Plasma cytokine level changes in preterm neonates with respiratory disorders in the early neonatal period
Aim. To investigate plasma cytokine profile changes in preterm neonates with respiratory disorders in the early neonatal period.Nikitina I.V., Inviyaeva E.V., Krog-Jensen O.A., Vtorushina V.V., Lenyushkina A.A., Krechetova L.V., Zubkov V.V., Degtyarev D.N.
Materials and methods. The study included 105 neonates born in our Center from January 2019 to November 2019. The study group consisted of 92 preterm neonates with gestational age (GA) 32–36 weeks who were admitted to the neonatal intensive care unit (NICU) on the first day of life (DOL). The study group was divided into subgroups of respiratory distress syndrome (RDS) (n=21), transient tachypnea of the newborn (TTN) (n=28), and congenital pneumonia (n=43). The control group included healthy early-term infants (GA 37 weeks).
Plasma cytokine profiles of the neonates were analyzed. Blood samples were taken on days 1, 3, and 7 of life in the study group and day 1 of life in the control group. Blood samples were collected into EDTA tubes. Plasma cytokine profiling included pro-inflammatory (IFNγ, TNFα, IL-1β, IL-2, IL-6, IL-12, IL-17) cytokines, anti-inflammatory (IL-4, IL-5, IL-10, IL-13) cytokines, chemokines (IL-8, MCP-1, MIP-1β), and growth factors (IL-7, G-CSF, GM-CSF).
Results. Among preterm neonates, plasma levels of cytokines at the beginning and the end of the early neonatal period differed from those on the first day of life and depended on the type of respiratory disorders. There were statistically significant changes for pro-inflammatory cytokines (IL-6, INF-γ, TNF-α), chemokines (IL-8, MCP-1, MIP-1β), and anti-inflammatory cytokine IL-10.
Conclusion. Plasma cytokine profiling in preterm neonates on the first DOL can be used for differential diagnosis and assessment of treatment effectiveness DOL7. An increase in IL-6 concentration on DOL1 is the most informative of the studied markers regarding differential diagnosis between congenital pneumonia, RDS, and TTN. The IL-6/IL-10 ratio can be used as an additional criterion for decision-making regarding discontinuation of antibiotic therapy in preterm infants with congenital pneumonia.
Keywords
Respiratory disorders are one of the most common causes of neonatal intensive care unit (ICU) admissions of neonates, regardless of their gestational age (GA). Gaining insight into the pathogenesis of preterm infant respiratory disorders presents significant difficulties due to the nonspecific clinical manifestations and the lack of reliable laboratory markers of the systemic inflammatory response in preterm infants with pronounced morphofunctional immaturity. At the same time, the effectiveness of treatment depends on the accuracy and timeliness of diagnosing respiratory disorders. Determination of the cytokine status of preterm infants can be helpful in terms of differential diagnosis of respiratory diseases.
Despite numerous published studies investigating cytokine levels in newborns with different GA [1–5], the dynamics of their concentration in the early neonatal period in various perinatal diseases remains poorly understood.
The present study aimed to investigate plasma cytokine profile changes in preterm neonates with infectious and non-infectious respiratory disorders in the early neonatal period.
Materials and methods
The study included 105 preterm neonates born at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia from January to November 2019. The study group consisted of 92 preterm neonates with GA 32–36 weeks admitted to the ICU on the first day of life. Patients in study group were divided into subgroups of respiratory distress syndrome (RDS) (n=21), transient tachypnea of the newborn (TTN) (n=28), and congenital pneumonia (n=43). The control group included healthy early-term infants (GA 37 weeks).
The criteria for exclusion from the study were a syndromic form of hereditary pathology, congenital defects requiring urgent surgical correction, and hemolytic disease of the newborn.
Parents of newborns provided signed informed consent for their children to take part in the study. The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Clinical evaluation of the study participants included chest X-ray, blood culture, acute-phase proteins (C-reactive protein, CRP), a complete blood count including leukocytes, platelets, neutrophils, and calculation of the neutrophilic index (NI). Based on the clinical, laboratory and instrumental examination findings at 72 hours of the child's life, a conclusion was made about the presence or absence of congenital infection. The spectrum of infectious pathology was presented exclusively by congenital pneumonia; there were no other foci of infection in the study participants. No bacterial, fungal, and viral pathogens were detected in sterile loci (blood, tracheal aspirate).
Differential diagnosis between congenital pneumonia, RDS, and TTN was made following clinical guidelines [6, 7].
The plasma cytokine profiles were analyzed on the first and seventh days of life. Blood samples were collected in EDTA vacuum tubes on the first day of life simultaneously with the clinical and laboratory examination upon admission of newborns to the ICU before starting specific therapy and then on the seventh day of life, also simultaneously with the standard clinical and laboratory investigation. Blood samples were taken from children of the control group on the first day of life before enteral nutrition.
Plasma cytokine analysis was performed on a flow-based laser Bio-Plex 200 system (Bio-Rad, USA) using the Bio-Plex Pro Human Cytokine 17-plex Assay commercial kit ( Bio-Rad, USA). Plasma cytokine profiling included proinflammatory (interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2, IL-5, IL-6, IL-12, IL-17), anti-inflammatory (IL-4, IL-10, IL-13), chemokines (IL-8, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory proteins (MIP)-1β and growth factors (IL-7, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF). According to the manufacturer's instructions of the Bio-Rad test system, for the preparation of EDTA plasma samples, blood samples were centrifuged at 4°C in two stages (at 1000g for 15 min and then at 10000g for 10 min) to remove platelets and sediments completely. Plasma samples were frozen and stored at -80°C until the analysis.
Statistical analysis
Statistical analysis was performed using Microsoft Office Excel 2007, MedCalc12 for Windows 7, and IBM SPSS Statistics version 23 (USA). The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov, Lilliefors, Shapiro–Wilk tests, and the data for skewness and kurtosis were explored.
It was found that numerical variables were not normally distributed. Therefore, the Kruskal–Wallis test was used to compare numerical data between groups, followed by pairwise comparison using the Mann–Whitney U-test with Bonferroni correction for multiple comparisons. Results are presented as median (Me) and interquartile range (Q1–Q3). Taking into account the specifics of patient groups (newborn children in the ICU), in particular, significant differences in body weight and height, as well as the practical focus of the study, to more detailed clinical characteristics of the groups, we present the minimum (min) and maximum (max) values for each variable [min–max]. Differences were considered statistically significant at p <0.05. categorical variables were compared by the Pearson's χ2 test with Yates' correction and Fisher's exact test when the expected cell count is less than 5. To compare paired variables (comparison of paired samples with repeated measurements on the 1st and 7th days of life), a Wilcoxon matched-pair test was used.
Results
The clinical characteristics of the study groups are presented in Table 1.
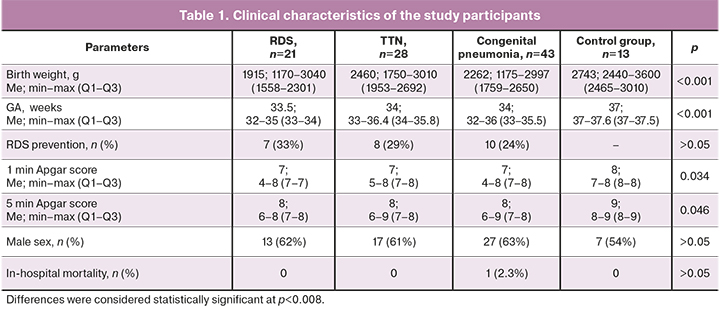
There were significant differences between newborns in the study and control groups regarding GA, birth weight, and 1- and 5-min APGAR scores. The child sex distribution in the study groups and subgroups was similar. The differences between the subgroups, shown in Table 1, are due to the general population frequencies of selected nosologies occurrence in children of different gestational ages [8]. There were no significant differences between subgroups, including in the frequency of antenatal steroid prophylaxis of RDS.
All neonates in the study group required respiratory therapy. Non-invasive respiratory treatment was carried out using three methods, including CPAP (continuous positive airway pressure) with the Infant Flow system and through high-flow cannulas and non-invasive mechanical ventilation. Non-invasive respiratory therapy was effective in all children in the TTN subgroup and did not require conventional or high-frequency oscillatory ventilation. Compared with infants of other subgroups, neonates with congenital pneumonia most often needed conventional mechanical ventilation and/or high-frequency ventilation (28% and 42%, respectively, p=0.002).
Antibiotic therapy for suspected infectious diseases was administered to 13/21 (62%) children in the RDS subgroup and 8/28 (28.5%) in the TTN subgroup until the clinical diagnosis was specified. All newborns with congenital pneumonia received antibiotic therapy from birth with two broad-spectrum antibiotics (ampicillin and gentamicin) (p=0.002).
The main complications during the neonatal period in preterm infants were disseminated intravascular coagulation syndrome (DIC) and intraventricular hemorrhage (IVH) of varying severity. The incidence of IVH was 9.5%, 10.7%, and 14% in the RDS, TTN, and congenital pneumonia subgroups, respectively (p=0.85). DIC was observed in newborns with congenital pneumonia in 23% of cases (10/43), compared with 9.5% (2/21) in the RDS subgroup and 3.5% (1/28) in the TTN subgroup (p=0.053). One preterm infant with GA of 33 weeks died of severe congenital pneumonia.
This work presents an analysis of only those cytokines in which changes in concentrations were observed. These are the proinflammatory cytokine IL-6, the chemokines IL-8, MCP-1, MIP-1β, and the anti-inflammatory cytokine IL-10. No statistically significant differences between groups were found in concentrations of IFNγ, TNFα, IL-1β, IL-2, IL-5, IL-12, IL-17, IL-4, IL-13, IL-7, G-CSF, and GM-CSF.
Peripheral venous blood concentrations of pro-inflammatory cytokines (IL-6, IL-8, MCP-1, MIP-1β) and anti-inflammatory IL-10 in preterm neonates with RDS, TTN, and congenital pneumonia on the first and seventh days of life are presented in Tables 2 and 3.
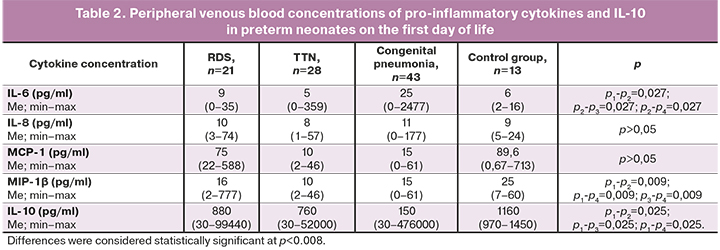
Plasma IL-6 levels in neonates with congenital pneumonia on the first day of life were significantly higher than in infants in the control group and preterm infants without infectious diagnosis (RDS and TTN subgroups). Further, plasma IL-6 levels decreased, and by the seventh day, there were no differences in its concentrations between the groups. Also, lower levels of MIP-1β were noted in preterm infants compared to that in children of the control group. Also, statistically significant differences were revealed in the concentrations of anti-inflammatory IL-10, with the maximum difference between children with congenital pneumonia and the control group on the first day of life.
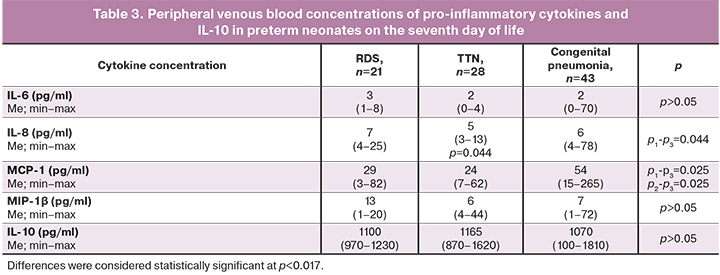
When assessing the dynamics of cytokine and chemokine levels in the subgroups of preterm infants on the seventh day of life, the main differences were noted for IL-6, MCP-1, MIP-1β, and IL-10 (Table 3).
Venous blood cytokine profiles of children with congenital pneumonia differed from those in children with non-infectious respiratory disorders. They were characterized by a decrease in the levels of IL-6, MCP-1 and an increase in IL-10 and MIP-1β concentrations during treatment (Figure). It should be noted that the reduction in IL-6 in neonates with congenital pneumonia occurred to the same values as in RDS, TTN. However, the level of MCP-1 remained higher on the seventh day in congenital pneumonia, compared with that in subgroups of non-infectious respiratory disorders (p=0.025).
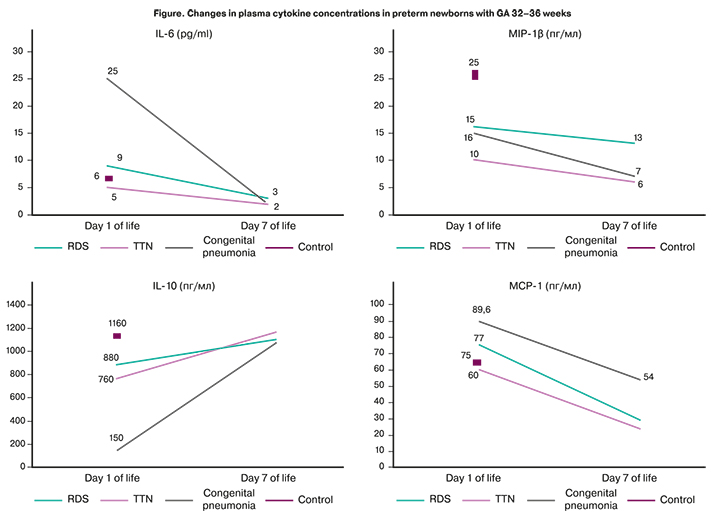
By the end of the early neonatal period, statistically significant differences in IL-10 levels between the subgroups leveled off against the background of treatment, which can indirectly characterize the effectiveness of specific therapy and restoration of the cytokine balance.
To assess the dynamics of cytokine levels within the subgroups (RDS, TTN, and congenital pneumonia), we compared the levels of cytokines on the seventh day with their baseline level on the first day of life separately for each subgroup. Statistical significance was revealed only for IL-6 and MCP-1 in children with congenital pneumonia (p˂0.001 and p=0.006, respectively) and IL-6 in children with RDS, which decreased on the seventh day of life compared with the baseline (p=0.026).
IL6, IL8, IFN-γ, TNF-α, MCP-1, and MIP1-β are markers of the pro-inflammatory immune responses. Changes in the balance of pro-inflammatory and anti-inflammatory cytokines in clinical practice are usually assessed using their ratios with IL-10, which are presented in Tables 4 and 5.
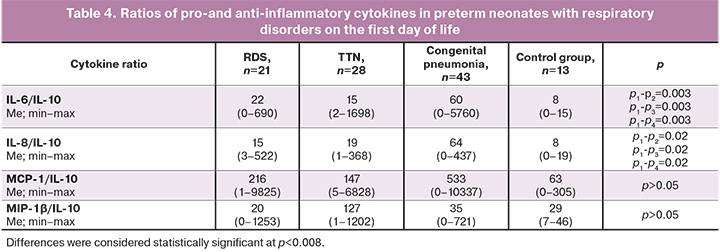
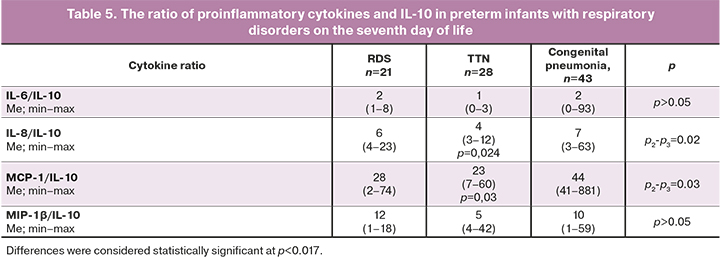
There was a persistence of the predominance of the pro-inflammatory immune reactions in newborns with congenital pneumonia, compared with children from the RDS and TTN subgroups (median IL-6/IL-10 ratio=60, IL-8/IL-10=64 against IL-6/IL-10=22, IL-8/IL-10=15 versus IL-6/IL-10=15, IL-8/IL-10=19, respectively, p=0.003, p=0.002). At the same time, on the first day of life, there was an increase in IL-6 concentration and its ratio with IL-10, which was not recorded on day 7. These changes most likely indicate the restoration of the balance between pro-and anti-inflammatory cytokines. However, a statistically significant difference in IL-8/IL-10 values between the subgroups of children with RDS and congenital pneumonia persists on the seventh day.
Differences in the concentration of TNF-α, IFN-γ, and the ratios of TNF-α/IL-10 and IFN-γ/IL-10 between groups and subgroups were not found either on the first or the seventh day of life.
Discussion
Our results confirm the findings of previously published studies. The overwhelming majority of them suggest the diagnostic value of IL-6 in infectious diseases in newborn infants. This cytokine is produced by various cells, including fibroblasts, keratinocytes, vascular endothelial cells, mast cells, macrophages, dendritic cells, and T and B cells [9] in response to tissue damage/or exposure to infections [10]. After interacting with specific receptors, IL-6 triggers a cascade of signaling reactions, mainly associated with the JAK/STAT3 activation [11], which promotes the transcription of genes that trigger various signaling pathways and, as a result, the production of other cytokines with different mediator functions. IL-6 also controls the synthesis of proteins involved in regulating the expression of immune response genes [12]. Such a large-scale interaction with various signaling molecules and the dependence of the expression of several genes on the activity of IL-6, according to many authors, determines the pleiotropic nature of this IL. Production of IL-6 stimulates both pro and anti-inflammatory components of the immune response [13], thus providing not only pro-inflammatory but also a regulatory effect. IL-6 controls the differentiation of monocytes into macrophages, increases the production of B-cell IgG [14].
IL-6 was shown to be no less significant and, in some cases, an earlier marker of neonatal infection and sepsis than CRP [1, 15]. A study by Leal et al., which included 96 newborns with different GA (from 27 to 42 weeks), reported that an increase in IL-6 occurs in both early and late neonatal sepsis [1].
An increase in proinflammatory cytokines, particularly IL-6, has also been described in non-infectious lung damage, RDS, and bronchopulmonary dysplasia (BPD) [2, 16]. However, two main features of studies of the cytokine profile in RDS are worth noting: 1) most of them were carried out in the period 2000–2010, and 2) the study group was most often extremely preterm infants. The cytokine response in this cohort is due to aseptic inflammation resulting from several damaging factors on immature lungs. They include surfactant deficiency, collapse and damage of the alveoli, the leakage of blood plasma proteins into the alveoli, and ventilator-associated lung damage during mechanical ventilation. Over the past decade, the rapid development of perinatal and neonatal technologies has reduced the incidence of severe mechanical ventilation-induced lung damage, and cases of severe RDS have become less and less common in routine neonatal practice in children born > 32 weeks of gestation. Our study included children with GA 32–36 weeks with a higher lung maturity and, accordingly, less pronounced damage to the alveoli, which caused the absence of an obvious cytokine reaction in children with RDS.
This study demonstrateв a statistically significant reduction in the level of IL-6 by the seventh day of life as the inflammatory process fades away during treatment in newborns with congenital pneumonia. Unidirectional but less pronounced changes were also noted in non-infectious, aseptic inflammation triggered during the development of RDS. In the course of TTN (retention of fetal fluid in the alveoli), respiratory failure in the vast majority of cases was moderately expressed, regressed during the first hours of life. It was not accompanied by pronounced fluctuations in IL-6 levels.
Chemokines (chemotactic cytokines) play a central role in regulating inflammation in various diseases, helping to attract macrophage monocytes, lymphocytes, eosinophils, and basophils to the inflammation focus. MIP-1β is one of the critical proteins of the CC family [17]. MCP-1 (or CCL2) regulates the migration and infiltration of monocytes, memory T lymphocytes and plays an essential role in the Th1-mediated immune response. An increase in MCP-1 is observed in various acute and chronic lung diseases in adults and children. A study by Murch [18] studied changes in chemokine concentrations in the tracheobronchial aspirate in very low birth weight infants. The concentration of chemokines increased in acute lung injury in children with very low body weight on mechanical ventilation and correlated with an unfavorable outcome. Baier et al. also demonstrated that the concentrations of MCP-1, MCP-2, MCP-3, and MIP-1β in the tracheal aspirate of very low birth weight infants with the course of RDS increase during the first week of life, while there was no noticeable increase in the concentration of MIP-1a. It was also found that an increase in the concentration of chemokines in the tracheal aspirate is associated with BPD development [17]. However, in a more recent study, the association of BPD with MCP-1 was not confirmed [19].
It should be noted that MCP-1 can increase both aseptic and pathogen-induced inflammation [20]. Besides, MCP-1 is not a protein-specific exclusively for the lung tissue. Its increase has been described in the case of damage to other organs and tissues [21, 22]. In our study, an increase in MCP-1 was observed on the first day of life in newborns with infectious respiratory disorders, compared with children in the control group and preterm neonates with RDS and TTN (although the difference did not reach statistical significance). It is important to note that MCP-1 remained significantly elevated on the seventh day of life, most likely associated with severe damage and inflammatory changes in the lung tissue in congenital pneumonia, which were not observed during RDS in children born at 32–36 weeks gestation. And unlike IL-6, the dynamics of MCP-1 by the seventh day of life was distinct and statistically significant only in infectious respiratory disorders - congenital pneumonia. The hypothesis of a more pronounced infectious, pathogen-induced inflammation in children with GA 32–36 weeks is also supported by clinical data. In the group of patients with congenital pneumonia, there was a higher need for invasive mechanical ventilation than in the RDS and TTN subgroups. This is also evidenced by the results of our earlier study, in which the level of MCP-1 was significantly higher in preterm infants with congenital infection, regardless of their GA [5].
IL-10 is an anti-inflammatory cytokine that suppresses inflammation in body tissues. According to our data, IL-10 levels in the control group were the highest and the lowest in newborns with congenital pneumonia, which is natural for active systemic inflammation.
Ratios of IL-6/IL-10 and IL-8/IL-10 were statistically significantly higher on the first day of life in newborns with congenital pneumonia compared with children from the control group and other subgroups. In dynamics, by the end of the early neonatal period, there was a decrease in IL-6/IL-10 in newborns with congenital pneumonia to the same figures as in children without an infectious diagnosis. The IL-8/IL-10 also decreased over time; however, it remained statistically significantly higher in neonates with congenital pneumonia. An increase in the level of IL-8 and its ratio with IL-10 by the end of the early neonatal period may indicate the persistence and/or absence of relief of the inflammatory process, despite antibiotic therapy. Unformed adaptive immunity (no changes in the content and dynamics of IFNγ, TNFα) in combination with changes in IL-8 indicate a general immune system immaturity and a pro-inflammatory orientation of immune responses.
Conclusion
The changes in cytokine levels in neonates of 32–36 weeks’ gestational age with different etiology of respiratory disorders (RDS, TTN, and congenital pneumonia) indicate other mechanisms of immune responses in these pathological conditions.
Comparison of cytokine levels in newborns with infectious and non-infectious disorders on the first day of life revealed statistically significant differences in concentrations of IL-6, MIP-1β, and IL-10. However, on the seventh day of life, significant differences were only in MCP-1 levels. Comparison of the cytokine levels changes showed a decrease in the levels of IL-6, MCP-1 and an increase in IL-10 and MIP-1β concentrations in newborns with congenital pneumonia, compared with children with RDS and TTN.
In the future, the assessment of IL-6/IL-10 and IL-8/IL-10 ratios may have diagnostic and prognostic significance since on the first day of life, both ratios in newborns with pneumonia were increased compared to other subgroups and the control group. At the same time, in dynamics, both indicators decreased to almost the same values as in children without an infectious diagnosis, which may indirectly indicate a decrease in the severity of the inflammatory process.
Thus, the most demonstrative of the studied marker was IL-6, which can be determined in routine clinical practice. Serum levels of IL-6, both as concentration and a ratio with IL-10, can be used for the early diagnosis of congenital pneumonia in preterm infants and assessing the changes in the course of the inflammatory process.
Determination of cytokine levels on the first day of life can be helpful in the differential diagnosis of respiratory disorders in preterm neonates and on the seventh day of life for an objective assessment of the treatment effectiveness. The ratio of proinflammatory cytokines to IL-10 can be used as an additional criterion for decision-making regarding discontinuation of antibiotic therapy in preterm infants with congenital pneumonia.
References
- Girón-Carrillo J.L., Cedillo-Rivera R., Velazquez J.R. Cytokine profile as diagnostic and prognostic factor in neonatal sepsis. J. Matern. Fetal Neonatal Med. 2019; 32(17): 2830-6. https://dx.doi.org/10.1080/14767058.2018.449828.
- Speer C.P. Neonatal respiratory distress syndrome: an inflammatory disease? Neonatology. 2011; 99(4): 316-9. https://dx.doi.org/10.1159/000326619.
- Varvarigou A.A., Thomas I., Rodi M., Economou I., Mantagos S., Mouzaki A. Respiratory distress syndrome (RDS) in premature infants is underscored by the magnitude of Th1 cytokine polarization. Cytokine. 2012; 58(3): 355-60. https://dx.doi.org/10.1016/j.cyto.2012.03.005.
- Zasada M., Lenart M., Rutkowska-Zapała M., Stec M., Mól N., Czyz O. et al. Analysis of selected aspects of inflammasome function in the monocytes from neonates born extremely and very prematurely. Immunobiology. 2018; 223(1): 18-24. https://dx.doi.org/10.1016/j.imbio.2017.10.019.
- Никитина И.В., Жукова А.С., Ванько Л.В., Вторушина В.В., Матвеева Н.К., Кречетова Л.В., Крючко Д.С., Ионов О.В., Зубков В.В., Дегтярев Д.Н. Особенности цитокинового статуса у недоношенных новорожденных с заболеваниями легких инфекционного и неинфекционного генеза. Неонатология: новости, мнения, обучение. 2018; 6(4): 16-23. [Nikitina I.V., Zhukova A.S., Vanko L.V. et al. Cytokine status of preterm newborns with infectious and noninfectious diseases. Neonatology: News, Opinions, Training. 2018; 7(4): 16-23. (in Russian)]. https://dx.doi.org/10.24411/2308-2402-2018-14002.
- Антонов А.Г., Байбарина Е.Н., Балашова Е.Н., Дегтярев Д.Н., Зубков В.В., Иванов Д.О., Ионов О.В., Карпова А.Л., Киртбая А.Р., Крохина К.Н., Крючко Д.С., Ленюшкина А.А., Ли А.Г., Малютина Л.В., Мебелова И.И., Никитина И.В., Петренко Ю.В., Рындин А.Ю., Рюмина И.И., Романенко А.В. Врожденная пневмония (клинические рекомендации). Неонатология: новости, мнения, обучение. 2017; 4: 133-48. [Antonov A.G., Baybarina E.N., Balashova E.N., Degtyarev D.N., Zubkov V.V. et al. Congenital pneumonia (clinical practice guidelines). Neonatology: News, Opinions, Training. 2017; 4: 133-48. (in Russian)]. https://dx.doi.org/10.24411/2308-2402-2017-00049.
- Володин Н.Н., ред. Ведение новорожденных с респираторным дистресс-синдромом. Клинические рекомендации. М.: Российская ассоциация специалистов перинатальной медицины, Ассоциация неонатологов; 2015. [Volodin N.N., Averin A.P., Antonov A.G., Baibarina E.N. et al. Respiratory Distress Syndrome in neonates (clinical practice guidelines). Russian association of perinatal medicine. 2016. (in Russian)].
- Никитина И.В., Донников А.Е., Крог-Йенсен О.А., Ленюшкина А.А., Быстрицкий А.А., Крючко Д.С., Ионов О.В., Зубков В.В., Дегтярев Д.Н. Генетические полиморфизмы у детей, ассоциированные с развитием врожденных инфекций. Акушерство и гинекология. 2019; 11: 175-85. [Nikitina I.V., Donnikov A.E., Krogh-Jensen O.A., Lenyushkina A.A., Bystritsky A.A., Kryuchko D.S., Ionov O.V., Zubkov V.V., Degtyarev D.N. Congenital infection-associated genetic polymorphisms in children. Obstetrics and Gynegology. 2019; 11: 175-85. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.175-185.
- Mauer J., Denson J.L., Brüning J.C. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015; 36(2): 92-101. https://dx.doi.org/ 10.1016/j.it.2014.12.008.
- Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014; 6(10): a016295. https://dx.doi.org/10.1101/cshperspect.a016295.
- Wang S.W., Sun Y.M. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (Review). Int. J. Oncol. 2014; 44(4): 1032-40. https://dx.doi.org/10.3892/ijo.2014.2259.
- Brocke-Heidrich K., Kretzschmar A.K., Pfeifer G., Henze C., Löffler D., Koczan D. et al. Interleukin-6-dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family-independent survival pathway closely associated with Stat3 activation. Blood. 2004; 103(1): 242-51. https://dx.doi.org/10.1182/blood-2003-04-1048.
- Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011; 1813(5): 878-88. https://dx.doi.org/10.1016/j.bbamcr.2011.01.034.
- Yang R., Masters A.R., Fortner K.A., Champagne D.P., Yanguas-Casás N., Silberger D.J. et al. IL-6 promotes the differentiation of a subset of naive CD8+ T cells into IL-21-producing B helper CD8+ T cells. J. Exp. Med. 2016; 213(11): 2281-91. https://dx.doi.org/10.1084/jem.20160417.
- Mirzarahimi M., Barak M., Eslami A., Enteshari-Moghaddam A. The role of interleukin-6 in the early diagnosis of sepsis in premature infants. Pediatr. Rep. 2017; 9(3): 7305. https://dx.doi.org/10.4081/pr.2017.7305.
- Speer C.P. Chorioamnionitis, postnatal factors and proinflammatory pesponse in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology. 2009; 95(4): 353-61. https://dx.doi.org/10.1159/000209301.
- Baier R.J., Majid A., Parupia H., Loggins J., Kruger T.E. CC chemokine concentrations increase in respiratory distress syndrome and correlate with development of bronchopulmonary dysplasia. Pediatr. Pulmonol. 2004; 37(2): 137-48. https://dx.doi.org/10.1002/ppul.10417.
- Murch S.H., Costeloe K., Klein N.J., MacDonald T.T. Early production of macrophage inflammatory protein-1 alpha occurs in respiratory distress syndrome and is associated with poor outcome. Pediatr. Res. 1996; 40(3): 490-7. https://dx.doi.org/10.1203/00006450-199609000-00020.
- Zhou D., Shi F., Xiong Y., Zhou M., Wan H., Liu H. Increased serum Th2 chemokine levels are associated with bronchopulmonary dysplasia in premature infants. Eur. J. Pediatr. 2019; 178(1): 81-7. https://dx.doi.org/10.1007/s00431-018-3266-z.
- Dong Y., Glaser K., Schlegel N., Claus H., Speer C.P. An underestimated pathogen: Staphylococcus epidermidis induces pro-inflammatory responses in human alveolar epithelial cells. Cytokine. 2019; 123: 154761. https://dx.doi.org/10.1016/j.cyto.2019.154761.
- Heuer L.S., Croen L.A., Jones K.L., Yoshida C.K., Hansen R.L., Yolken R. et al. An exploratory examination of neonatal cytokines and chemokines as predictors of autism risk: The Early Markers for Autism Study. Biol. Psychiatry. 2019; 86(4): 255-64. https://dx.doi.org/10.1016/j.biopsych.2019.04.037.
- Pappas A., Shankaran S., McDonald S.A., Carlo W.A., Laptook A.R., Tyson J.E. et al. Blood biomarkers and 6- to 7-year childhood outcomes following neonatal encephalopathy. Am. J. Perinatol. 2020 Oct 10. https://dx.doi.org/10.1055/s-0040-1717072.
Received 26.03.2021
Accepted 05.05.2021
About the Authors
Irina V. Nikitina, Ph.D., Leading Researcher at the Neonatal Intensive Care Unit №2 of the Institute of Neonatology and Pediatrics, Associate Professor at the Departmentof Neonatology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)531-44-44, ex. 2700, 2697. Е-mail: i_nikitina@oparina4.ru.
ORCID: 0000-0002-1103-1908; Researcher ID: AAH-3465-2019; SCOPUS Author ID: 57189233499. 117997, Russia, Moscow, Oparina str., 4.
Eugenia V. Inviyaeva, Ph.D. (bio.sci.), Senior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel: +7(495)438-11-83. E-mail: e_inviyaeva@oparina4.ru. ORCID: 0000-0001-9878-3637. 117997, Russia, Moscow, Oparina str., 4.
Olga A. Krogh-Jensen, Ph.D., Neonatologist at the Neonatal Intensive Care Unit №2 of the Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia; Associate Professor at the Department of Neonatology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University).
Tel.: +7(926)014-01-35. E-mail: o_krogh@oparina4.ru. ORCID: 0000-0002-5178-5659. 117997, Russia, Moscow, Oparina str., 4.
Valentina V. Vtorushina, Ph.D., Immunologist at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-11-83. E-mail: v_vtorushina@oparina4.ru. ORCID: 0000-0002-8406-3206. 117997, Russia, Moscow, Oparina str., 4.
Anna A. Lenyushkina, Ph.D., Head of the Neonatal Intensive Care Unit №2 of the Institute of Neonatology and Pediatrics, Associate Professor at the Department
of Neonatology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)531-44-44, ex. 2700, 2697. E-mail: a-lenushkina@yandex.ru.
ORCID: 0000-0001-8929-2991. 117997, Russia, Moscow, Oparina str., 4.
Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-11-83.
E-mail: l_krechetova@oparina4.ru. 117997, Russia, Moscow, Oparina str., 4.
Viktor V. Zubkov, Dr. Med. Sci., Professor, Director of the Institute of Neonatology and Pediatrics, Head of the Department of Neonatology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor at the Department of Neonatology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University).
E-mail: victor.zubkov@mail.ru. ORCID ID: 0000-0002-9697-9596. 117997, Russia, Moscow, Oparina str., 4.
Dmitriy N. Degtyarev, Dr. Med. Sci., Professor, Deputy Director for Research, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Head of the Department
of Neonatology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University). Tel.: +7(495)438-25-33. E-mail: d_degtiarev@oparina4.ru.
ORCID: 0000-0001-8975-2425. 117997, Russia, Moscow, Oparina str., 4.
For citation: Nikitina I.V., Inviyaeva E.V., Krog-Jensen O.A., Vtorushina V.V., Lenyushkina A.A., Krechetova L.V., Zubkov V.V., Degtyarev D.N. Plasma cytokine level changes in preterm neonates with respiratory disorders in the early neonatal period.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 8: 133-142 (in Russian)
https://dx.doi.org/10.18565/aig.2021.8.133-142



