The possibility of using bulbar conjunctival vessel biomicroscopy in the diagnosis of placental disorders in pregnancy
Objective. To determine the state of the major components of the microcirculatory bed (MCB) in uncomplicated pregnancy and in different types of obstetric diseases leading to placental insufficiency (PI).Bloshchinskiy S.A., Zhmerenetskiy K.V., Bloshchinskaya I.A.
Subjects and methods. The investigation enrolled 50 pregnant women diagnosed with sub- and decompensated PI that was caused by thrombophilia (n = 24) or severe preeclampia (n = 26). Thirty women with uncomplicated pregnancy at 28–36 weeks’ gestation and 20 non-pregnant women were additionally examined. Bulbar conjunctival vessel biomicroscopy (BCVB) was used to evaluate the MCB.
Results. Hyperdynamic microcirculatory changes were established in uncomplicated pregnancy. In placental abnormalities, there was thrombophilia-induced atonic microcirculation and preeclampsia-induced spastic atonic and ischemic microcirculatory disorders. Pregnant women with PI had signs of thrombotic readiness in the microcirculation system: impaired venous outflow; blood corpuscle aggregation, and a slowing blood flow in all MCB components.
Conclusion. The findings can be used in the diagnosis of PI of different genesis in order to choose a rational management tactics for a pregnant woman.
Keywords
Microcirculation (MC) is a fundamental process in the functioning of the cardiovascular system; it plays a leading role in the trophic provision of tissues, and maintains tissue metabolism. Tissue cells receive nutrition by way of MC and get rid of metabolites due to changing blood flow consistent with the tissue needs [1–3].
The structure of the microcirculatory bed (MCB) in different organs has its peculiarities and depends on the function of the organ though there are certain regularities. MC disorders underlie many diseases or develop secondarily to them. MC impairments are included in many pathological processes and separate pathological forms of different diseases as an important pathogenic component [4].
MC condition plays a key role in a favorable pregnancy outcome starting from the period of periconceptional preparation of the endometrium, nidation of the ovum, embryo development and creation of favorable conditions for a fetus development at all the stages of pregnancy. The autohemodilution processes, increase of the circulating blood volume, new formations of circulatory bed, coagulation potential changes aimed at a favorable pregnancy outcome are the most significant components of MC condition. Whereas, rheological changes of the blood, activation of the processes of intravascular thrombus formation in the MC system, vascular tonus changes with blood stagnation in venules under the condition of a low vascular resistance and slow blood flow can result in unfavorable pregnancy outcomes [1, 5, 6].
Biomicroscopy of bulbar conjunctiva vessels (BMBCV) allows opening «an information window» into the vascular system of a man. This method gives a possibility to directly assess many morphofunctional parameters and MCB organization, MC processes, and partially, the condition of tissue environment [4, 7, 8].
The condition of MCB has not been sufficiently studied both in normal pregnancy and in different obstetrical pathologies.
The aim of this study was to determine the condition of MCB in uncomplicated pregnancy and in different types of obstetrical pathology leading to placental insufficiency (PI).
Materials and Methods
The study included 50 pregnant women with the confirmed diagnoses of sub- and decompensated forms of PI at 28–36 weeks gestation. These patients comprised the main group (MG). The diagnosis was made on the basis of ultrasound examination which revealed oligoamnios, fetal growth retardation, the findings of dopplerometry on uterus-placental and fetal-placental circulation disorders, cardiotocography pathological findings, meconium-stained fluids detected at amnioscopy; this examination was performed according to the clinical recommendations on obstetrics of the Russian Society of Obstetricians and Gynecologists [9]. On the basis of suggested genesis of PI, the MG was divided into subgroups. The subgroup MG-1, included 24 pregnant women with PI due to thrombophilia. The patients of MG-1 had high thrombogenic risk factor determined by family and personal case histories, results of examinations aimed at revealing genetic and acquired thrombophilia; they also had characteristic changes in homeostasis at the time of making a diagnosis.
The criteria of thrombophilia included the presence of anti-phospholipid syndrome (AFS), factor V Leiden mutation, prothrombin, hyperhomocysteinemia, their combination, or combinations with genes polymorphism; three or more homozygotic forms of genes polymorphism; five or more heterozygotic forms of genes polymorphism [10].
Subgroup MG-II comprised 26 pregnant women with the confirmed diagnosis of PI due to severe preeclampsia development. In addition, we examined 30 women with the same gestation period compared to the women of the same age of the MG with uncomplicated pregnancy and its favorable outcome. The control group (CG) enrolled 20 non-pregnant women who did not receive hormonal contraceptives and after their menstrual cycle was over. The women of CG had a similar age and somatic status as the women of MG and CG. Exclusion criteria were severe somatic pathology, infectious complication of pregnancy, diabetes mellitus.
MC study was conducted with BMBCV method using the apparatus diagnostic complex including a slit lamp 2B providing visualization of MCB of the conjunctiva and video camera. The received data were processed by the system analyzing videos “EPOS МC” with the following automatic processing of MC parameters [4]. BMBCV with magnification 96×determined the following MC parameters: arteriole diameter (dA in mkm), venule (dV in mkm); arterial-venous ratio (A/V); a number of capillaries per unit of conjunctiva square (cap/mm2); signs of aggregation in arterioles, venules, and capillaries (scores), circulation velocity in arterioles, venules and capillaries, (mm/s). This non-invasive method could be used many times to detect signs of thrombotic activity including venous outflow disorder in MC and signs of aggregation of blood components [11]. Videotaping of the study results allowed performing real time comparative analysis of multiple examinations of every pregnant woman.
Statistical processing of the findings was conducted using Statistica 7.0 software package; the arithmetic mean (M) and the average error (±m) were calculated. For comparison of the groups, we used Student’s t-test, Fisher criterion and probability level (p). The differences between the compared values were statistically reliable at the level p < 0.05. The analysis of mutual dependence was performed using the Spearman correlation coefficient.
Results
It was revealed that by the third trimester of pregnancy women of both control group (CG) and group of comparison (GC) developed significant changes in arterial-venous relations and partial structural changes of microvessels with an increase in a gathering part of MCB (Table 1).
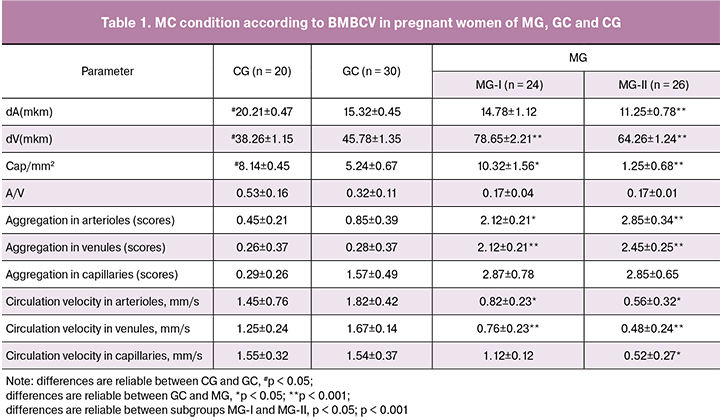
There are signs of hyperdynamic circulatory changes of MC primarily of the functional character. Arteriole tonus increase (p < 0.001) is accompanied by circulation velocity both in inflow and outflow part of MCB. There are initial signs of increased aggregation of blood components (Fig. 1).
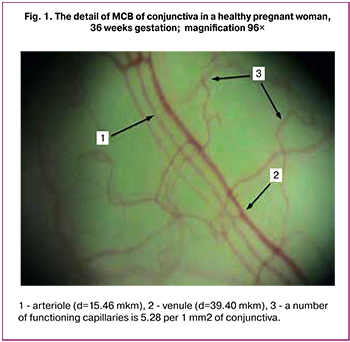 In subgroup MG-1, there are signs of pronounced disorders of MCB (Table 1.) Normal parameters of the central hemodynamics in MCB are accompanied by a decrease in diameter of inflow vessel with a significant increase in venules diameter (p < 0.001), circulation velocity decrease in both arterioles (p < 0.05) and venules (p < 0.001). These impairments in combination with blood cells aggregation in all parts of MCB in the women of MG-1 can lead to the development of thrombotic situation in MC (Fig. 2).
In subgroup MG-1, there are signs of pronounced disorders of MCB (Table 1.) Normal parameters of the central hemodynamics in MCB are accompanied by a decrease in diameter of inflow vessel with a significant increase in venules diameter (p < 0.001), circulation velocity decrease in both arterioles (p < 0.05) and venules (p < 0.001). These impairments in combination with blood cells aggregation in all parts of MCB in the women of MG-1 can lead to the development of thrombotic situation in MC (Fig. 2).
In blood outflow disorders caused by changes in blood rheology and aggregation of blood components, microvessels turn into passive carriers of blood. It results in redistribution of blood in tissues that does not meet the functional needs of cells in different zones and is due to those anatomic relations that determine the resistance to circulation; namely, microvessels with big diameter, a smaller number of branch knots, less extension or lower angle of branching prevail.
Due to the above mentioned morphofunctional peculiarities, those microvessels receive a larger portion of blood flow and signs of stasis develop there. We noticed that in the MG-1 a significant increase in capillary bed occurred (p < 0.05); it may have a compensatory character at the initial stages but under the conditions of a pronounced blood flow velocity restriction in venules (p < 0.001) and increase in blood cells aggregation activity (p < 0.001) it may lead to shunting of blood flow in MC. Later, blood flow takes place in a smaller number of capillaries limiting adjacent micro regions in regard to metabolism.
The most significant changes of MCB were observed in PI caused by the development of severe preeclampsia (subgroup MG-II). Spastic condition of inflow part of MCB (p < 0.001), reactive restructuring of venules (p < 0.001), decrease of (A/V) ratio by 1.9 times are accompanied by the signs of severe MC insufficiency (Table 1). Density decrease in functioning capillaries (p < 0.001), that is rarefaction or exhaustion of the capillary bed reduces a total square of functioning vessels by four times, increases vascular resistance, causes inadequate perfusion leading to organs and tissue ischemia (Fig. 3).
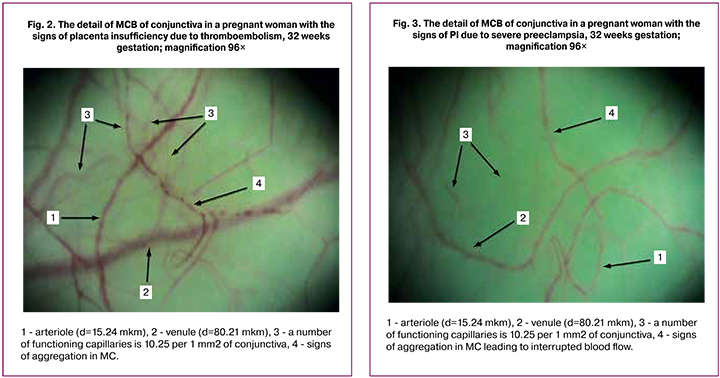
Maximum manifestations of aggregation activity of blood elements in all parts of MCB are accompanied by the restriction of blood flow velocity in all parts of MCB by more than three times, finally resulting in organs dysfunction/insufficiency. Only women of MG-II developed perivascular changes characterized by turbid conjunctiva due to swelling and separate hemorrhages.
Correlation analysis confirmed a direct correlation between venules diameter and blood corpuscles aggregation: in arterioles (r = +0.75) and in venules (r = +0.87) in MG -I; in arterioles (r = +0.85) and in venules (r = +0.72) in MG-II.
Discussion
The results of the conducted study can draw attention to the MCB condition both in the period of adaptation to pregnancy and in pregnancy pathology development leading to an unfavorable outcome both for a mother and a fetus.
The changes revealed in MCB in GC compared to CG are completely consistent with hyperdynamic disorders in MC [1, 4]. Those changes reflect primarily functional, reversible shifts in MC without causing microcirculatory insufficiency. MCB changes are explained by the adaptation processes of MCB to new hemodynamics conditions in pregnancy connected with autohemodilution causing increase in volume of circulating blood, rheology parameters changes, decrease of peripheral blood flow resistance under the conditions of increased coagulation potential and decrease in fibrinolytic activity [1].
Pregnant women with clinical manifestations of PI caused by thrombophilia (subgroup MG-1) demonstrated signs of atonic forms and types of MC, which had a functional character of MCB disorders at the initial stages [4]. BMBCV performed in those pregnant women revealed signs of thrombotic activity in MC which was manifested by venous congestion accompanied by blood corpuscles aggregation in all parts of MCB. A slow blood flow which was revealed both in inflow and outflow of MC can cause thrombotic microangiopathy and all its fatal outcomes [10–14].
Examination of MCB condition in pregnant women with clinical manifestations of preeclampsia (subgroup MG-II) revealed spastic, atonic and ischemic disorders of MCB reflecting structural-functional and, as a rule, irreversible MCB disorders leading to microcirculatory insufficiency [4, 15–19]. In MG-II, signs of MCB disorders were characterized by blood vessels systemic impairment in MC. Along with a significant increase in the tonus of inflow part of MCB, we observed a marked dilation of venules, a high aggregation activity of blood components accompanied by a slow blood flow in all parts of MC. Unlike the parameters in group MG-1, there was a critical restriction of functional capillaries square, signs of a high permeability and break-ability of vascular walls.
The patients of group MG-I had MC disorders with the signs of thrombotic activity development of a reversible type as all the changes were connected with the disorders of coagulation link of homeostasis that can be corrected by anticoagulation therapy [9, 10, 20, 21]. The women of group MG-II demonstrated critical MCB disorders, but readiness for thrombotic situation was secondary due to a deep damage to vascular endothelium when term birth was only reasonable. Prolongation of pregnancy in this condition of MCB can result in structural degenerative form with organic disorders of MC [10, 22].
Conclusion
The received data are consistent with the data on pathogenic mechanisms of PI development and give good evidence to start anticoagulation therapy in thrombophilia during pregnancy and confirm the absence of necessity to prolong pregnancy in case of severe preeclampsia.
Revealed peculiarities of MCB condition can be used in diagnosing different stages of placental problems. They provide a rational management of pregnancy with a favorable outcome both for a mother and a fetus. Non-invasive character of this method allows recommending it to be used many times. Videotaping helps to compare the received data in dynamics.
References
1. Abdo I., George R.B., Farrag M., Cerny V., Lehmann C. Microcirculation in pregnancy. Physiol. Res. 2014; 63(4): 395-408.
2. Xu Z., Jiang H., Tao A., Wu S., Yan W., Yuan J. et al. Variability of variability of bulbar conjunctiva microorganisms in healthy people using biomicroscopy of functional slit lamps (FSLB). Microvasc. Res. 2015; 101(3): 15-9.
3. Муравьев А.В., Михайлов П.В., Тихомирова И.А. Микроциркуляция и гемореология: точки взаимодействия. Регионарное кровообращение и микроциркуляция. 2017; 2: 90-100. [Muravev A.V., Mikhailov P.V., Tikhomirova I.A. Microcirculation and hemorheology: points of interaction. Regional blood circulation and microcirculation. 2017; 2: 90-100. (in Russian)]
4. Сиротин Б.З., Жмеренецкий К.В. Микроциркуляция: влияние лекарственных препаратов. Хабаровск: КГУП «Хабаровская краевая типография»; 2010. 128с. [Syrotin B.Z., Zhmerenetsky K.V. Microcirculation: the influence of drugs. Khabarovsk: Khabarovsk Regional Printing House; 2010. 128p. (in Russian)]
5. Baltajian K., Hecht J.L., Wenger J.B., Salahuddin S., Verlohren S., Perschel F.H. et al. Placental lesions of vascular insufficiency are associated with anti-angiogenic state in women with preeclampsia. Hypertens. Pregnancy. 2014; 33(4): 427-39.
6. Марченко А.И., Ярема В.И., Королюк Г.М. Исследование физических свойств крови и изменение коагуляционного звена гемостаза, ДВС-синдром. Хирург. 2016; 6: 30-5. [Marchenko A.I., Yarema V.I., Korolyuk G.M. Investigation of the physical properties of blood and the change in the coagulation unit of hemostasis, DIC. Surgeon. 2016; 6: 30-5. (in Russian)]
7. Wright I.M., Latter J.L., Dyson R.M., Levi C.R., Clifton V.L. Videomicroscopy as a tool for investigation of the microcirculation in the newborn. Physiol. Rep. 2016; 4(19): e12941.
8. Воробьев Б.И., Воробьев В.Б., Зибарев А.Л. Эффективность модифицированной бульбарной микроскопии, как достоверного современного метода оценки дебюта гемостазиологических катастроф. Международный журнал прикладных и фундаментальных исследований. 2013; 5: 31-6. [Vorobyov B.I, Vorobyov V.B, Zibarev, AL The effectiveness of modified bulbar microscopy as a reliable modern method for assessing the debut of hemostasiological catastrophes. International Journal of Applied and Basic Research. 2013; 5: 31-6. (in Russian)]
9. Макацария А.Д., Бицадзе В.О., Хизроева Д.Х., Хамани Н.М. Плацентарная недостаточность при осложненной беременности и возможности применения дипиридамола. Акушерство, гинекология и репродукция. 2016; 10(4): 72-82. [Makatsariya A.D., Bitsadze V.O., Khizroeva D.Kh., Khamani N.M. Placental insufficiency in complicated pregnancy and the possibility of using dipyridamole. Obstetrics, gynecology and reproduction. 2016; 10(4): 72-82. (in Russian)]
10. Макацария А.Д., Бицадзе В.О., Хизроева Д.Х. Тромботические микроангиопатии в акушерской практике. М.: ГЭОТАР-Медиа; 2017. 295c. [Makatsariya A.D., Bitsadze V.O., Khizroeva D.Kh. Thrombotic microangiopathy in obstetric practice. M .: GEOTAR-Media; 2017. 295p. (in Russian)]
11. Баркаган З.С. Клинико-патогенетические варианты, номенклатура и основы диагностики гематогенных тромбофилий. Проблемы гематологии и переливания крови. 1996; 3: 5-15. [Barkagan Z.S. Clinical and pathogenetic variants, nomenclature and basics of diagnosis of hematogenous thrombophilia. Problems of hematology and blood transfusion. 1996; 3: 5-15. (in Russian)]
12. Battinelli E.M., Marshall A., Connors J.M. The role of thrombophilia in pregnancy. Thrombosis. 2013; 2013: 516420.
13. Croles F.N., Nasserinejad K., Duvekot J.J., Kruip M.J., Meijer K., Leebeek F.W. Pregnancy, thrombophilia, and the risk of a first venous thrombosis: systematic review and bayesian meta-analysis. BMJ. 2017; 359: j4452.
14. Нестерова Э.А., Путилова Н.В. Роль родительско-плодовой тромбофилии в формировании тяжелых форм плацентарной недостаточности. Акушерство и гинекология. 2014; 12: 5-9. [Nesterova E.A., Putilova N.V. The role of parent-fruit thrombophilia in the formation of severe forms of placental insufficiency. Obstetrics and gynecology. 2014; 12: 5-9. (in Russian)]
15. Walker C.K., Ashwood P., Hertz-Picciotto I. Pre-eclampsia, placental insufficiency, autism and antiphospholipid antibodies are the answer. JAMA Pediatr. 2015; 169(6): 606-7.
16. Walker C.K., Krakowiak P., Baker A., Hansen R.L., Ozonoff S., Hertz-Picciotto I. Pre-eclampsia, placental insufficiency and autism, disturbance of spectrum or delay in development. JAMA Pediatr. 2015; 169(2): 154-62.
17. Rusavy Z., Pitrova B., Korecko V., Kalis V. Changes in capillary diameters in pregnancy-induced hypertension. Hypertens. Pregnancy. 2015; 34(3): 307-13.
18. Сергеевa O.Н., Чеснокова Н.П., Понукалина Е.В., Рогожина И.Е., Глухов T.Н. Патогенетические связи между эндотелиальной дисфункцией и нарушениями коагуляционного потенциала крови во время беременности, осложненной преэклампсией. Вестник Российской академии медицинских наук. 2015; 70(5): 599-603. [Sergeeva O.N., Chesnokova NP, Ponukalina E.V., Rogozhina I.E., Glukhov T.N. Pathogenetic links between endothelial dysfunction and impaired blood coagulation potential during pregnancy, complicated by preeclampsia. Bulletin of the Russian Academy of Medical Sciences. 2015; 70 (5): 599-603.(in Russian)]
19. Boeldt D.S., Bird I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017; 232(1): 27-44.
20. Хруслов М.В., Жабин С.Н., Боева М.И., Иванов С.В., Пашина И.В., Авагова С.А. Изучение условий нормального течения беременности у женщин с наследственными тромбофилиями. Акушерство, гинекология и репродукция. 2015; 9(2): 6-11. [Khruslov M.V., Zhabin S.N., Boyeva M.I., Ivanov S.V., Pashina I.V., Avagova S.A. Study of conditions for the normal course of pregnancy in women with hereditary thrombophilia. Obstetrics, gynecology and reproduction. 2015; 9 (2): 6-11. (in Russian)]
21. Мельников А.П., Петрухин В.А., Половинкина И.А. Рациональная антикоагулянтная терапия при беременности. Российский вестник акушера-гинеколога. 2010; 1: 23-8. [Melnikov A.P., Petrukhin V.A., Polovinkina I.A. Rational anticoagulant therapy during pregnancy. Russian Bulletin of the obstetrician-gynecologist. 2010; 1: 23-8.(in Russian)]
22. Hunt K., Kennedy S.H., Vatish M. Definitions and reporting of placental insufficiency in biomedical journals: a review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016; 205: 146-9.
Received 13.04.2018
Accepted 20.04.2018
About the Authors
Bloshchinskiy, Stepan A., head of the gynecological Department, Non-state health care institution «Road Clinical Hospital at Khabarovsk-1 Station» Open Joint Stock Company “Russian Railways». 680022, Russia, Khabarovsk, Voronezhskaya str. 49. Tel. +74212410945E-mail: Stepan.Bloshchinskiy@dkb-dv.ru. ORCID ID https://orcid.org/0000-0002-2802-9986
Zhmerenetskiy, Konstantin V., MD, Correspondent member of RAS, Rector Far Eastern State Medical University, Ministry of Health of the Russian Federation,
680000, Russia, Khabarovsk, Muravieva-Amurskogo str. 35. Tel. +74212305311. E-mail: rec@mail.fesmu.ru. ORCID ID https://orcid.org/0000-0002-3316-8796
Bloshchinskaya, Irina A., MD, professor of the Department of Obstetrics and Gynecology Far Eastern State Medical University, Ministry of Health of the Russian Federation.
680000, Russia, Khabarovsk, Muravieva-Amurskogo str. 35. Tel. +74212754800. E-mail: nauka@mail.fesmu.ru. ORCID ID https://orcid.org/0000-0002-1888-7071.
For citations: Bloshchinskiy S.A., Zhmerenetskiy K.V., Bloschinskaya I.A. The possibility of using bulbar conjunctival vessel biomicroscopy in the diagnosis of placental disorders in pregnancy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (12): 36-41. (in Russian)
http://dx.doi.org/10.18565/aig.2018.12.36-41



