Immunogenetic mechanisms of recurrent pathological vaginal discharge in women of reproductive age
Uruymagova A.T., Mezhevitinova E.A., Donnikov A.E.
Objective: To identify immunogenetic factors that predispose women of reproductive age to recurrent pathological vaginal discharge.
Materials and methods: This study enrolled 173 women of reproductive age: 59 with episodic pathological vaginal discharge, 74 with recurrent pathological discharge, and 40 healthy women (control group). Genotyping of single nucleotide polymorphisms (SNPs) in immune-regulatory genes (CD14, CTLA4, IFN-γ, IGF-2, IL-10, IL-12β, IL-18, IL-1α, IL-1β, IL-1R, IL-2, IL-4, IL-4R, IL-6, IL-6R, IL-8, TGFβ1, TNF, and MBL2) and genes associated with the neuroendocrine stress response (CRH, CRHR1, and CRHR22) was performed using PCR-based SNP typing. The expression of cytokine (IL-1β, IL-10, IL-18, and TNF-α) and innate immunity receptor (TLR4) mRNAs in vaginal samples was measured by real-time RT-PCR. Statistical analysis included comparisons of allele/genotype frequencies, risk ratio (RR) calculations with 95% confidence intervals, χ² test, Mann–Whitney test, and multivariate logistic regression (significance at p<0.05).
Results: Recurrent pathological vaginal discharge was associated with alterations in local immune response. In the group with recurrent inflammatory discharge, there was significantly increased expression of the anti-inflammatory cytokine IL-10 and decreased IL-18 expression compared to episodic cases, indicating an imbalance in immune regulation. Patients with non-inflammatory recurrences exhibit a different immune profile, characterized by elevated levels of the pro-inflammatory cytokines TNF-α and IL-10. Statistically significant associations were identified between genetic variants and disease recurrence. The GG genotype of the IL-10 -1082 G>A polymorphism increased the risk of recurrent pathological discharge of both inflammatory and non-inflammatory origins, whereas the GG genotypes of the TNF-α -238 G>A polymorphism and CRHR1 (rs17689966) polymorphism significantly increased the likelihood of recurrence in cases of non-inflammatory discharge.
Conclusion: Recurrence of pathological vaginal discharge is driven by a combination of local immune imbalance and genetic predisposition. Polymorphisms in immune-related genes (IL-10, TNF, and CRHR1) may serve as molecular genetic markers of recurrence risk, paving the way for personalized prevention and treatment strategies.
Authors' contributions: Uruymagova A.T., Mezhevitinova E.A., Donnikov A.E. – conception and design of the study, obtaining data for analysis, review of relevant literature, analysis of the obtained data, drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Uruymagova A.T., Mezhevitinova E.A., Donnikov A.E. Immunogenetic mechanisms of
recurrent pathological vaginal discharge in women of reproductive age.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (7): 103-111 (in Russian)
https://dx.doi.org/10.18565/aig.2025.138
Keywords
Pathological vaginal discharges, whether of inflammatory or non-inflammatory origin, continue to pose a significant challenge in gynecology owing to their widespread prevalence and tendency to recur [1, 2]. One contributing factor is the imbalance between Lactobacillus spp. and opportunistic pathogens (OPs). Specific manifestations of these disturbances include aerobic vaginitis (AV) and bacterial vaginosis (BV). According to WHO data, the prevalence of BV among women of reproductive age is approximately 30%, with over half of the patients experiencing recurrence within 3–6 months after treatment [1, 3]. Recurrences are attributed to the persistence of Gardnerella, Atopobium, and biofilm formation, as well as behavioral factors such as frequent partner changes, douching, smoking, contraceptive use, and immunosuppression [4, 5]. To date, no single determinant has been identified that fully explains individual susceptibility to recurrence.
The significance of recurrent vaginal infections lies not only in their frequency, but also in their considerable negative impact on reproductive health and patients' quality of life. These conditions increase the risk of inflammatory diseases of the pelvic organs and infertility [6]. An established association exists between BV, particularly in conjunction with human papillomavirus (HPV), and the risk of cervical neoplasia [7]. Additionally, vaginal dysbiosis in IVF patients is correlated with lower implantation and pregnancy success rates [8]. Furthermore, BV and AV heighten the likelihood of obstetric complications, including miscarriage, preterm birth, chorioamnionitis, and postpartum endometritis [9, 10]. This emphasizes the urgent need for novel approaches for prevention and prognosis.
Traditionally, BV is viewed as anaerobic dysbiosis characterized by minimal inflammation, whereas AV is characterized by pronounced inflammatory symptoms [11, 12]. However, these conditions can coexist or occur sequentially, resulting in mixed forms. The diversity of microbial factors significantly complicates recurrence prevention.
In the past decade, there has been a growing interest in the role of innate immunity and genetic predisposition in the pathogenesis of vaginal infections. Several studies have confirmed the impact of polymorphisms in innate immunity genes (TLR2, TLR4, TLR7, and TLR9) and cytokines (IL-1β and IL-6) on vaginal microbiota and the risk of BV [13, 14]. According to Murphy K. et al. (2024), genetic variations in immune regulatory genes (cytokines and TLRs) are associated with BV in women from various racial backgrounds [15]. A meta-analysis by Kalia N. et al. (2020) indicated that the rs1800450 polymorphism in MBL2 increases the risk of recurrent vaginal infections by more than 3.5 times [16]. However, De Seta F. et al. (2007) reported that this polymorphism did not demonstrate a clear association with recurrent BV [17].
Thus, the immunogenetic mechanisms underlying the recurrence of pathological vaginal discharges remain inadequately studied and necessitate a comprehensive approach that considers both clinical and genetic factors influencing the immune response to microbial invasion.
This study aimed to identify immunogenetic factors that predispose women of reproductive age to recurrent pathological vaginal discharge.
Materials and methods
Study design and participants
This study was conducted at the Scientific Polyclinic Department of the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia from 2019 to 2022. The study comprised 173 women of reproductive age, including patients with complaints of pathological vaginal discharge (study group) and those without pathological discharge (control group). The group allocation was based on clinical and microscopic criteria.
Due to the frequent combination of various microorganisms (aerobes, anaerobes, Candida) and conflicting data on inflammation in BV, patients were classified not by nosological forms but by the presence of symptoms (pathological discharge) and signs of inflammation.
Three groups were identified: group I, episodic pathological discharge (n=59); group II, recurrent (≥3 episodes per year, n=74); and group III, comparison group (healthy women, n=40). Groups I and II were subdivided based on the presence of inflammation: subgroup "a" (inflammatory: leukocytosis, itching, hyperemia) and subgroup "b" (non-inflammatory, without inflammation). Thus: Ia – episodic inflammatory (n=27), Ib – episodic non-inflammatory (n=32), IIa – recurrent inflammatory (n=38), IIb – recurrent non-inflammatory (n=36). This allowed us to study the impact of recurrence rate and current inflammatory status simultaneously.
The sample size was preliminarily calculated based on data from previous studies on similar topics. To detect differences between groups with an estimated prevalence of studied polymorphisms around 30% in the control group and an increase to 60% in the case group, with a study power of 80% and a significance level of α=0.05, at least 41 participants needed to be included in each group. The study included 74 women with recurrent pathological discharge (cases), 59 women with episodic discharge, and 40 women in the control group, which met the sample size calculation requirements and provided sufficient statistical power.
Inclusion criteria were age 18–49 years; for groups I–II, presence of clinical signs of pathological discharge; and recurrent course, defined as ≥3 exacerbation episodes within 12 months (according to ISSVD recommendations, 2023).
Exclusion criteria included pregnancy, sexually transmitted infections (STIs), Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Mycoplasma genitalium, etc.; presence of Candida spp. >104 in vaginal microbiota; severe concomitant somatic or endocrine diseases, immunodeficiency states; and systemic antibiotic or immunomodulator intake within 4 weeks before study inclusion.
All the participants provided written informed consent. The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P.
Examination and laboratory methods
All patients underwent standard clinical and gynecological examinations. The presence of an inflammatory reaction in the vaginal mucosa was assessed based on gynecological examination (vaginal mucosal hyperemia detected during speculum examination) and microscopic examination of vaginal discharge (leukocyte reaction was evaluated based on the ratio of leukocytes to epithelial cells, with leukocyte predominance at a ratio of 5:1 and above indicating inflammation). Additionally, STI screening was performed using polymerase chain reaction (PCR). Upon detection, the patients received appropriate treatment and were excluded from the study.
Molecular genetic studies
DNA samples were extracted from venous blood and vaginal swabs for genetic typing in all participants. DNA was extracted from the blood using the standard phenol-chloroform method. Single nucleotide polymorphisms (SNPs) were genotyped by allele-specific PCR with real-time detection (using equipment and reagents from DNA Technology LLC, Russia). The following gene polymorphisms were studied: IL-10 (promoter SNP -1082 G>A, rs1800896), TNF-α (promoter SNP -238 G>A, rs361525), MBL2 (exon 1 polymorphisms: rs5030737, rs1800450, rs1800551), CRH gene (encoding corticotropin-releasing hormone; SNP rs6996265, rs3176921) and its receptors CRHR1 (rs17689966) and CRHR2 (rs2267717). Alleles were designated according to the accepted nomenclature; for example, for IL-10 -1082 G>A, the A allele corresponds to the "low-producing" cytokine variant. Genotypes in the groups were checked for compliance with the Hardy–Weinberg law.
For gene expression analysis, total RNA was extracted from vaginal swabs and reverse transcribed (RT) to cDNA. Quantitative real-time PCR was performed to assess the relative transcript levels of key cytokines and immune response factors, IL-1B, IL-10, IL-18, TNF, GATA3, and CD68, as well as the innate immunity receptor TLR4. The β-actin gene (ACTB) was used as an endogenous expression control. The relative mRNA expression levels (relative to the average level in the control group) were calculated using the 2-ΔΔCt method.
Statistical analysis
Statistical analysis was conducted using SPSS Statistics 26 (USA) and Microsoft Excel. Continuous variables showing a normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1–Q3) was reported. Frequencies and percentages were reported for categorical variables [n (%)]. Normality of continuous variable distribution was checked using the Shapiro–Wilk test. When comparing three or more groups for continuous variables with a normal distribution, an analysis of variance was used. In the first stage, Fisher’s F-test was applied with homogeneous variances. The Kruskal–Wallis test was used with subsequent post-hoc pairwise comparisons to compare three or more independent groups for continuous variables. Differences in categorical variables were assessed using the Pearson's χ² test or Fisher's exact test. Differences were considered statistically significant at p<0.05.
Results
Clinical characteristics of the study groups
The study included 173 women with pathological vaginal discharge. The mean age of the participants was 32.2 (7.2) years. The groups did not differ in age, somatic and gynecological diseases, number of pregnancies, age at sexual debut, or sexual behavior characteristics (p>0.05) (Tables 1 and 2).
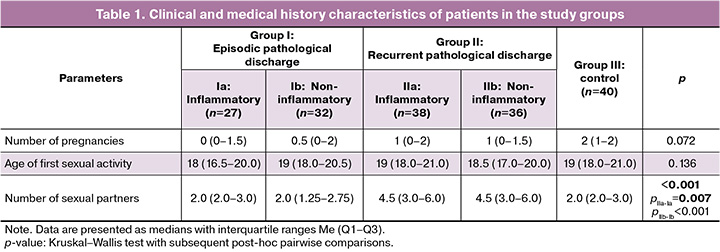
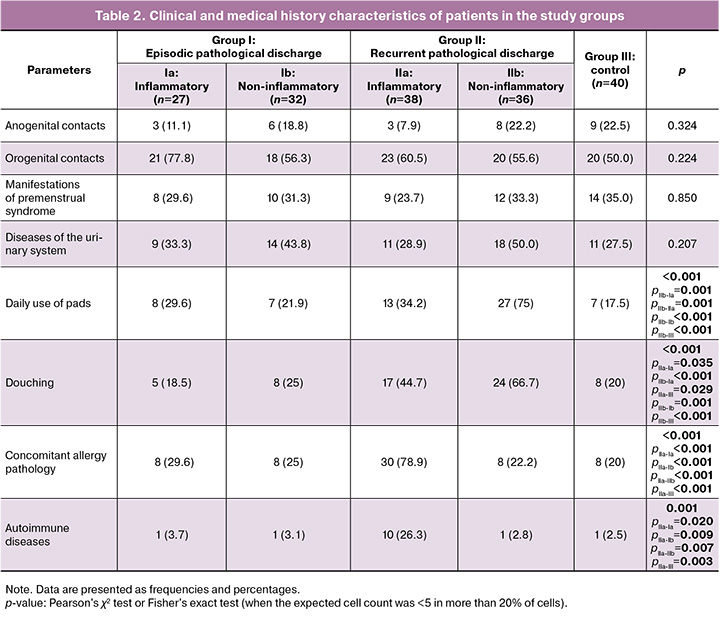
However, the analysis of medical history and lifestyle revealed significant differences between episodic and recurrent cases. The mean number of sexual partners was significantly higher in patients with recurrence than in those with episodic discharge (p<0.001). In addition, women with recurrent noninflammatory discharges more frequently used daily pads (75% (27/36)) and regularly practiced douching (67% (24/36)), whereas in single episodes, these rates were 22% (7/32) and 25% (8/32), respectively (p<0.001).
For the group of women with recurrent inflammatory discharges, a high frequency of associated allergies (food or drug allergy, bronchial asthma, pollinosis, and atopic dermatitis) and autoimmune diseases was characteristic. Allergic conditions were present in 80% (30/38) of patients in subgroup IIa compared with 30% (8/27) in subgroup Ia (episodic inflammatory discharges). Autoimmune diseases were noted in every fourth patient in subgroup IIa (26% (10/38)) and in only 4% (1/27) in subgroup Ia. No such association was found in the subgroup of patients with recurrent noninflammatory discharges. These data suggest a possible role for immune factors, including predisposition to allergic and autoimmune reactions, in the development of recurrent inflammatory processes.
Local immune status
Analysis of immune response gene expression in vaginal smears revealed significant differences between episodic and recurrent pathological discharges. Inflammatory processes were characterized by increased expression of TLR4 and IL-10 compared to those in the control group (p<0.05). The level of TLR4 mRNA did not differ between episodic (Ia) and recurrent (IIa) cases (p>0.05), but that of IL-10 was higher in recurrences (p<0.05). In subgroup IIa, a reduction in pro-inflammatory IL-18 was observed, 1.6 times lower than that in controls and 1.3 times lower than that in episodic cases (p<0.05). Expression of the Th2 cell marker GATA3 and macrophage marker CD68 was also decreased in inflammatory discharges, indicating the suppression of local defense mechanisms (p<0.05) (Fig. 1).
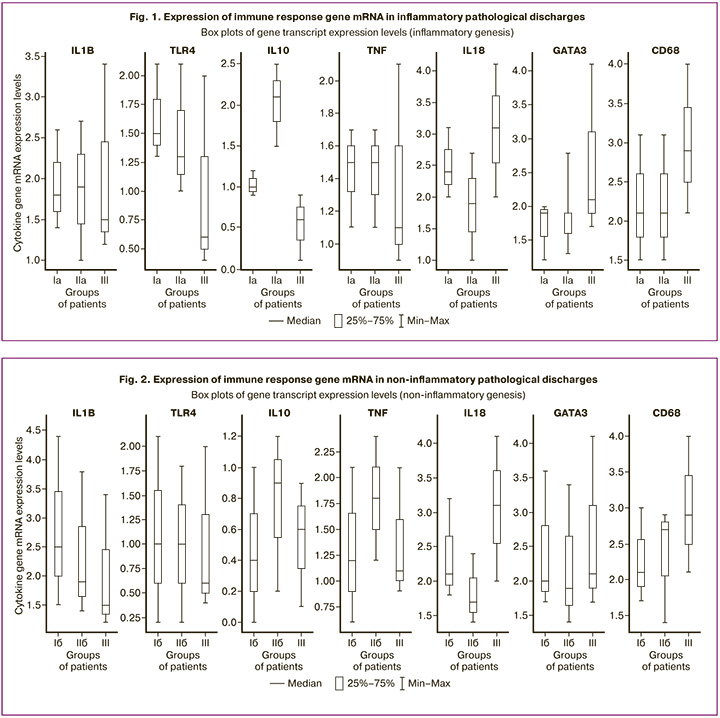
The inflammation index (II) in women with recurrent inflammatory discharges (IIa) was significantly higher than that in the control group [83.9 (80.2–91.1) vs. 1.0 (0.0–31.8), p<0.001] and episodic cases [68.5 (60.6–79.3), p<0.001]. This reflects an enhanced local inflammatory response and accumulation of innate immune cells during repeated episodes.
In the group with non-inflammatory discharges, a different pattern was observed: during recurrences (IIb), the level of anti-inflammatory IL-10 was twice as high as in episodic cases [0.9 (0.2–1.2) vs. 0.4 (0.0–1.0), p=0.027], and higher than in the comparison group [0.6 (0.1–0.9), p=0.037]. Recurrences also showed increased expression of TNF-α [1.8 (1.2–2.4) vs. 1.2 (0.6–2.1) in episodic cases, p=0.009; and 1.1 (0.9–2.1) in the comparison group, p=0.005]. No significant changes in IL-1β and TLR4 expression were observed in non-inflammatory processes (Fig. 2).
The II in noninflammatory discharges was significantly higher during recurrent episodes [65.1 (55.6–99.0) vs. 29.6 (20.6–36.4) in episodic cases, p<0.01].
Thus, recurrent pathological discharges are characterized by paradoxical activation of both anti- and pro-inflammatory cytokines against a background of IL-18 deficiency, which may indicate disruption of local antimicrobial mechanisms (IL-18 participates in NK cell activation and Th1 response). Hyperproduction of IL-10 during recurrence may be a compensatory reaction to inflammation or a manifestation of immune tolerance to chronic microbial persistence.
Genetic markers of predisposition to recurrent pathological discharges
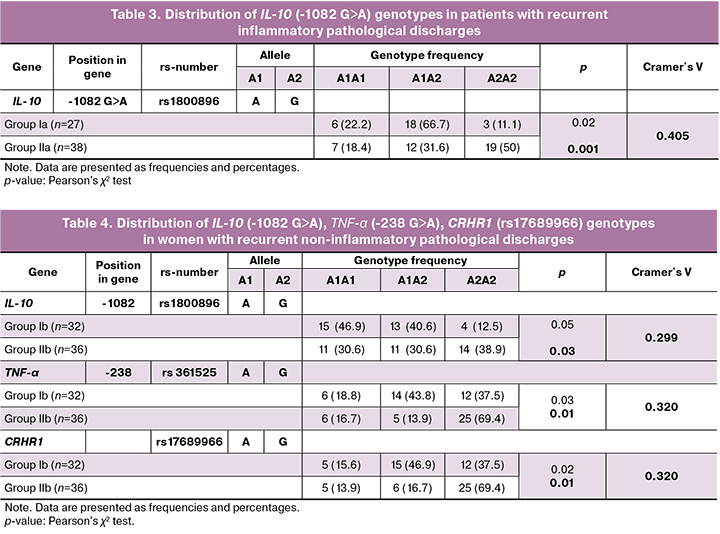
An important part of the study was the analysis of the genetic predisposition to recurrence. Significant associations were found between polymorphisms in IL-10, TNF-α, and CRHR1 and the course of the disease (Tables 3 and 4). The GG genotype for IL-10 (-1082 G>A) polymorphism was significantly more common in recurrences, both of inflammatory origin [50% (19/38) in subgroup IIa vs. 11% (3/27) in episodic cases Ia, OR=2.0 (95% CI 1.3; 2.8), p=0.001], and non-inflammatory origin [39% (14/36) in subgroup IIb vs. 12.5% (4/32) in subgroup Ib, OR=1.8 (95% CI 1.2; 2.6), p=0.03]. This genotype can be considered as a marker of predisposition to the recurrence of pathological discharges.
A polymorphism in the TNF-α gene (-238 G>A) also showed a significant association. The GG genotype was predominant in women with recurrent noninflammatory discharges [69.4% (25/36) vs. 37.5% (12/32) in episodic cases, OR=1.9 (95% CI 1.1; 3.2), p=0.01]. Thus, TNF-α polymorphisms may contribute to the development of recurrent non-inflammatory vaginitis.
The CRHR1 gene polymorphism (rs17689966) was also associated with recurrences, mainly of a non-inflammatory nature [69.4% (25/36) vs. 37.5% (12/32), OR=1.9 (95% CI 1.1; 3.2), p=0.01]. The GG genotype for this polymorphism is associated with an increased tendency for repeated episodes of noninflammatory discharges.
Calculating the strength of the association revealed that the IL-10 (-1082 G>A) polymorphism had a relatively strong association with recurrent inflammatory discharges (Cramer's V=0.405) and a moderate link with recurrent non-inflammatory types (Cramer's V=0.299). Polymorphisms in TNF-α and CRHR1 also demonstrated a moderate association (Cramer's V=0.320) with recurrent non-inflammatory pathological discharges (Tables 3 and 4).
Thus, the genetic background of patients significantly influences their susceptibility to specific immune response types and development of recurrent disease. The identified immunological and genetic markers (increased IL-10 and TNF-α, IL-18 deficiency, and GG genotypes for IL-10, TNF-α, and CRHR1) can be used for personalized prognosis of recurrences and for developing individualized preventive and therapeutic measures.
Discussion
Our study revealed that recurrent pathological vaginal discharge is caused by a combination of local immune disorders and genetic factors. In patients experiencing recurrences, we identified an imbalance of cytokines: elevated levels of IL-10 and TNF-α, along with reduced levels of IL-18. This imbalance reflects a disruption in the local immune regulation. Although bacterial vaginosis (BV) was previously considered a non-inflammatory condition, modern data indicate the presence of hidden inflammation in this disease [18, 19]. Our results confirmed these findings; even clinically non-inflammatory episodes were accompanied by an increase in the pro-inflammatory cytokine TNF-α. The simultaneous increase in IL-10 levels suggests a dual immune response, with the body attempting to suppress inflammation while counteracting microbial persistence.
An increase in IL-10 levels in patients with recurrence may indicate chronic subclinical inflammation and the development of local tolerance to pathogens. Pathogens such as Trichomonas can induce IL-10 and TGF-β expression to suppress innate immunity [18]. Similar mechanisms have been described in recurrent vulvovaginal candidiasis, in which an excess of anti-inflammatory cytokines and a deficiency in the Th1 response contribute to the persistence of infection [20]. Thus, hyperproduction of IL-10 serves as a common mechanism that creates a local immunodeficiency background and predisposes individuals to chronic infections.
Simultaneously, we observed a decrease in IL-18 levels during inflammatory recurrences, which plays a crucial role in activating cellular immunity and producing IFN-γ. Low IL-18 levels during repeated episodes of inflammation may indicate depletion in innate immunity mechanisms. This depletion can result from the prolonged stimulation of Toll-like receptors, followed by a decrease in cytokine production. A reduction in IL-18 levels undermines the protection of mucous membranes and contributes to the chronicity of infections.
The increase in TNF-α during non-inflammatory recurrences confirms that, even in the absence of pronounced inflammation, inflammatory cascades are activated. Moderately elevated TNF-α levels can sustain chronic subclinical inflammation, potentially increasing susceptibility to other infections, such as human immunodeficiency virus, as demonstrated in the meta-analysis by Atashili J. et al. [21]. Controlling excessive TNF-α production may be a valuable adjunct strategy for treating these patients.
The genetic associations we identified were aligned with the concept of hereditary predisposition to vaginal infections. The association of the IL-10 (-1082 G>A) polymorphism with recurrence can be explained by functional differences among the alleles, which is linked to increased IL-10 production, which contributes to immune tolerance to pathogens [22]. Similar patterns have been previously identified for polymorphisms in IL-1β and IL1RN genes in the context of BV [23]. Our data underscore the significance of IL-10 polymorphism in recurrent vaginal discharge and are corroborated by recent work by Murphy K. et al., who established a connection between immunoregulatory gene polymorphisms and the presence of BV [15].
According to our findings, the TNF-α (-238 G>A) polymorphism is associated with recurrent non-inflammatory discharge, with the GG genotype predominating among patients with this form of disease. This genotype is likely linked to moderately increased TNF-α production, which contributes to chronic low-intensity inflammation and maintenance of microbial dysbiosis. Although the functional significance of this specific variant has not been extensively studied, the general role of TNF-α polymorphisms in inflammatory processes has been well established.
Of particular interest is the association between the CRHR1 (rs17689966) polymorphism and non-inflammatory recurrences. This gene encodes a receptor that is involved in the body’s response to stress. Our findings coincide with those of Ryckman et al. who demonstrated the impact of CRHR1 polymorphisms on the risk of developing BV under stress and smoking [24]. Literature indicates that the rs17689966 polymorphism does not alter basal cortisol levels but affects immunity in response to external stressors [25]. Thus, CRHR1 acts as a modifier for the influence of stress on the local immune response in the vagina.
Our results suggest that persistent recurrences of vaginal infections are driven not only by pathogen persistence, but also by genetically determined immune response characteristics. Individual variations in inflammation regulation influence patient susceptibility to chronic vaginal dysbiosis and recurrence.
The obtained data hold a direct practical value. The identified immunogenetic markers (IL-10, TNF-α, and CRHR1) can be used to personalize preventive and therapeutic approaches. Patients with hyperproduction of IL-10 may benefit from immunostimulatory agents that enhance Th1 response and antimicrobial protection (e.g., local immunomodulators). In cases of excessive TNF-α production, the administration of anti-inflammatory medications is warranted. For individuals with the CRHR1 polymorphism, special attention should be paid to stress management and potential medications that modulate the activity of the hypothalamic-pituitary-adrenal axis.
Conclusion
The recurrence of pathological vaginal discharge is associated with a combination of local immune imbalance and hereditary predisposition. Polymorphisms in immune genes (IL-10, TNF, and CRHR1) can serve as molecular genetic markers of the risk of recurrence. Incorporating immunogenetic analysis into clinical practice will enable a personalized approach for patient management. For instance, women at a high risk of recurrence may benefit from enhanced preventive measures, including extended courses of probiotics, local estrogens, and immunotherapy agents. This approach will increase the effectiveness of prevention, reduce recurrence rates, and ultimately improve reproductive health, while alleviating the burden on the healthcare system.
References
- Coudray M.S., Madhivanan P. Bacterial vaginosis – a brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 245: 143-8. https://dx.doi.org/10.1016/j.ejogrb.2019.12.035
- Donders G.G.G., Bellen G., Grinceviciene S., Ruban K., Vieira-Baptista P. Aerobic vaginitis: no longer a stranger. Res. Microbiol. 2017; 168(9-10): 845-58. https://dx.doi.org/10.1016/j.resmic.2017.04.004
- Sherrard J., Wilson J., Donders G., Mendling W., Jensen J.S. European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int. J. STD AIDS. 2018; 29(13): 1258-72. https://dx.doi.org/10.1177/0956462418785451
- Geng N., Wu W., Fan A., Han C., Wang C., Wang Y. et al. Analysis of the risk factors for aerobic vaginitis: a case-control study. Gynecol. Obstet. Invest. 2015. https://dx.doi.org/10.1159/000431286
- Muzny C.A., Sunesara I.R., Austin E.L., Mena L.A., Schwebke J.R. Bacterial vaginosis among African American women who have sex with women. Sex. Transm. Dis. 2013; 40(9): 751-5. https://dx.doi.org/10.1097/OLQ.0000000000000004
- Hunt S., Vollenhoven B. Pelvic inflammatory disease and infertility. Aust. J. Gen. Pract. 2023; 52(4): 229-33. https://dx.doi.org/10.31128/AJGP-09-22-6576
- Gillet E., Meys J.F.A., Verstraelen H., Verhelst R., Sutter P. de, Temmerman M. et al. Association between bacterial vaginosis and cervical intraepithelial neoplasia: systematic review and meta-analysis. PLOS One. 2012; 7(10): e45201. https://dx.doi.org/10.1371/journal.pone.0045201
- Ji L., Peng C., Bao X. Effect of vaginal flora on clinical outcome of frozen embryo transfer. Front. Cell. Infect. Microbiol. 2022; 12: 987292. https://dx.doi.org/10.3389/fcimb.2022.987292
- Nguyen A.T.C., Nguyen N.T.L., Hoang T.T.A., Nguyen T.T., Tran T.T.Q., Tran D.N.T. et al. Aerobic vaginitis in the third trimester and its impact on pregnancy outcomes. BMC Pregnancy Childbirth. 2022; 22(1): 432. https://dx.doi.org/10.1186/s12884-022-04761-5
- Dingens A.S., Fairfortune T.S., Reed S., Mitchell C. Bacterial vaginosis and adverse outcomes among full-term infants: a cohort study. BMC Pregnancy Childbirth. 2016; 16(1): 278. https://dx.doi.org/10.1186/s12884-016-1073-y
- Workowski K.A., Bolan G.A.; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015; 64(RR-03): 1-137.
- Donders G.G., Vereecken A., Bosmans E., Dekeersmaecker A., Salembier G., Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG. 2002; 109(1): 34-43. https://dx.doi.org/10.1111/j.1471-0528.2002.00432.x
- Taylor B.D., Darville T., Ferrell R.E., Ness R.B., Kelsey S.F., Haggerty C.L. Cross-sectional analysis of Toll-like receptor variants and bacterial vaginosis in African-American women with pelvic inflammatory disease. Sex. Transm. Infect. 2014; 90(6): 436-41. https://dx.doi.org/10.1136/sextrans-2014-051524
- Mackelprang R.D., Scoville C.W., Cohen C.R., Ondondo R.O., Bigham A.W., Celum C., et al. Toll-like receptor gene variants and bacterial vaginosis among HIV-1 infected and uninfected African women. Genes Immun. 2015; 16(5): 362. https://dx.doi.org/10.1038/gene.2015.13
- Murphy K., Shi Q., Hoover D.R., Adimora A.A., Alcaide M.L., Brockmann S. et al. Genetic predictors for bacterial vaginosis in women living with and at risk for HIV in the United States. Am. J. Reprod. Immunol. 2024; 91(1): e13845. https://dx.doi.org/10.1111/aji.13845
- Kalia N., Singh J., Rauniyar A.K., Kaur M. A meta-analysis of mannose-binding lectin gene polymorphisms with the risk of recurrent vulvovaginal infections. Sci. Rep. 2020; 10(1): 6079. https://dx.doi.org/10.1038/s41598-020-63261-8
- De Seta F., Maso G., Piccoli M., Bianchini E., Crovella S., De Santo D. et al. The role of mannose-binding lectin gene polymorphisms in women with recurrent bacterial vaginosis. Am. J. Obstet. Gynecol. 2007; 197(6): 613.e1-e3. https://dx.doi.org/10.1016/j.ajog.2007.04.009
- Kalia N., Singh J., Kaur M. Immunopathology of recurrent vulvovaginal infections: new aspects and research directions. Front. Immunol. 2019; 10: 2034. https://dx.doi.org/10.3389/fimmu.2019.02034
- Usyk M., Schlecht N.F., Pickering S., Williams L., Sollecito C.C., Gradissimo A. et al. molBV reveals immune landscape of bacterial vaginosis and predicts human papillomavirus infection natural history. Nat. Commun. 2022; 13(1): 233. https://dx.doi.org/10.1038/s41467-021-27628-3
- Ge G., Yang Z., Li D. Distinct host immune responses in recurrent vulvovaginal candidiasis and vulvovaginal candidiasis. Front. Immunol. 2022; 13: 959740. https://dx.doi.org/10.3389/fimmu.2022.959740
- Atashili J., Poole C., Ndumbe P.M., Adimora A.A., Smith J.S. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008; 22(12): 1493-501. https://dx.doi.org/10.1097/QAD.0b013e3283021a37
- Larsson L., Rymo L., Berglundh T. Sp1 binds to the G allele of the -1087 polymorphism in the IL-10 promoter and promotes IL-10 mRNA transcription and protein production. Genes Immun. 2010; 11(2): 181-7. https://dx.doi.org/10.1038/gene.2009.103
- Cauci S., Santolo M. di, Casabellata G., Ryckman K., Williams S.M., Guaschino S. Association of interleukin-1beta and interleukin-1 receptor antagonist polymorphisms with bacterial vaginosis in non-pregnant Italian women. Mol. Hum. Reprod. 2007; 13(4): 243-50. https://dx.doi.org/10.1093/molehr/gam002
- Ryckman K.K., Simhan H.N., Krohn M.A., Williams S.M. Predicting risk of bacterial vaginosis: the role of race, smoking and corticotropin-releasing hormone-related genes. Mol Hum Reprod. 2009; 15(2): 131-7. https://dx.doi.org/10.1093/molehr/gan081
- White S., Acierno R., Ruggiero K.J., Koenen K.C., Kilpatrick D.G., Galea S. et al. Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J. Anxiety Disord. 2013; 27(7): 678-83. https://dx.doi.org/10.1016/j.janxdis.2013.08.003
Received 27.05.2025
Accepted 18.06.2025
About the Authors
Ada T. Uruymagova, PhD student at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, V.I. Kulakov NMRC for OGP, Ministry of Health of Russia, 4 Ac. Oparina str., Moscow, 117997, Russia, +7(925)704-33-97, ada.uruimagova@yandex.ru, https://orcid.org/0000-0001-5650-3377Elena A. Mezhevitinova, Dr. Med. Sci., Leading Researcher at the Scientific and Outpatient Department, V.I. Kulakov NMRC for OGP, Ministry of Health of Russia,
4 Ac. Oparina str., Moscow, 117997, Russia, mejevitinova@mail.ru, https://orcid.org/0000-0003-2977-9065
Andrey E. Donnikov, PhD, Head of the Laboratory of Molecular Genetic Methods, V.I. Kulakov NMRC for OGP, Ministry of Health of Russia,
4 Ac. Oparina str., Moscow, 117997, Russia, https://orcid.org/0000-0003-3504-2406



