1) Siberian State Medical University, Ministry of Health of Russia, Tomsk, Russia;
2) Research Institute of Medical Genetics, Tomsk National Research Medical Center, Russian Academy of Sciences, Tomsk, Russia;
3) I.D. Evtushenko Regional Perinatal Center, Tomsk, Russia
Fetal growth restriction (FGR) remains one of the important obstetric problems, being a risk factor for antenatal and neonatal mortality and morbidity. The timely prediction of FGR is one of the most important measures in reducing adverse pregnancy outcomes. However, despite numerous studies, practical obstetrics still lacks highly sensitive and specific prognostic biomarkers for this disease.
This paper analyzes open-access modern scientific literature data in the PubMed, Cochrane, and eLibrary databases, which characterize the role of biomarkers in predicting the risk of FGR. Meta-analyses demonstrate that beta-human chorionic gonadotropin, alpha-fetoprotein, free estriol, human chorionic somatomammotropin hormone 1, pappalysin 1, inhibin α-subunit, and placental growth factor are considered the most promising predictive biomarkers for FGR. However, the data obtained by most authors suggest that the use of individual biomarkers has insufficient sensitivity and specificity in stratifying the risk of FGR. The most promising direction in this area is the creation of models for complex multiparametric screening based on the study of maternal risk factors, the levels of biomarkers (both proteomic and molecular genetic ones) in conjunction with ultrasound data.
Conclusion: Thus, studies focused on the search for new biomarkers in order to develop a comprehensive screening program that is of high prognostic value in identifying the risk of FGR are highly relevant and determine personalized patient management tactics in the future.
Authors’ contributions: Izhoykina E.V., Gavrilenko M.M., Trifonova E.A. – material collection and processing, writing the text; Stepanov I.A., Kutsenko I.G., Stepanov V.A. – editing.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The article has been prepared without sponsorship.
For citation: Izhoykina E.V., Trifonova E.A., Kutsenko I.G., Stepanov I.A., Gavrilenko M.M., Stepanov V.A. Feasibility of predicting fetal growth restriction, by identifying plasma biomarkers.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (2): 18-24 (in Russian)
https://dx.doi.org/10.18565/aig.2022.269
fetal growth restriction
biomarkers
screening
Задержка роста плода (ЗРП) – осложнение беременности, при котором плод не может достигнуть генетически детерминированного потенциала роста по отношению к гестационному сроку согласно этнической группе и половой принадлежности. ЗРП встречается с частотой от 5 до 22% среди доношенных новорожденных и от 18 до 24% – недоношенных детей [1]. Данное осложнение беременности входит в группу «больших акушерских синдромов» и ассоциируется с повышенной антенатальной и неонатальной смертностью и заболеваемостью, является вероятным фактором риска нарушения состояния здоровья в постнатальном периоде [2–4]. Представленные обстоятельства определяют актуальность своевременной диагностики ЗРП.
Согласно данным исследований последних лет, одним из значимых механизмов развития ЗРП является нарушение процессов плацентации: неполное ремоделирование спиральных артерий приводит к их дальнейшей неспособности адекватно расширяться для усиления кровотока, вследствие чего снижается перфузия плаценты, возникает плацентарная ишемия, характеризующаяся сниженной оксигенацией, что ведет к образованию свободных радикалов, оксидативному стрессу и, в конечном счете, к эндотелиальной дисфункции [5–7]. Вышеописанные звенья патогенеза ЗРП характерны и для развития других «больших акушерских синдромов»: преэклампсии, преждевременных родов, преждевременного разрыва плодных оболочек, внутриутробной гибели плода и привычного невынашивания беременности. В случае ЗРП дисфункция плаценты коррелирует с изменением уровня биомаркеров, что представляет интерес при выявлении беременных с риском развития этого осложнения беременности.
При поиске сывороточных молекулярных маркеров, ассоциированных с развитием ЗРП, использованы следующие варианты запроса в поисковой строке трех баз данных (PubMed, Cochrane, eLibrary): «задержка роста плода», «скрининг», «биомаркеры», «fetal growth restriction», «fetal growth retardation», «intrauterine growth restriction», «screening», «biomarkers». Поиск в PubMed выдал 796 статей, Cochrane – 27, eLibrary – 863. В дальнейший анализ были включены результаты 47 исследований, в которых сообщалось об одноплодной беременности, закончившейся рождением новорожденного с задержкой роста, без хромосомной патологии или пороков развития. Дополнительно был проведен анализ работ, описывающих результаты полногеномного исследования экспрессии генов в плаценте или изучение транскрипционной активности отдельных генов, кодирующих представленные биомаркеры.
Результаты обобщенного анализа рассмотренных работ позволяют выделить 26 биомаркеров, показавших статистически значимые (р<0,05) ассоциации с риском развития ЗРП (таблица). Наиболее изученными биомаркерами (результаты представлены в 2 работах и более) являются: лептин (Leptin), β-хорионический гонадотропин человека (β-HCG), паппализин 1 (РАРР-А), α-фетопротеин (AFP), α-субъединица ингибина (Inhibin-А), плацентарный фактор роста (PIGF), связанная с fms рецепторная тирозинкиназа 1 (sFlt-1), растворимая форма Е-кадгерина (sE-cad), фактор некроза опухоли (TNF), интерлейкин-6 (IL-6), С-реактивный белок (CRP). Важно отметить, что направления изменения этих биомаркеров в различных исследованиях согласуются между собой, за исключением данных, приведенных для лептина. Вероятно, противоречивые результаты, полученные для этого протеина, обусловлены особенностями критериев включения/исключения из исследования, в частности первоначальными значениями индекса массы тела женщин и массовой доли жировой ткани в структуре тела, которые оказывают влияние на уровень лептина.
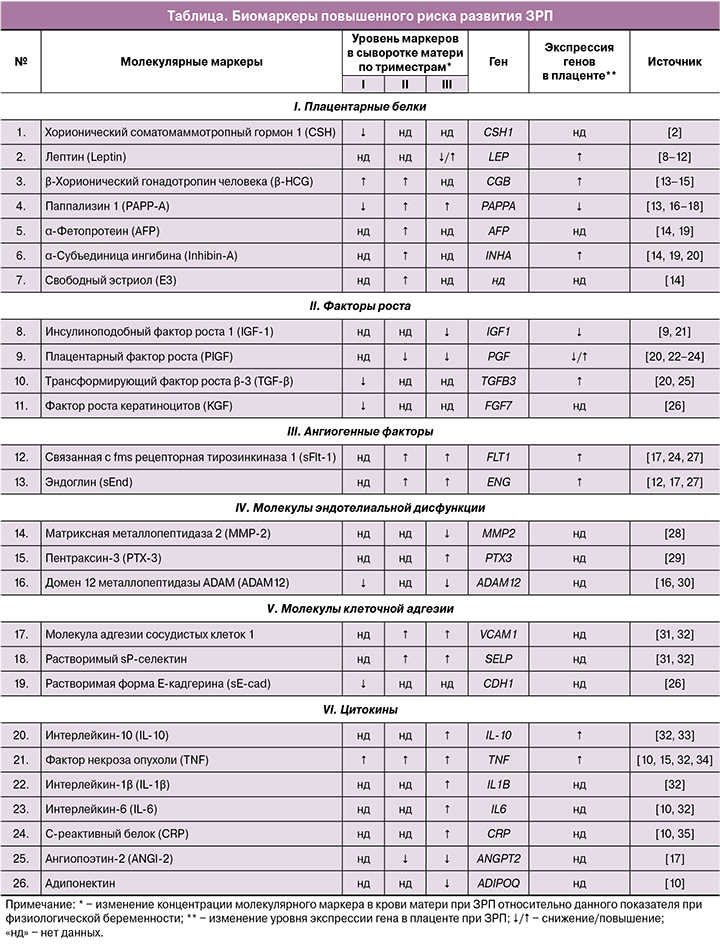
В последнее десятилетие большое внимание уделяется изучению таких маркеров, как β-HCG, РАРР-А, AFP, Inhibin-А, что связано с их широким использованием в программах скрининга хромосомных аномалий плода. Наряду с этим, высокой актуальностью обладают также полученные рядом авторов данные, демонстрирующие корреляционные связи уровней представленных биомаркеров с развитием ЗРП. Стоит отметить, что многие из рассматриваемых работ посвящены исследованию провоспалительных цитокинов – TNF, IL-1β и -6, что отражает большой научный интерес к иммунологической теории развития ЗРП [36].
Учитывая представленное многообразие описанных биомаркеров ЗРП, особое значение представляют результаты обобщенного количественного анализа данных (метаанализа), полученные в отдельных исследованиях ассоциации биомаркеров с изучаемой патологией беременности. Так, с 2015 г. в базах данных PubMed и Cochrane опубликовано 220 результатов метаанализов, связанных с ЗРП, из которых только пять посвящены изучению биомаркеров в прогнозировании развития данной патологии. Результаты последних позволяют нам выделить следующие биомаркеры, продемонстрировавшие высокодостоверную ассоциацию с развитием ЗРП: β-HCG, свободный эстриол (Е3), AFP, Inhibin-А, РАРР-А, хорионический соматомаммотропный гормон 1 (CSH), PIGF [2, 37–40]. Ниже приведена их более подробная характеристика.
Биомаркеры, ассоциированные с развитием задержки роста плода, по данным метаанализов β-HCG – гонадотропный гормон, начинающий вырабатываться цитотрофобластом с 6−7-й недели гестации. Его уровень определяет благополучное течение беременности при условии постепенного увеличения его в крови матери в I триместре. Однако при беременности, осложненной ЗРП, отмечается преждевременная ускоренная дифференцировка ворсинчатого цитотрофобласта, чем объясняется высокий уровень β-HCG в сыворотке матери в I триместре беременности [41].
РАРР-А – гликопротеин, продуцируемый цитотрофобластом. Гормон появляется в материнской сыворотке на ранних сроках гестации, и постепенно уровень его увеличивается вплоть до ее окончания, тем самым определяя успешное завершение беременности [42]. Уровень РАРР-А входит в стратификацию риска ЗРП, проводимую в программе Аstraia при выполнении пренатального скрининга I триместра. Показано, что при нормально протекающей беременности формирование плаценты происходит благодаря строго контролируемому балансу между пролиферацией клеток трофобласта и их апоптозом. Однако ЗРП характеризуется дисбалансом между этими процессами, приводя к образованию зон некроза в синцитиотрофобласте, что определяет уменьшение уровня РАРР-А [5].
Е3 – стероидный гормон, который также имеет большое значение для нормального течения беременности. Путем сложных биохимических превращений из дегидроэпиандростерона сульфата, образующегося в печени плода, происходит синтез Е3 в плаценте [42]. При нарушении ремоделирования сосудистой стенки происходят уменьшение поставки кислорода и замедление биохимических реакций, в том числе уменьшается синтез Е3 в плаценте, что отражается на его уровне в крови матери [3].
Inhibin-А – гормон белковой природы. Во время беременности синтезируется клетками цитотрофобласта [42]. Включен в пренатальный скрининг II триместра для выявления хромосомной патологии плода [37]. Ряд авторов отмечают высокие уровни гормона при ЗРП в ответ на повышение активности провоспалительных цитокинов. Другие исследователи связывают высокие уровни гормона с ответной компенсаторной реакцией на нарушение ремоделирования спиральных артерий.
AFP – гликопротеин, начало синтеза которого совпадает с началом эмбрионального гемопоэза на 3−4-й неделе, происходящего в желточном мешке. К концу I триместра основным местом выработки становятся гепатоциты печени плода. Определение высокого уровня AFP в сыворотке крови беременных характерно большей частью для скрининга пороков развития плода (крестцово-копчиковая тератома, гастрошизис, дефекты нервной трубки и др.) [37]. Однако в случае ЗРП при отсутствии у него пороков развития также отмечаются высокие показатели AFP, что, возможно, связано с особенностями плодового гематопоэза.
В аспекте диагностики ЗРП на ранних сроках гестации исследуются уровни описываемых выше биомаркеров [42, 43]. Отмечено, что отклонения от средних величин данных показателей, нередко регистрируемые при нормальном кариотипе плода, в значительном проценте случаев оказываются ассоциированными с различными акушерскими осложнениями, возникающими в результате нарушения плацентации, в том числе с ЗРП [37, 38]. Так, результаты метаанализов показывают, что вес новорожденного менее 5-го процентиля ассоциируется со значениями PAPP-A и Е3 в I и во II триместрах менее 0,5 МоМ [38, 39]. При этом параллельно отмечается повышение показателей AFP, β-HCG, Inhibin-А [38].
CSH представляет собой полипептидный гормон, синтез которого происходит в синцитиотрофобласте. При физиологической беременности уровень его повышается и является фактическим отражением увеличения массы плаценты и ее белково-синтетической функции, что напрямую влияет на рост и развитие плода [42]. Как известно, неполноценная перестройка спиральных артерий миометрия приводит к плацентарной гипоперфузии и подавлению функции плаценты, что закономерно вызывает снижение значения CSH менее 10 процентиля [2].
PIGF – один из белков семейства факторов роста эндотелия сосудов. Вырабатывается преимущественно трофобластом и обладает выраженным ангиогенным потенциалом, необходимым для физиологического течения гестации. Показано, что беременность, осложненная ЗРП, часто характеризуется антиангиогенным уклоном со снижением уровня PIGF менее 12 пг/мл [2]. Гипотезу антиангиогенного состояния при ЗРП подтверждают результаты многочисленных исследований и метаанализа, продемонстрировавшие повышение соотношения sFlt-1/PIGF более 33, что обусловливает его высокую прогностическую значимость при оценке риска ЗРП [40].
Авторы проведенных метаанализов единогласно отмечают, что изолированное использование одного из представленных биомаркеров для прогнозирования ЗРП обладает низкими чувствительностью и специфичностью [2, 37, 38]. Так, в метаанализе Heazell A. et al. (2019), включавшем в общей сложности 175 426 беременных, показана чувствительность для Е3 0,35 (95% ДИ 0,28−0,43), CSH 0,39 (95% ДИ 0,30−0,49), PIGF 0,24 (95% ДИ 0,15−0,38) [2].
Для улучшения прогнозирования рождения ребенка с задержкой роста предлагается использовать комплексный анализ, учитывающий индивидуальные для каждой пациентки факторы риска, уровень молекулярных маркеров в совокупности с данными инструментальных методов исследований (рисунок). В Российской Федерации с 2021 г. внедрен безвыборочный комбинированный скрининг на ЗРП в I триместре беременности с использованием программного обеспечения Аstraia, разработанного Фондом медицины плода (Fetal Medicine Foundation). Однако следует отметить, что эффективность его в отношении прогнозирования ЗРП для российской популяции до конца не изучена.

В последнее десятилетие с использованием новейших молекулярно-генетических технологий у женщин с ЗРП и физиологическим течением беременности было проведено несколько полногеномных исследований экспрессии генов в плаценте, а также изучена транскрипционная активность отдельных генов, кодирующих рассматриваемые в данном обзоре биомаркеры [11, 12, 15, 20, 21, 24, 33]. Из 26 генов, кодирующих анализируемые нами белки, лишь 11 (LEP, СGB, PAPPA, INHA, IGF1, PGF, TGFB3, FLT1, ENG, IL-10, TNF) показали ассоциацию с ЗРП на уровне транскриптома плацентарной ткани (таблица), что является дополнительным доказательством роли данных биомаркеров в молекулярном патогенезе ЗРП и позволяет выделить их в качестве наиболее значимых для дальнейшего изучения. Примечательно, что направления изменения уровня РНК и белка для большинства генов согласуются между собой, за исключением показателей для таких биомаркеров, как РАРР-А и TGF-β [13, 16–18]. Данный факт отражает перспективность использования данных РНК-биомаркеров наряду с белковыми для разработки новой стратегии прогнозирования ЗРП, поскольку представляется маловероятным, что будет найден единственный биомаркер, который сам по себе обладает достаточной предиктивной значимостью.
Заключение
ЗРП сопряжена с повышенной неонатальной заболеваемостью и смертностью, а также сопровождается четырехкратным повышением риска мертворождения, что обуславливает высокую актуальность поиска прогностических биомаркеров данной патологии. Проведенный анализ литературы последних лет выявил 26 биомаркеров, вовлеченных в различные патофизиологические механизмы ЗРП, включающие эндотелиальную дисфункцию, оксидативный стресс, ангиогенез. Высокую статистическую значимость (р≤0,001) в анализируемых работах показали РАРР-А, Е3, AFP, Inhibin-А, sFlt-1, MMP-2, IL-10, IL-1β, CRP, ANGI-2. Стоит отметить, что на сегодняшний день клинический анализ рассмотренных в настоящем обзоре биомаркеров не является целесообразным как отдельный прогностический алгоритм ввиду низкой чувствительности и специфичности, однако он может использоваться в сочетании с другими предикторами, такими как ультразвуковые параметры, новые РНК-биомаркеры ЗРП и/или материнские факторы риска, которые будут обнаружены в последующих исследованиях.
Таким образом, проведение когортных исследований в области поиска как протеомных, так и РНК-биомаркеров ЗРП с целью разработки мультипараметрической модели скрининга данной патологии на ранних сроках беременности является необходимым и обоснованным.
- Российское общество акушеров-гинекологов. Клинические рекомендации «Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода)». 2020. [Russian Society of Obstetricians and Gynecologists. Clinical guidelines “Inadequate fetal growth requiring maternal medical care (fetal growth restriction)”. 2020. (in Russian)].
- Heazell A.E., Hayes D.Jl., Whitworth M., Takwoingi Y., Bayliss S.E., Davenport C. Biochemical tests of placental function versus ultrasound assessment of fetal size for stillbirth and small-for-gestational-age infants. Cochrane Database Syst. Rev. 2019; 5(5): CD012245. https://dx.doi.org/10.1002/14651858.
- Levine T.A, Grunau R.E., McAuliffe F.M., Pinnamaneni R.M., Foran A.,Alderdice F.A. Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics. 2015; 135(1): 126-41.https://dx.doi.org/10.1542/peds.2014-1143.
- Colella M., Frérot A., Novais A.R.B., Baud O. Neonatal and long-term consequences of fetal growth restriction. Curr. Pediatr. Rev. 2018; 14(4): 212-8. https://dx.doi.org/10.2174/1573396314666180712114531.
- Хачатрян З.В., Кан Н.Е., Макарова Н.П. Современные представления о молекулярных механизмах формирования задержки роста плода. Акушерство и гинекология. 2019; 10: 22-6. https://dx.doi.org/10.18565/aig.2019.10.22-26. [Khachatryan Z.V., Kan N.E., Makarova N.P. Present views on molecular mechanisms of formation of fetal growth restriction. Obstetrics and Gynecology. 2019; (10): 22-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.10.22-26.
- Burton G.J., Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018; 218(Suppl. 2): S745-61.https://dx.doi.org/10.1016/j.ajog.2017.11.577.
- Zur R.L., Kingdom J.C., Parks W.T., Hobson S.R. The placental basis of fetal growth restriction. Obstet. Gynecol. Clin. North Am. 2020; 47(1): 81-98. https://dx.doi.org/10.1016/j.ogc.2019.10.008.
- Zareaan E., Heidarpour M., Kargarzadeh E., Moshfeghi M. Association of maternal and umbilical cord blood leptin concentrations and abnormal color Doppler indices of umbilical artery with fetal growth restriction. Int. J. Reprod. Biomed. 2017; 15(3): 135-40.
- Ferrero S., Mazarico E., Valls C., Di Gregorio S., Montejo R., Ibáñez L. et al. Relationship between fetal growth restriction and maternal nutrition status measured by Dual-Energy X-Ray absorptiometry, leptin, and insulin-like growth factor. Gynecol. Obstet. Invest. 2015; 80(1): 54-9.https://dx.doi.org/10.1159/000371761.
- Visentin S., Lapolla A., Londero A.P., Cosma C., Dalfrà M., Camerin M. et al. Adiponectin levels are reduced while markers of systemic inflammation and aortic remodelling are increased in intrauterine growth restricted mother-child couple. Biomed. Res. Int. 2014; 2014: 401595. https://dx.doi.org/10.1155/2014/401595.
- Madeleneau D., Buffat C., Mondon F., Grimault H., Rigourd V., Tsatsaris V.et al. Transcriptomic analysis of human placenta in intrauterine growth restriction. Pediatr. Res. 2015; 77(6): 799-807. https://dx.doi.org/10.1038/pr.2015.40.
- Zhang C., Ding J., Li H., Wang T. Identification of key genes in pathogenesis of placental insufficiency intrauterine growth restriction. BMC Pregnancy Childbirth. 2022; 22(1): 77. https://dx.doi.org/10.1186/s12884-022-04399-3.
- Cignini P., Savasta L.M., Gulino F.A., Vitale S.G., Mangiafico L., Mesoraca A., Giorlandino C. Predictive value of pregnancy-associated plasma protein-A (PAPP-A) and free beta-hCG on fetal growth restriction: results of a prospective study. Arch. Gynecol. Obstet. 2016; 293(6): 1227-33. https://dx.doi.org/10.1007/s00404-015-3947-z.
- Boonpiam R., Wanapirak C., Sirichotiyakul S., Sekararithi R., Traisrisilp K., Tongsong T. Quad test for fetal aneuploidy screening as a predictor of small-for-gestational age fetuses: a population-based study. BMC Pregnancy Childbirth. 2020; 20(1): 621. https://dx.doi.org/10.1186/s12884-020-03298-9.
- Kiyokoba R., Uchiumi T., Yagi M., Toshima T., Tsukahara S., Fujita Y. et al. Mitochondrial dysfunction-induced high hCG associated with development of fetal growth restriction and pre-eclampsia with fetal growth restriction. Sci. Rep. 2022; 12(1): 4056. https://dx.doi.org/10.1038/s41598-022-07893-y.
- Yu N., Cui H., Chen X., Chang Y. First trimester maternal serum analytes and second trimester uterine artery Doppler in the prediction of preeclampsia and fetal growth restriction. Taiwan. J. Obstet. Gynecol. 2017; 56(3): 358-61. https://dx.doi.org/10.1016/j.tjog.2017.01.009.
- Zamarian A.C.P., Araujo E.Jn., Daher S., Rolo L.C., Moron A.F., Nardozza L.M.M. Evaluation of biochemical markers combined with uterine artery Doppler parameters in fetuses with growth restriction: a case-control study. Arch. Gynecol. Obstet. 2016; 294(4): 715-23. https://dx.doi.org/10.1007/s00404-016-4024-y.
- Sifakis S., Androutsopoulos V.P., Pontikaki A., Velegrakis A., Papaioannou G.I., Koukoura O. et al. Placental expression of PAPPA, PAPPA-2 and PLAC-1 in pregnacies is associated with FGR. Mol. Med. Rep. 2018; 17(5): 6435-40. https://dx.doi.org/10.3892/mmr.2018.8721.
- Yazdani S., Rouholahnejad R., Asnafi N., Sharbatdaran M., Zakershob M., Bouzari Z. Correlation of pregnancy outcome with quadruple screening test at second trimester. Med. J. Islam. Repub. Iran. 2015; 29: 281.
- Awamleh Z., Gloor G.B., Han V.K.M. Placental microRNAs in pregnancies with early onset intrauterine growth restriction and preeclampsia: potential impact on gene expression and pathophysiology. BMC Med. Genomics. 2019; 12: 91. https://dx.doi.org/10.1186/s12920-019-0548-x.
- Nawathe A.R., Christian M., Kim S.H., Johnson M., Savvidou M.D., Terzidou V. Insulin-like growth factor axis in pregnancies affected by fetal growth disorders. Clin. Epigenetics. 2016; 8: 11. https://dx.doi.org/10.1186/s13148-016-0178-5.
- Lesmes C., Gallo D.M., Gonzalez R., Poon L.C., Nicolaides K.H. Prediction of small-for-gestational-age neonates: screening by maternal serum biochemical markers at 19-24 weeks. Ultrasound Obstet. Gynecol. 2015; 46(3): 341-9. https://dx.doi.org/10.1002/uog.14899.
- Komwilaisak R., Tangkiratichai P. Maternal serum angiogenic growth factors in intrauterine growth restriction versus normal pregnancies. J. Med. Assoc. Thai. 2017; 100(2): 119-24.
- Ravikumar G., Mukhopadhyay A., Mani C., Kocchar P., Crasta J., Thomas T. et al. Placental expression of angiogenesis-related genes and their receptors in IUGR pregnancies: correlation with fetoplacental and maternal parameters. J. Matern. Fetal Neonatal Med. 2019; 28: 1-8. https://dx.doi.org/10.1080/14767058.2019.1593362.
- Хачатрян З.В., Кан Н.Е., Вторушина В.В., Кречетова Л.В., Харченко Д.К., Мантрова Д.А., Тютюнник В.Л. Роль трансформирующего фактора роста β в формировании задержки роста плода Акушерство и гинекология. 2019; 11: 107-12. [Khachatryan Z.V., Kan N.E., Vtorushina V.V., Krechetova L.V., Kharchenko D.K., Mantrova D.A., Tyutyunnik V.L. Role of transforming growth factor-β in the formation of fetal growth restriction. Obstetrics and Gynecology. 2019; (11): 107-12. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.107-112.
- Красный А.М., Хачатурян А.А., Вторушина В.В., Кречетова Л.В., Кан Н.Е., Тютюнник В.Л. Содержание растворимой формы Е-кадгерина и фактора роста кератиноцитов в плазме крови при задержке роста плода. Акушерство и гинекология. 2020; 6: 37-42. [Krasnyi A.M., Khachaturyan A.A., Vtorushina V.V., Krechetova L.V., Kan N.E., Tyutyunnik V.L. Plasma levels of soluble E-cadherin and the keratinocytes growth factor in intrauterine growth restriction. Obstetrics and Gynecology. 2020; (6): 37-42. (in Russian)].https://dx.doi.org/10.18565/aig.2020.6.37-42.
- Raia-Barjat T., Prieux C., Gris J-C., Chapelle C., Laporte S., Chauleur C. Angiogenic factors for prediction of preeclampsia and intrauterine growth restriction onset in high-risk women: AngioPred study. J. Matern. Fetal Neonatal Med. 2019; 32(2): 248-57. https://dx.doi.org/ 10.1080/14767058.2017.1378325.
- Şahin B., Soyer-Çalışkan C., Çelik S., Hatırnaz Ş., Tinelli A. Midregional pro-adrenomedullin and matrix metalloproteinase-2 levels in intrauterine growth restriction and small gestational age pregnancies: biochemical diagnostic difference. J. Matern. Fetal Neonatal Med. 2021; 34(12): 1999-2005.https://dx.doi.org/10.1080/14767058.2020.1846707.
- Ibrahim M.I., Ammar E.M., Ramy A., Ellaithy M.I., Abdelrahman R.M., Elkabarity R. The association between pentraxin 3 in maternal circulation and pathological intrauterine fetal growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015; 185: 1-8. https://dx.doi.org/10.1016/j.ejogrb.2014.11.010.
- Andres F., Wong G.P., Walker S.P., MacDonald T.M., Keenan E., Cannon P. et al. A disintegrin and metalloproteinase 12 (ADAM12) is reduced at 36 weeks' gestation in pregnancies destined to deliver small for gestational age infants. Placenta. 2022; 117: 1-4. https://dx.doi.org/10.1016/j.placenta.2021.11.001.
- Docheva N., Romero R., Chaemsaithong P., Tarca A.L., Bhatti G., Pacora P. et al. The profiles of soluble adhesion molecules in the "great obstetrical syndromes". J. Matern. Fetal Neonatal Med. 2019; 32(13): 2113-36. https://dx.doi.org/10.1080/14767058.2018.1427058.
- Berbets A., Koval H., Barbe A., Albota O., Yuzko O. Melatonin decreases and cytokines increase in women with placental insufficiency. J. Matern. Fetal Neonatal Med. 2021; 34(3): 373-8. https://dx.doi.org/10.1080/14767058.2019.1608432.
- Medina-Bastidas D., Guzmán-Huerta M., Borboa-Olivares H., Ruiz-Cruz C., Parra-Hernández S., Flores-Pliego A. et al. Placental microarray profiling reveals common mRNA and lncRNA expression patterns in preeclampsia and intrauterine growth restriction Int. J. Mol. Sci. 2020; 21(10): 3597.https://dx.doi.org/10.3390/ijms21103597.
- Azizieh F.Y., Raghupathy R.G. Tumor necrosis factor-α and pregnancy complications: a prospective study. Med. Princ. Pract. 2015; 24(2): 165-70. https://dx.doi.org/10.1159/000369363.
- Karlı P., Özdemir A.Z., Ayan D. Maternal serum and fetal cord blood C-reactive protein levels but not procalcitonin levels are increased in idiopathic intrauterine gowth restriction. Med. Sci. Monit. 2019; 25: 6512-7.https://dx.doi.org/10.12659/MSM.917397.
- Dai F.F., Hu M., Zhang Y.W., Zhu R.H., Chen L.P., Li Z.D. et al. Maternal serum and fetal cord blood C-reactive protein levels but not procalcitonin levels are increased in idiopathic intrauterine gowth restriction. Expert Rev. Mol. Med. 2022; 24: e26. https://dx.doi.org/10.1017/erm.2022.18.
- Pylypjuk C.L., Monarrez-Espino J. False-positive maternal serum screens in the second trimester as markers of placentally mediated complications later in pregnancy: a systematic review and meta-analysis. Dis. Markers. 2021; 2021: 5566234. https://dx.doi.org/10.1155/2021/5566234.
- Heazell A.E.P., Whitworth M., Duley L., Thornton J.G. Use of biochemical tests of placental function for improving pregnancy outcome. Cochrane Database Syst. Rev. 2015; 2015(11): CD011202. https://dx.doi.org/10.1002/14651858.CD011202.pub2.
- Morris R.K., Bilagi A., Devani P., Kilby M.D. Association of serum PAPP-A levels in first trimester with small for gestational age and adverse pregnancy outcomes: systematic review and meta-analysis. Prenat. Diagn. 2017; 37(3): 253-65. https://dx.doi.org/10.1002/pd.5001.
- Chen W., Wei Q., Liang Q., Song S., Li J. Diagnostic capacity of sFlt-1/PlGF ratio in fetal growth restriction: a systematic review and meta-analysis. Placenta. 2022; 127: 37-42. https://dx.doi.org/10.1016/j.placenta.2022.07.020.
- Kiyokoba R., Uchiumi T., Yagi M., Toshima Т., Tsukahara S., Fujita Y. et al. Mitochondrial dysfunction-induced high hCG associated with development of fetal growth restriction and pre-eclampsia with fetal growth restriction. Sci. Rep. 2022; 12: 4056.
- Costa M.A. The endocrine function of human placenta: an overview. Reprod. Biomed. Online. 2016; 32(1): 14-43. https://dx.doi.org/10.1016/j.rbmo.2015.10.005.
- Yu N., Cui H., Chen X., Chang Y. First trimester maternal serum analytes and second trimester uterine artery Doppler in the prediction of preeclampsia and fetal growth restriction. Taiwan. J. Obstet. Gynecol. 2017; 56(3): 358-61.https://dx.doi.org/10.1016/j.tjog.2017.01.009.
Received 14.11.2022
Accepted 17.01.2023
Ekaterina V. Izhoykina, Laboratory Assistant, Department of Obstetrics and Gynecology, Siberian State Medical University, Ministry of Health of the Russia,
+7(923)441-12-18,
katushkabig@mail.ru, 634050, Russia, Tomsk, Moskovsky Trakt str., 2.
Ekaterina A. Trifonova, PhD, Senior Researcher, Research Institute of Medical Genetics, Tomsk Scientific Research Center RAS, +7(3822)51-33-34,
ekaterina.trifonova@medgenetics.ru, 634050, Russia, Tomsk, Naberezhnaya Reki Ushayka str., 10.
Irina G. Kutsenko, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Siberian State Medical University, Ministry of Health of Russia, +7(3822)90-11-01, 17-36,
kutsenko.ig@ssmu.ru, 634050, Russia, Tomsk, Moskovsky Trakt str., 2.
Igor A. Stepanov, PhD, Chief Physician, I.D. Evtushenko Regional Perinatal Center, +7(3822)91-50-11,
StepanovIA@opc.tomsk.ru,
634063, Russia, Tomsk, Ivana Chernykh str., 96/1.
Maria M. Gavrilenko, Ph.D. student, Research Institute of Medical Genetics, Tomsk Scientific Research Center RAS, +7(3822)51-33-34,
maria.gavrilenko@medgenetics.ru, 634050, Russia, Tomsk, Naberezhnaya Reki Ushayka str., 10.
Vadim A. Stepanov, Academician of RAS, Dr. Bio. Sci., Professor, Director of the Research Institute of Medical Genetics, Tomsk Research Center RAS, +7(3822)51-33-34,
vadim.stepanov@medgenetics.ru, 634050, Russia, Tomsk, Naberezhnaya Reki Ushayka str., 10.
Corresponding author: Ekaterina V. Izhoykina,
katushkabig@mail.ru




