%, and 15%, respectively; p=0.003). The comparison of cardiovascular anomalies in the second fetus (donor fetus in the TTTS group and lower birth weight fetus in the sFGR group) did not show statistically significant differences. These changes are probably due to uneven blood flow from one fetus to another in the group of complicated monochorionic twins.
When studying the pathology of the gastrointestinal tract (GIT) of newborns, it was revealed that intrauterine peritonitis and necrotizing enterocolitis in both newborns were significantly more often diagnosed in groups 1 and 2. Clinical manifestations of intestinal obstruction were not detected in the control group, but in groups 1 and 2 they were 2.15% and 1.5%, respectively, which was not statistically significant, p=0.0553. The high incidence of gastrointestinal diseases is probably due to the characteristic features of intrauterine blood supply and the high degree of prematurity of newborns in groups 1 and 2.
Analysis of the early neonatal period showed a significant difference in stay in the neonatal intensive care unit (NICU) between groups 1 and 2 (p<0.0001). The time spent by children in neonatal departments was significantly longer in the complicated twin groups due to the degree of prematurity and high morbidity.
Discussion
Recent studies have been devoted to the search for molecular determinants of the development of complications in monochorionic multiple pregnancies, with the aim of timely prevention and improvement of perinatal outcomes. Researchers have determined the multidirectional nature of the secretion of vasculogenic and angiogenic factors in complicated monochorionic multiple pregnancies [16, 17]. We have shown that an increase in the maternal blood levels of VEGF-R1, VEGF-C, and HIF-1α increases the risk of early neonatal mortality, and a significant increase in HIF-1α concentration may lead to stillbirth.
sVEGF-R1 is known to be a negative regulator of angiogenesis, inhibiting excessive blood vessel formation. Angiogenic VEGF-C promotes the formation of fetal anastomoses. Due to the imbalance of these factors, the process of vasculogenesis and angiogenesis of the placenta is disturbed, leading to the formation of specific complications in monochorionic multiple pregnancies. Neonatal morbidity and mortality increase in the absence of timely treatment or in cases of incorrectly chosen management strategies.
HIF-1α is a subunit of a heterodimeric transcription factor that is induced under hypoxia [18]. With unbalanced blood flow between fetuses and the development of TTTS and sFGR, the level of this protein is elevated, increasing stillbirth rates. Alterations in VEGF-R1 and ANPGT2 concentrations in the maternal blood increase the risk of fetal growth restriction.
Analysis of the early neonatal period showed a high incidence of respiratory, cardiovascular, and GI morbidity in newborns in the TTTS and sFGR groups. This is probably because of premature birth and intrauterine deficits. For example, due to uneven blood flow in the monochorionic placenta, there is an increase in the circulating blood volume in the bloodstream of one fetus and a decrease in the other fetus. This redistribution of blood leads to a decrease in fetal cardiac output, which, with prolonged progression of the underlying disease, contributes to the formation of congenital heart defects in the fetus such as pulmonary atresia and coarctation of the aorta, pathologically affecting the gestational development of all systems (CNS and GI).
Conclusion
Current ideas regarding the pathogenesis of complications in monochorionic multiple pregnancies are diverse. At the same time, no specific markers for the development of sFGR and TTTS have been identified, and, most importantly, no predictors of adverse perinatal outcomes have been identified. Nevertheless, the study of vasculogenic and angiogenic factors in the most common complications of monochorionic twin pregnancies suggests their determinant role in the development of these complications and outlines the vectors of scientific search aimed at improving the effectiveness of obstetric management, including fetoscopic interventions.
References
- Cheong-See F., Schuit E., Arroyo-Manzano D., Khalil A., Barrett J., Joseph K.S. et al. Prospective risk of stillbirth and neonatal complications in twin pregnancies: systematic review and meta-analysis. BMJ. 2016; 354: i4353.https://dx.doi.org/10.1136/bmj.i4353.
- Mackie F.L., Morris R.K., Kilby M.D. The prediction, diagnosis and management of complications in monochorionic twin pregnancies: the OMMIT (Optimal Management of Monochorionic Twins) study. BMC Pregnancy Childbirth. 2017;17(1):153. https://dx.doi.org/10.1186/s12884-017-1335-3.
- D’Antonio F., Benlioglu C., Sileo F.G., Thilaganathan B., Papageorghiou A., Bhide A., Khalil A. Perinatal outcomes of twin pregnancies affected by early twin-twin transfusion syndrome: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2020; 99(9): 1121-34. https://dx.doi.org/10.1111/aogs.13840.
- Wang X., Li L., Yuan P., Zhao Y., Wei Y. Placental characteristics in different types of selective fetal growth restriction in monochorionic diamniotic twins. Acta Obstet. Gynecol. Scand. 2021;100(9):1688-93. https://dx.doi.org/10.1111/aogs.14204.
- Valsky D.V., Eixarch E., Martinez J.M., Crispi F., Gratacós E. Selective intrauterine growth restriction in monochorionic twins: pathophysiology, diagnostic approach and management dilemmas. Semin. Fetal. Neonatal. Med. 2010;15(6):342-8. https://dx.doi.org/10.1016/j.siny.2010.07.002.
- Wu J., He Z., Gao Y., Zhang G., Huang X., Fang Q. Placental NFE2L2 is discordantly activated in monochorionic twins with selective intrauterine growth restriction and possibly regulated by hypoxia. Free Radic. Res. 2017;51(4):351-9. https://dx.doi.org/10.1080/10715762.2017.1315113.
- Ivanov D., Mazzoccoli G., Anderson G., Linkova N., Dyatlova A., Mironova E.et al. Melatonin, its beneficial effects on embryogenesis from mitigating oxidative stress to regulating gene expression. Int. J. Mol. Sci. 2021;22(11):5885.https://dx.doi.org/10.3390/ijms22115885.
- Demir R., Kayisli U., Seval Y., Celik-Ozenci C., Korgun E., Demir-Weusten A., Huppertz B. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta. 2004;25(6):560-72. https://dx.doi.org/10.1016/j.placenta.2003.11.011.
- Lyall F., Greer I.A., Boswell F., Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br. J. Obstet. Gynaecol. 1997;104(2):223-8.https://dx.doi.org/10.1111/j.1471-0528.1997.tb11050.x.
- Kaufmann P., Mayhew T.M., Charnock-Jones D.S. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25(2-3):114-26. https://dx.doi.org/10.1016/j.placenta.2003.10.009.
- Tammela T., Zarkada G., Nurmi H., Jakobsson L., Heinolainen K., Tvorogov D. et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat. Cell Biol. 2011;13(10):1202-13.https://dx.doi.org/10.1038/ncb2331.
- He Y., Smith S.K., Day K.A., Clark D.E., Licence D.R., Charnock-Jones D.S. Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol. Endocrinol. 1999;13(4):537-45. https://dx.doi.org/10.1210/mend.13.4.0265.
- Wang X.-H., Xu S., Zhou X.-Y., Zhao R., Lin Y., Cao J. et al. Low chorionic villous succinate accumulation associates with recurrent spontaneous abortion risk. Nat. Commun. 2021;12(1):3428. https://dx.doi.org/10.1038/s41467-021-23827-0.
- Gunatillake T., Yong H.E., Dunk C.E., Keogh R.J., Borg A.J., Cartwright J.E. et al. Homeobox gene TGIF-1 is increased in placental endothelial cells of human fetal growth restriction. Reproduction. 2016;152(5):457-65.https://dx.doi.org/10.1530/REP-16-0068.
- Taylor M. Validation of the Quintero staging system for twin-twin transfusion syndrome. Obstet Gynecol. 2002;100(6):1257-65. https://dx.doi.org/10.1016/S0029-7844(02)02392-X.
- Basavaraja R., Drum J.N., Sapuleni J., Bibi L., Friedlander G., Kumar S. et al. Downregulated luteolytic pathways in the transcriptome of early pregnancy bovine corpus luteum are mimicked by interferon-tau in vitro. BMC Genomics. 2021;22(1):452. https://dx.doi.org/10.1186/s12864-021-07747-3.
- Mert I., Sargın Oruc A., Yuksel S., Cakar E.S., Buyukkagnıcı U., Karaer A., Danısman N. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012;38(4):658-64. https://dx.doi.org/10.1111/j.1447-0756.2011.01771.x.
- Ge L., Yu D., Su R., Cao Y. [Effects of hypoxia-inducible factor 1α on hypoxic tolerance of human amniotic mesenchymal stem cells]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32(3):264-9. https://dx.doi.org/10.7507/1002-1892.201710104.
Received 17.10.2023
Accepted 05.12.2023
About the Authors
Kristina A. Gladkova, MD, Ph.D, Senior Researcher at the Fetal Medicine Unit, Institute of Obstetrics, Head of the 1st Obstetric Department of Pregnancy Pathology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)321-10-07,k_gladkova@oparina4.ru, https://orcid.org/0000-0001-8131-4682, 117997, Russia, Moscow, Ac. Oparin str., 4.
Zulfiya S. Khodzhaeva, Dr. Med. Sci., Professor, Deputy Director of Obstetrics Institute, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-07-88, z_khodzhaeva@oparina4.ru, https://orcid.org/0000-0001-8159-3714,
117997, Russia, Moscow, Ac. Oparin str., 4.
Viktoriya A. Sakalo, MD, Ph.D, Junior Researcher at the Department of Pregnancy Pathology, Institute of Obstetrics, Obstetrician-Gynecologist at the 1st Obstetric Department of Pregnancy Pathology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(929)588-72-08, v_sakalo@oparina4.ru, https://orcid.org/0000-0002-5870-4655, 117997, Russia, Moscow, Ac. Oparin str., 4.
Ekaterina R. Frolova, PhD Student at the High-Risk Pregnancy Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(985)473-85-47, kattirella@gmail.com, https://orcid.org 0000-0003-2817-3504, 117997, Russia, Moscow, Ac. Oparin str., 4.
Marika N. Shakaya, Neonatologist the A.G. Antonov Neonatal Intensive Care Unit, Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-22-77, +7(903)769-61-60, 117997, Russia, Moscow, Ac. Oparin str., 4; Teaching Assistant at the N.F. Filatov Department of Neonatology, Faculty of Pediatrics, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, 119991, Russia, Moscow, Trubetskaya str., 8-2, +7(499)248-01-81, dr.shakaya@mail.ru, m_shakaya@oparina4.ru, https://orcid.org/0000-0002-3838-3321
Аnna R. Kirtbaya, Dr. Med. Sci., Clinical Care Supervisor at the A.G. Antonov Neonatal Intensive Care Unit, Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
+7(495)438-22-77, a_kirtbaya@oparina4.ru; Professor at the N.F. Filatov Department of Neonatology, Faculty of Pediatrics, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, 119991, Russia, Moscow, Trubetskaya str., 8-2, +7(499)248-01-81, +7(903)769-61-60, https://orcid.org/0000-0002-7628-8157



