Relationship between polymorphism in NOS3, AGTR1, TLR9, DRD4 genes and severity of congenital pneumonia in newborns
Objective. To identify variable region gene polymorphisms associated with the severity of congenital pneumonia in newborns.Ionov O.V., Donnikov A.E., Bezlepkina M.B., Nikitina I.V., Balashov E.N., Kirtbaya A.R., Kryuchko D.S., Baibarina E.N.
Material and Methods. The study comprised 101 newborns with confirmed congenital pneumonia requiring respiratory support after birth. The infants were divided into three groups. Group I (n = 63) included newborns whose respiratory therapy was limited to nasal non-invasive support including CPAP or non-invasive mechanical ventilation in the bi-level CPAP (Biphasic) mode. Newborns in group II (n = 25) required endotracheal intubation and invasive ventilation. Group III (n = 13) included newborns requiring aggressive invasive ventilation (МАР> 12 Н2O и FiO2> 0.5) and high-frequency oscillatory ventilation (HFOV). All patients were genotyped to identify DNA polymorphism is specific loci.
Results. We identified a statistically significant association between the polymorphic locus of the NOS3 gene: -786 (р = 0.028), the AGTR1 polymorphic locus: 1166 A>C (p = 0.009), the TLR9 polymorphic locus: 1486T>C (р = 0.022) of the DRD4 polymorphic locus : 521C>T (р = 0.04) and severity of the disease, need for aggressive invasive ventilation, and high-frequency oscillatory ventilation (HFOV).
Conclusions. Severity of congenital pneumonia in newborns requiring aggressive invasive ventilation (MAP> 12H2O and FiO2> 0.5) and HFOV was associated with polymorphism in NOS3 genes (synthesis of endogenous nitric oxide), AGTR1 (angiotensin converting receptor enzyme), TLR9 (toll-like receptor 9 is a membrane protein from the group of toll-like receptors playing an essential role in the innate immunity), and DRD4 (encodes a subtype of D4 receptor dopamine). Model experiments investigating targeted proteins and interaction pathways of the altered gene products are needed to confirm the predictive value and clinical significance of the studied polymorphism.
Keywords
In recent decades, there has been an ongoing search to develop new genetic markers that could improve risk prediction for individual patients with various diseases. Respiratory distress syndrome (RDS) and congenital pneumonia, the most common respiratory diseases in newborn infants, are multifactorial diseases. There has been gaining research evidence to support a genetic predisposition to these respiratory diseases [1-3]. In a study comparing the variable region gene polymorphisms in newborn infants with and without respiratory disorders, Khamidullina LI reported that susceptibility to respiratory distress in newborn infants was associated with the Ala/Ala genotype (OR = 2.10) and the Ala allele (OR = l. 89) of polymorphic Ala114Val locus of the GSTP1 gene. In boys, a predisposition to respiratory distress was associated with haplotype *2A (and, apparently, with haplotype *2B, because the 102T3801C locus (rs4646903)) of the CYP1A1 gene was analyzed (OR = 1.96) [4].
It was also found that the predisposition marker for developing RDS was the haplotype 11Thr + 160Thr of the SFTPD gene (OR = 3.18), and the propensity for developing RDS complicated by pneumonia was associated with the allele A of the polymorphic variant C-163A (rs762551) of the CYP1A2 gene (OR = 1.24) and the Thr/Met genotype of the polymorphic locus Thr11Met of SFTPD gene (OR = 2.67) [4]. In another study, it was found that the Ins / Ins genotype of the insertion-deletion polymorphism of the ACE gene (p = 0.011) and the number of tandem repeats in the 4th intron of the surfactant protein B (SFTPB) were markers of resistance to developing neonatal respiratory disorders. At the same time, the Del allele of the polymorphic locus of the ACE gene is a marker of the susceptibility of newborn infants to respiratory distress (p = 0.014). In addition, it was found that the genotype C/C of the polymorphic locus 32C> T of the surfactant protein D gene (SFTPD) is a marker of resistance to developing infectious complications of RDS (p = 0.0090), while the allele A of the polymorphic locus –627C>A of the interleukin 10 gene is associated with the development of infectious RDS complications [1].
We raised the question of why children with such a common disease as congenital pneumonia demonstrate different degrees of respiratory impairment. Some neonates with congenital pneumonia are successfully managed only with non-invasive respiratory support, others develop severe respiratory distress and required traditional mechanical ventilation (MV), and some have such severe respiratory distress that it requires aggressive ventilation parameters, high oxygen concentration, and high-frequency oscillatory ventilation (HFOV). We hypothesized that the carriage of allelic variants of genes is associated with severe respiratory distress in congenital pneumonia. In the available literature, we did not find evidence related to genetic markers of the severity of respiratory distress in newborn infants with congenital pneumonia. This study was aimed to investigate this issue.
An objective of this study was to identify variable region gene polymorphisms associated with the severity of congenital pneumonia in newborn infants.
Materials and methods
We examined the variable region gene polymorphisms in peripheral blood samples collected from101 newborn infants with a confirmed diagnosis of congenital pneumonia on their first day of life. The newborn infants were treated at the A.G. Antonov Neonatal Intensive Care Unit, Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia (Director of the Institute of Neonatology and Pediatrics Zubkov V.V., Head of the A.G. Antonov Neonatal Intensive Care Unit Ionov O.V.) from 1.10.2013 to 31.12.2014. All infants required respiratory therapy. The diagnosis of congenital pneumonia was made in accordance with the protocol for the diagnosis of infectious diseases adopted in the department [5].
The study participants were divided into three groups. Group I (n = 63) included newborn infants whose respiratory therapy was limited to nasal non-invasive support, including CPAP or non-invasive mechanical ventilation in the bi-level CPAP (Biphasic) mode. Newborn infants in group II (n = 25) required endotracheal intubation and traditional mechanical ventilation. Group III (n = 13) included newborn infants requiring aggressive mechanical ventilation (МАР > 12 Н2O и FiO2 > 0.5) and high-frequency oscillatory ventilation (HFOV).
Clinical indications for non-invasive respiratory support at birth were: for premature babies born at 32 weeks’ gestation and earlier - as a preventive measure; for babies born at 33 weeks’ gestation and later – respiratory distress with respiratory severity score greater than three as measured by Silverman Respiratory Severity Score.
The criteria for switching children from non-invasive respiratory therapy to traditional mechanical ventilation were respiratory distress with respiratory severity score greater than three as measured by Silverman Respiratory Severity Score or the need to increase oxygen saturation by more than 30% for infants born before 28 weeks’ gestation and by more than 40% for newborns born after 28 weeks’ gestation to maintain oxygen saturation at 90–95%.
The indications for switching to HFOV were aggressive modes of traditional mechanical ventilation: the mean airway pressure (MAP) more than 12 cm H2O and the need to increase oxygen saturation by more than 50% to maintain oxygen saturation at 90–95%.
DNA was extracted from peripheral blood samples that were collected in EDTA blood collection tubes using the PREP-GS Genetics reagent kit (DNA-Technology LLC, Russia). After extraction, the sample volume was 100 μl. The DNA concentration was determined using a DNA mini-fluorimeter (Nofer, the USA) and was, on average, 50–100 µg/ml.
Polymerase chain reaction (PCR) and determination of the melting temperature of oligonucleotide samples were performed using a DT-964 amplifier (DNA-Technology LLC, Russia).
PCR genotyping was conducted by analyzing the melting curves using a modified method of adjacent (kissing) probes at the Molecular Genetics Diagnostic Laboratory of the V.I. Kulakov NMRC for OG&P (Head of the Laboratory Donnikov A.E.) using commercial test systems manufactured by DNA-Technology LLC, Russia.
Infants whose mothers received antenatal corticosteroid prophylaxis were excluded from the study. Characteristics of the groups are presented in Table 1.

Statistically significant differences were observed between the groups in terms of gestational age and birth weight. However, severe respiratory distress was associated with more favorable anthropometric parameters and higher gestational age.
Results
The genotype allele frequencies in the groups are presented in Table 2.


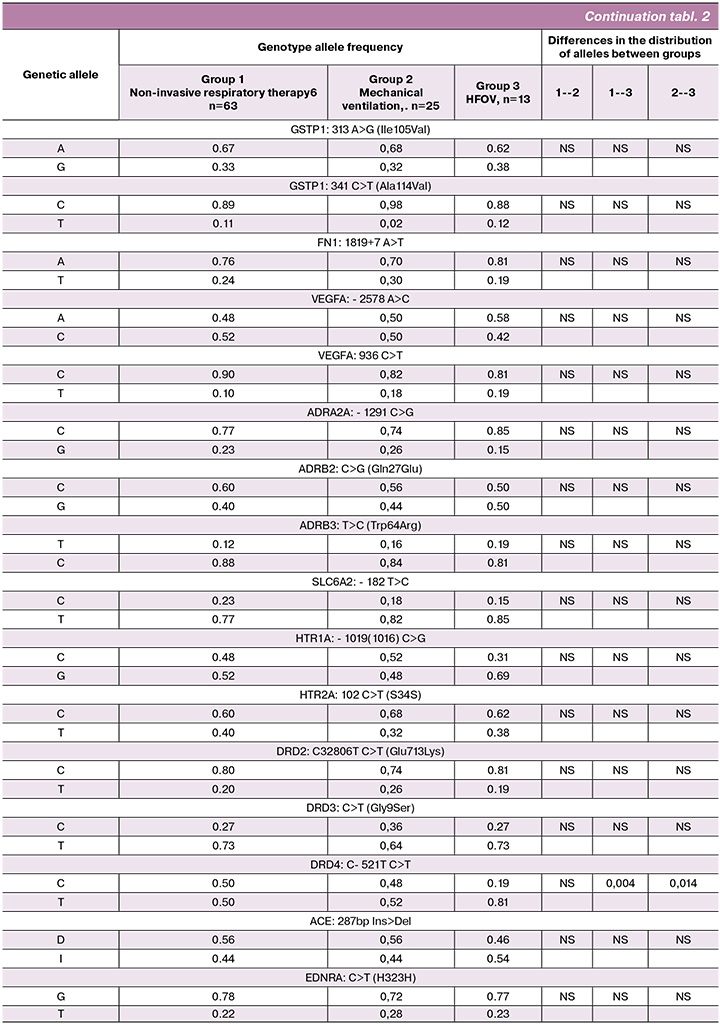
The distribution of alleles of the studied polymorphic loci in groups I and II did not differ. Therefore, we found it possible to pool groups I and II together. Thus, we compared gene polymorphisms between the two groups of patients: group I (pooled group) comprised 88 newborn infants receiving non-invasive respiratory therapy and traditional mechanical ventilation and group II included 13 newborns on HFOV.
Characteristics of the groups are presented in table 3.

The findings of the study showed statistically significant associations between the polymorphism in NOS3 (synthesis of endogenous nitric oxide), AGTR1 (angiotensin-converting enzyme receptor), TLR9 (toll-like receptor 9 - membrane protein, included in the group of toll-like receptors are involved the innate immunity ), DRD4 (encodes a subtype of the D4 dopamine receptor) genes and the severe course of the disease, which dictated the need for aggressive modes of mechanical ventilation and transition to HFOV in newborns with congenital pneumonia. These findings are shown in table 4.
Comparison of the frequencies of alleles of the -786 T/C locus of the NOS3 gene showed a statistically significant association of the C allele with severe respiratory distress and the need for aggressive mechanical ventilation in patients with congenital pneumonia (p = 0.028) (Fig. 1).
The distribution of alleles of the polymorphic locus AGTR1: 1166 A> C was statistically significantly different between groups (p = 0.009). According to the autosomal recessive model, the C/C genotype is associated with the need for aggressive ventilation in children with congenital pneumonia (OR = 19.1 (3.1-119.3), p = 5 × 10-5) (Fig. 2).

Comparison of the frequencies of alleles of the TLR9 polymorphic locus: 1486T> C showed a statistically significant association of allele C with a severe course of congenital pneumonia and the need for HFOV (p = 0.022), but when analyzing the distribution of genotypes, borderline significant results were obtained (Fig. 3), which did not allow us to determine the model of inheritance of this trait.
The −521 C>T polymorphism of the DRD4 gene also demonstrated a significant association with severe congenital pneumonia (p = 0.04 for the distribution of alleles and p = 0.01 for the C/T and T/T genotypes), which indirectly may confirm the role of the dopamine system in pathogenesis of the respiratory distress (Fig. 4).
Discussion
The severity of congenital pneumonia and respiratory distress as its main clinical manifestation is associated with many factors. The modern theory of the pathogenesis of respiratory distress in congenital pneumonia suggests its multifactorial nature with polygenic determinants, as well as complex interactions of both genetically determined and exogenous factors in the development of the disease.
Nitric oxide (NO) is a modulator of apoptosis and inflammatory cascade that affects endothelial permeability; it is synthesized by nitric oxide synthase (NOS) through oxidation of L‐arginine to L‐citrulline. In humans, three different types of NOS isoforms have been isolated, including neuronal NOS (nNOS) encoded by the NOS1 gene, inducible NOS (iNOS) encoded by the NOS2 gene, and endothelial NOS (eNOS) encoded by the NOS3 gene, expressed mainly in the epithelium of the respiratory tract.
NO mediates multiple physiological functions, including neurotransmission, immunoregulation, angiogenesis, antiplatelet activity, and surfactant maturation or secretion. The abundance of variants of physiological functions, strict control of NO production is crucial for its selective actions. In our study, we identified the association of the C allele of the -786 T/C locus of the NOS3 gene with a severe manifestation of respiratory distress and the need for HFHV in newborns with congenital pneumonia (p = 0.028). NOS3, participating in oxidative stress reactions and in the synthesis of NO, which has a relaxing effect on the endothelium, apparently affects the endothelial permeability, causing a change in the ventilation-perfusion ratio, which leads to the need for aggressive parameters of respiratory therapy. We also found that according to the autosomal recessive model, the NOS3 genotype: -786 T/T is protective: the odds ratio (OR) of the need for HFOV for these patients is 0.24 (0.05-1.14), p = 0.056.
The actions of angiotensin II are mediated by its interactions with the AT1 receptor. The ligand-receptor complex activates NADPH-oxidase, which forms superoxide, which, in turn, interacts with the vasorelaxation factors NO. Chao Y et al. reported that low shear stress induces reactive oxygen species via AT1R/eNOS/NO cascade [6]. It is known that reactive oxygen species are constantly formed in the cell, but the realization of oxidative stress and, as a result, disruption of the membrane lipid bilayer can also depend on the density of AGTR1 receptors. In turn, massive cellular damage in congenital pneumonia aggravates disease severity requiring more aggressive parameters of respiratory therapy.
Toll-like receptors recognize pathogens and play a key role in the innate immune system. Genetic polymorphisms in TLR-encoding genes have been implicated in the development of infections, malignant neoplasms, and autoimmune diseases [7]. Studies investigating TLR9 gene polymorphism and its association with chorioamnionitis and congenital infections reported that the presence of a variant T allele in a common SNP (rs352140) in the TLR9 gene whose product recognizes bacterial DNA is associated with an increased likelihood of placental inflammation [8]. When comparing the distribution of the alleles of the TLR9 polymorphic locus: 1486T> C (rs187084), we obtained a statistically significant association of allele C with the severity of pneumonia and the need for aggressive mechanical ventilation (p = 0.022). TLR9 seems to have a role in the development of the inflammatory response and correlates with the severity of congenital pneumonia.
The DRD4 gene encodes the D4 subtype of the dopamine receptor. The D4 subtype is a G-protein coupled receptor which inhibits adenylyl cyclase. As a result, dopamine receptors are common drug targets for the treatment of schizophrenia and Parkinson’s disease. Mutations in this gene are associated with various behavioral phenotypes, including autonomic nervous system dysfunction, attention deficit/hyperactivity disorder, and autism. In our study, the DRD4: 521C> T gene polymorphism also showed a significant association with severe congenital pneumonia (p = 0, 04 for the distribution of alleles and p = 0.01 for genotypes C/T and T/T), which may indirectly imply the role of the dopamine system in the development of the disease.
The limitations of the study are its retrospective, observational design and the absence of a control group, making it challenging to extrapolate its results to other populations. We also investigated a limited population of newborn infants (the study included newborns from the European part of Russia and the South Caucasus) and a limited panel of genes.
Model experiments investigating targeted proteins and interaction pathways of the altered gene products are needed to confirm the underlying biological mechanism of the effects of the identified polymorphism. Considering the statistical significance of the obtained results, further studies are needed to validate the clinical significance of these findings.
Conclusion
Thus, the severe course of congenital pneumonia in newborns dictating the need for aggressive modes of mechanical ventilation and transition to HFOV is associated with the polymorphism of the NOS3, AGTR1, TLR9, and DRD4 genes. Model experiments investigating targeted proteins and interaction pathways of the altered gene products are needed to confirm the predictive value and clinical significance of the studied polymorphism.
References
- Богданова Р.З., Фатыхова А.И., Данилка К.В., Викторов В.В., Викторова Т.В. Генетические маркеры дыхательных расстройств у новорожденных. Вопросы практической педиатрии. 2008; 6(3): 12-6. [Bogdanova R.Z., Fatichova A.I., Danilko K.V., Viktorov V.V., Viktorova T.V. Genetic Markers of Respiratory Disorders in Newborns. Voprosi practicheskoj pediatrii/Questions of practical Pediatrics. 2008; 6 (3):12-16. (in Russian)].
- Poterjoy B.S., Vibert Y., Sola-Visner M., McGowan J., Visner G., Nogee L.M. Neonatal respiratory failure due to a novel mutation in the surfactant protein C gene. J.Perinatol. 2010; 30(2): 151-3.
- Огородова Л.М., Петрова И.В., Иванчук И.И., Деев И.А., Фрейдин М.Б. Роль полиморфизма генов NO-синтаз в развитии бронхиальной астмы у детей. Педиатрия. Журнал имени Г.Н. Сперанского. 2007; 86(4): 14-8. [Ogorodova L.M., Petrova I.V., Ivanchuk I.I., Deev I.A., Frejdin M.B. The role of NO-synthase Polymorphism in the Development of Bronchial Asthma in Children. Pediatrja/Pediatrics. 2007; 86(4):14-18. (in Russian)].
- Хамидуллина Л.И., Данилко К.В., Викторова Т.В., Файзуллина Р.М., Викторов В.В. Генетические маркеры предрасположенности к развитию дыхательных нарушений у новорожденных. Вопросы диагностики в педиатрии. 2012; 5: 26-30. [Hamidullina L.I., Danilko K.V., Viktorova T.V., Faizullina R.M., Viktorov V.V. Genetic Markers of Predisposition to the Development of Respiratory Disorders in Newborns. Voprosi diagnostiki v pediatrii/The Questions of Diagnostics in Pediatrics. 2012; 5: 26-30. (in Russian)].
- Ионов О.В., Никитина И.В., Зубков В.В., Митрохин С.Д., Крохина К.Н., Киртбая А.Р., Левадная А.В.Любасовская Л.А., Рюмина И.И., Дегтярев Д.Н., Крючко Д.С. Порядок обследования новорожденных с подозрением на инфекционную патологию и правила назначения антибактериальной терапии, принятые в ОРИТН ФГБУ НЦАГиП им. В.И. Кулакова. Неонатология: новости, мнения, обучение. 2014; 1: 95-106. [The Procedure of Examination of Newborns suspected of infectious Pathology and Rules of Antibacterial Therapy, adopted at Department of Resuscitation and Intensive Therapy of Newborn at Kulakov Research Center of Obstetrics, Gynecology and Perinatology. 2014; 1: 95-106. Neonatologija: novosti, mnenija, obuchenije/Neonatology: news, opinions, training. 2014; 1: 95-106. (in Russian)].
- Chao Y., Ye P., Zhu L., Kong X., Qu X., Zhang J. et al. Low shear stress induces endothelial reactive oxygen species via the AT1R/eNOS/NO pathway. J.Cell. Physiol. 2018; 233(2): 1384-95. https://dx.doi.org/10.1002/jcp.26016.
- Yusuf J.H., Kaliyaperumal D., Jayaraman M., Ramanathan G., Devaraju P. Genetic selection pressure in TLR9 gene may enforce risk for SLE in Indian Tamils. Lupus. 2017; 26(3): 307-10. https://dx.doi.org/10.1177/0961203316659151.
- Karody V., Reese S., Kumar N., Liedel J., Jarzembowski J., Sampath V. A toll-like receptor 9 (rs352140) variant is associated with placental inflammation in newborn infants. J. Fetal Neonatal Med. 2016; 29(13): 2210-6. https://dx.doi.org/10.3109/14767058.2015.1081590.
Received 15.02.2019
Accepted 22.02.2019
About the Authors
Ionov, Oleg V., PhD, head of the A.G. Antonov Neonatal Intensive Care Unit, Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia, Associate Professor at the Department of Neonatology, Faculty of Pediatrics of I.M. Sechenov First MSMU.117997, Russia, Moscow, Oparina str. 4. Tel.: +74954382277. E-mail: o_ionov@oparina4.ru ID orcid.org/0000-0002-4153-133X
Donnikov, Andrey E., PhD, head of the Molecular Genetics Diagnostic Laboratory, Clinical Pathologist , V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
117997, Russia, Moscow, Oparina str. 4. E-mail: a_donnikov@oparina4.ru ID orcid.org/0000-0003-3504-2406
Bezlepkina, Maria B., MD, anesthesiologist at the A.G. Antonov Neonatal Intensive Care Unit, Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. 117997, Russia, Moscow, Oparina str. 4. Tel.: +74954382277. E-mail: masha.bezlepkina@mail.ru
Nikitina, Irina V., PhD, leading researcher at the Neonatal Intensive Care Unit №2, Institute of Neonatology and Pediatrics, Associate Professor at the Department of Neonatology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. 117997, Russia, Moscow, Oparina str. 4. Tel.: +74954382277. E-mail: i_nikitina@oparina4.ru
Balashova, Ekaterina N., PhD, clinical care supervisor at the A.G. Antonov Neonatal Intensive Care Unit, Institute of Neonatology and Pediatrics, Associate Professor at the Department of Neonatology, Faculty of Pediatrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
117997, Russia, Moscow, Oparina str. 4. Tel.: +74954382277. E-mail: e_balashova@oparina4.ru ID orcid.org/0000-0002-3741-0770
Kirtbaya, Аnna R., PhD, clinical care supervisor at the A.G. Antonov Neonatal Intensive Care Unit, Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia, Associate Professor at the Department of Neonatology, Faculty of Pediatrics , I.M. Sechenov First MSMU.
117997, Russia, Moscow, Oparina str.4. Tel.: +74954382277. E-mail: a_kirtbaya@oparina4.ru ID orcid.org/0000-0002-7628-8157
Kryuchko, Daria S., MD, deputy director for Science of National Medical Research Center for Children’s Health of Minzdrav of Russia. Professor at the Department of Neonatology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. 117997, Russia, Moscow, Oparina str.4. Tel.: +74991340796. E-mail: kryuchko.DS@nczd.ru
ID orcid.org/0000-0001-9047-6050
Baibarina, Elena N., MD, professor, chief researcher at the Neonatal Intensive Care Unit, Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P
of Minzdrav of Russia. 117997, Russia, Moscow, Oparina str. 4. Tel.: +74954382277. E-mail: baibarina@mail.ru
For citation: Ionov O.V., Donnikov A.E., Bezlepkina M.B., Nikitina I.V., Balashov E.N., Kirtbaya A.R., Kryuchko D.S., Baibarina E.N. Relationship between polymorphism in NOS3, AGTR1, TLR9, DRD4 genes and severity of congenital pneumonia in newborns. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (5): 102-11. (in Russian)
https://dx.doi.org/10.18565/aig.2019.5.102-111



