Balance of pro-inflammatory and anti-inflammatory cytokines of fetal membranes of patients with prelabor rupture of membranes at term
Kaganova M.A., Spiridonova N.V.
Objective: To investigate the mRNA expression levels of pro-inflammatory (IL-1β, IL-6, IL-8, IL-17A, IL-18, IL-23, TNF-α, and IFN-γ) and anti-inflammatory (IL-4, IL-10, TGFβ) cytokines in the membranes of patients with prelabor rupture of membranes at term (PROM).
Materials and methods: During a clinical study at the Samara State Medical University, 40 pregnant women with full-term pregnancies were studied. The inclusion criteria were singleton pregnancy, gestational age 37.0-41.0 weeks without non-obstetric comorbidities, exacerbation of chronic and acute inflammatory diseases, and obstetric complications (placental insufficiency, preeclampsia). All patients delivered via cesarean section. Indications for planned surgery were abnormal fetal position and presentation, and postoperative uterine scar (control group, n=16). An additional indication for emergency cesarean section was premature rupture of the membranes (study group, n=24). The mRNA expression levels of IL-1β, IL-6, IL-8, IL-17A, IL-18, IL-23, TNFα, IFN-γ, IL-4, IL-10, and TGF-β were determined using RT-PCR (RT-PCR DNA technology).
Results: Expression of the mRNA genes IL-1β, IL-8, IL-18, and TNFα was observed in almost all subjects in both groups. The expression of IL-17 mRNA was not determined in any patient; IL-23 – in 7/24 (29.2%) of the study group and 3/16 (18.8%) of the control group; IL-4 expression – in 5/24 (20.8%) and 4/16 (25.0%), respectively, at an extremely low level. Expression of IL-6 and IFN-γ mRNA genes was observed slightly more often in 16/24 (66.7%) and 17/24 (70.8%) patients in the study group and in 6/16 (37.5%) and 7/16 (43.7%) patients in the control group. Significant differences between the groups were observed only for the expression of IL-8 gene mRNA – 105.44 (23.7; 648.93) RU and 33.49 (20.0; 116.6) RU and TGFβ – 216.63 (129.5; 329.9) RU and 112.5 (36.7; 182.4) RU in the study group and control group, respectively.
Conclusion: In patients with PROM, the main changes in the fetal membranes are a 3.1-fold increase in the expression of the IL-8 gene mRNA, which ensures the migration of immunocompetent cells (p=0.022), and a 1.9-fold increase in the expression of the TGFβ gene mRNA, which is responsible for epithelial-mesenchymal transition and thinning of the fetal membranes (p=0.024),
Authors' contributions: Spiridonova N.V., Kaganova M.A. – conception and design of the study; Kaganova M.A. – material collection and processing, statistical analysis, drafting of the manuscript; Spiridonova N.V. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Samara State Medical University, Ministry of Health of Russia (Ref. No: 207 dated May 20, 2020).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Kaganova M.A., Spiridonova N.V. Balance of pro-inflammatory and anti-inflammatory cytokines of fetal membranes of patients with prelabor rupture of membranes at term.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (3): 57-62 (in Russian)
https://dx.doi.org/10.18565/aig.2023.293
Keywords
The pathogenesis of prelabor rupture of membranes (PROM) remains incompletely understood, particularly with regard to its occurrence at term and preterm. It is unclear whether the mechanisms of PROM are identical across both term and preterm pregnancies or if they involve distinct processes. A comprehensive understanding of the pathogenesis of PROM in term pregnancies is crucial for developing effective strategies to manage this complication, including the prevention of preterm birth. Immunological imbalance plays a significant role in membrane rupture, contributing to both infectious and inflammatory processes as well as aseptic inflammation during normal childbirth [1].
In PROM at term, several key factors have been identified, including elevated levels of pro-inflammatory cytokines in both the blood serum and amniotic fluid [2], increased expression of innate immune receptors [3, 4], upregulation of matrix metalloproteinases accompanied by a decrease in their inhibitors [5, 6], and increased levels of vascular growth factors [7]. Cytokine production is typically transient and regulated by various stimuli at the transcriptional and translational levels, and its effects are typically localized to specific areas [8]. Consequently, assessing cytokine levels via enzyme-linked immunosorbent assay at loci distant from fetal membranes may provide less informative results compared to local determination. Modern molecular biological techniques, such as reverse transcription-polymerase chain reaction (RT-PCR) of mRNA genes directly from the studied lesion, offer opportunities to investigate the changes in immunological parameters across various pathological and physiological processes.
This study aimed to examine the mRNA expression levels of both pro-inflammatory (interleukin (IL)-1β, IL-6, IL-8, IL-17A, IL-18, IL-23, tumor necrosis factor (TNF)α, interferon (IFN)-γ) and anti-inflammatory (IL-4, IL-10, transforming growth factor (TGF)β) cytokines in the fetal membranes of patients experiencing prelabor rupture of membranes at term.
Materials and methods
This clinical study included 40 pregnant women with full-term pregnancies who were treated at the clinical base of SamSMU, Ministry of Health of Russia (N.I. Pirogov State Clinical Hospital No. 1). These 40 pregnant women were divided into a study group (n=24) and a control group (n=16).
The criteria for inclusion in the study group were gestational age 37.0–41.0 weeks, PROM, and indications for cesarean section (malposition or presentation of the fetus and/or uterine scar after cesarean section).
Criteria for inclusion in the control group were pregnancy 37.0–41.0 weeks without rupture of membranes and planned cesarean section (improper position or presentation of the fetus and/or uterine scar after cesarean section) were included in the control group.
The exclusion criteria were pregnant women in high-risk groups (according to the procedure for providing medical care in the field of “obstetrics and gynecology” (Order of the Ministry of Health of Russia dated November 1, 2012, No. 572n) with somatic or obstetric complications including diabetes mellitus, gestational diabetes, high blood pressure, intrauterine growth restriction, vaginal bleeding, placenta previa, suspected fetal macrosomia, intrahepatic cholestasis, multiple pregnancies, meconium staining of amniotic fluid, acute infection and exacerbation of chronic infection, and the presence of colpitis.
Using quantitative RT-PCR, we determined the mRNA expression levels of pro-inflammatory genes (IL-1β, IL-4, IL-6, IL-8, TNFα, IL-17A, IL-18, IL-23, and IFN-γ ) and anti-inflammatory (IL-4, IL-10, TGFβ) cytokines in the fetal membranes of all those examined at the laboratory of molecular genetic methods of DNA-Technology LLC, according to the manufacturer’s instructions (DNA-Technology, Russia). Samples of fetal membranes were obtained intraoperatively under sterile conditions within the surgical field using a conchotome with a working surface diameter of 9.4 mm midway between the placenta and internal os. The obtained samples were placed in Eppendorf tubes containing a transport medium (Proba-NK, manufactured by NPO DNA-Technology LLC). Normalization of the level of gene expression was performed using the method of comparison of indicator cycles (ΔCq method) for a reference gene B2M in relative units (RU), reflecting the abundance of the transcript relative to the normalization factor. The median (Me) was chosen as a measure of the central tendency of the quantitative characteristics, and the upper (Q1) and lower (Q3) quartiles were chosen as the interval estimates.
Written informed consent was obtained from all the participants prior to the start of the study. The study was reviewed and approved by the Research Ethics Committee of the Samara State Medical University, Ministry of Health of Russia (Ref. No: 207 dated May 20, 2020). The gestational age was calculated based on the first ultrasound scan or the first day of the last menstrual period.
Statistical analysis
Statistical analysis was performed using Statistica 10.0 and SPSS 13. Quantitative variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. The nonparametric Mann–Whitney U test was used to compare quantitative variables that did not meet the assumption of normality. The Pearson χ² test was used to compare categorical variables, and in the case of two-way contingency tables, the Yates correction was used. Differences were considered statistically significant at a significance level of p<0.05.
Results
The clinical and demographic characteristics of the study participants are presented in Table 1.
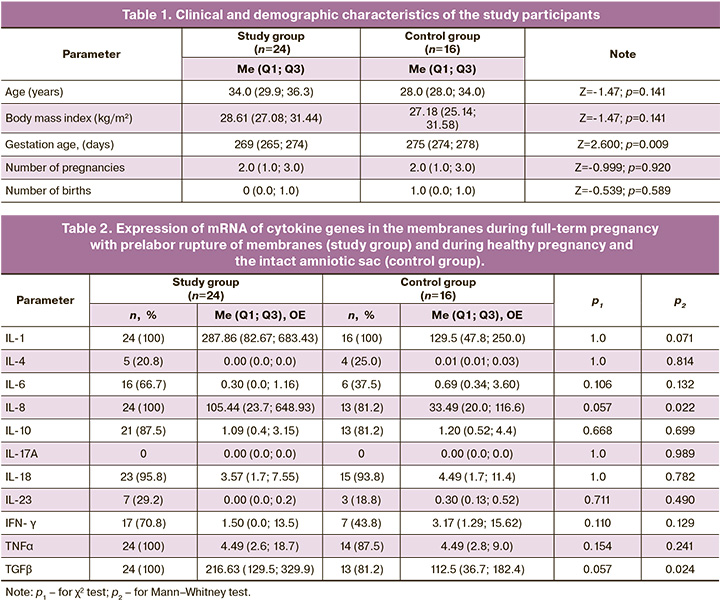
The groups were comparable in terms of age and constitutional characteristics: 8/24 (33.3%) pregnant women with a body mass index > 30 kg/m2 in the study group and 6/16 (37.5%) in the control group (p=0.946). The gestational age at delivery was significantly shorter in the study group than in the control group, which is understandable because patients in the control group were admitted for scheduled delivery after 39 weeks; in the study group, the time of rupture of membranes required earlier delivery dates. There were no significant differences in parity between the groups. The frequency of first birth in the study group was 16/24 (66.7%) in the control group and 7/16 (43.8%) in the control group (p=0.267).
The mRNA expression of cytokine genes in the fetal membranes is presented in Table 2.
The table shows that there were no significant differences in the frequency of the mRNA expression of cytokine genes for the studied parameters. Expression of the mRNA genes for most pro-inflammatory cytokines (IL-1β, IL-8, IL-18, TNFα) was observed in almost all patients in both groups, with the exception of IL-17, which was not detected in any patient. IL-23 was detected only in 7/24 (29.2%) patients in the study group and in 3/16 (18.8%) patients in the control group at an extremely low quantitative ratio. Expression of IL-6 and IFN-γ mRNA was observed more often in 16/24 (66.7%) and 17/24 (70.8%) patients in the study group and 6/16 (37.5%) and 7/16 (43.8%) patients in the control group, respectively. Of the anti-inflammatory cytokines studied, the most frequently expressed mRNA genes were IL-10 – 21/24 (87.0%) and 13/16 (81.2%) in the study and control groups, respectively, and TGFβ – 24/24 (100.0%) and 13/16 (81.2%), respectively. Expression of IL-4 mRNA is not typical for fetal membranes and was detected only in 5/24 (20.8%) and 4/16 (25.0%) cases at an extremely low level.
Significant differences between the groups were obtained only for the expression of the mRNA genes of the universal chemoattractant IL-8 – 105.44 (23.7; 648.93) RU and 33.49 (20.0; 116.6) RU and the anti-inflammatory cytokine TGFβ – 216 .63 (129.5;329.9) RU and 112.5 (36.7;182.4) RU in the study and control groups, respectively.
No statistically significant differences were observed among the other parameters studied.
Discussion
Membranes play a crucial role in facilitating communication between myometrial cells and the fetus. They have a significantly larger receptor surface than the placenta and are involved in maintaining amniotic fluid homeostasis. Hadley et al. (2018) found that exosomes from amnion epithelial cells cause an increased inflammatory response in the uterine decidua, while placental cells do not have the same effect. Exosomes from embryonic cells can signal the onset of labor by inducing aseptic inflammation in the decidua [8]. TGFβ, an anti-inflammatory cytokine responsible for controlling cell proliferation and differentiation, plays a significant role in aseptic inflammation. It triggers epithelial-mesenchymal transition, which normally occurs during labor in full-term pregnancies and prematurely in various pathological conditions [9, 10]. As pregnancy progresses and in response to proinflammatory cytokines or oxidative stress, the amnion undergoes the epithelial-mesenchymal transition more frequently, leading to the weakening of fetal membranes due to remodeling [11, 12]. Additionally, amniotic mesenchymal cells are more sensitive to proinflammatory stimuli, which further promotes membrane rupture and the production of uterotonic factors, including prostaglandins. Therefore, precise and timely changes in the phenotype of amnion cells are crucial for maintaining pregnancy and initiating labor. It is believed that epigenetic events, such as oxidative stress, proinflammatory stimuli, and placental and fetal membrane ischemia, impact gene expression patterns through histone modification and reduction in miRNA expression, ultimately contributing to the proinflammatory phenotype of amnion and decidua cells during pregnancy [10]. In our study, we observed a significant increase in the expression of TGF-β mRNA in the membranes during premature rupture, which was almost twice as high as that in the control group. This finding may be an important element in understanding the pathogenesis of PROM at term.
Several studies have shown an increase in the mRNA expression of pro-inflammatory cytokines during labor and the rupture of fetal membranes [1, 14]. In our study, we found that full-term pregnancy, regardless of fetal bladder integrity, is characterized by a consistent level of mRNA expression of the pro-inflammatory cytokines IL-1β, IL-18, TNFα, and anti-inflammatory IL-10. However, we did not find any significant differences between the groups. We also observed that IL-6 mRNA expression was not present in any of the patients in our study (16/24 (66.7%) in the study group and 6/16 (37.5%) in the control group; p=0.106). Most sources suggest that IL-6 is a marker of the septic process and does not play a key role in labor and rupture of membranes [1, 15, 16], which our results indirectly confirm. Although the patients were not in labor, the expression of IL-6 mRNA was not significantly different between the groups, which may indicate a subclinical inflammatory process in the fetal membranes. However, no clinical signs were observed in either the mother or newborn.
According to the literature, IL-8 in fetal membranes plays a significant role in the pathogenesis of premature rupture of membranes (PROM) during term pregnancy [17], and our study supports this finding. In patients with PROM, we observed an increase in the expression of mRNA for the IL-8 gene, which is responsible for the migration of neutrophils and macrophages. IL-8 also increases the expression of matrix metalloproteinases in fetal membranes. In our study, we found an increase in the mRNA expression of the IL-8 gene, which likely activated the expression of matrix metalloproteinase 8, leading to the breakdown of the extracellular matrix and subsequent membrane separation and rupture.
In full-term uncomplicated pregnancy, it is not typical for fetal membranes to express IL-17 mRNA, and it is not involved in the pathogenesis of PROM. IL-17 mRNA expression has been observed in studies of preeclampsia and fetal growth restriction [14]. It has also been shown that IL-17, along with other pro-inflammatory cytokines such as TNFα and IFN-γ, has embryotoxic and antitrophoblastic effects [15]. IL-17 is activated in the presence of lipopolysaccharides (components of the bacterial cell wall) or during autoimmune processes [16].
Conclusion
Regardless of the integrity of the fetal bladder, we observed a high frequency of mRNA expression of the genes IL-1, IL-8, IL-18, and TNFα (100%) in the fetal membranes. We did not detect any IL-17 mRNA expression and observed very low levels of IL-4 mRNA expression. During PROM, the expression of IL-8 mRNA, which acts as a chemoattractant for neutrophils and macrophages and activates metalloproteinases, was 3.1 times higher in fetal membranes (p=0.022). Additionally, there was a 1.9-fold increase in the activation of TGF-β mRNA expression, which promotes epithelial-mesenchymal transition in fetal membranes (p=0.024). As a result, fetal membranes become more sensitive to the effects of proinflammatory cytokines and prostaglandins, leading to thinning and weakening of the membranes.
References
- Menon R., Moore J.J. Fetal membranes, not a mere appendage of the placenta, but a critical part of the fetal-maternal interface controlling parturition. Obstet. Gynecol. Clin. North Am. 2020; 47(1): 147-62. https://dx.doi.org/10.1016/j.ogc.2019.10.004.
- Болотских В.М., Айламазян Э.К., ред. Преждевременное излитие околоплодных вод: теория и практика. Санкт-Петербург: Эко-Вектор; 2018. 191с. [Bolotskikh V.M., Ajlamazjan Je.K., eds. Premature discharge of amniotic fluid: theory and practice. St Petersburg: Eco-Vector; 2018. 191p. (in Russian)].
- Доброхотова Ю.Э., Трофимов Д.Ю., Щеголев А.И., Бурменская О.В., Веселовская Ю.С., Митрофанова Ю.В., Оленев А.С., Пастарнак А.Ю., Гогичаев Т.К. Особенности экспрессии мРНК гена прогестерониндуцированного блокирующего фактора в плаценте при преждевременных родах. Акушерство и гинекология. 2017; 7: 62-7. [Dobrokhotova Yu.E., Trofimov D.Yu., Shchegolev A.I., Burmenskaya O.V., Veselovskaya Yu.S., Mitrofanova Yu.V., Olenev A.S., Pastarnak A.Yu., Gogichaev T.K. Placental PIBF1 gene mRNA expression during preterm labor. Obstetrics and Gynecology. 2017; (7): 62-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.7.62-7.
- Доброхотова Ю.Э., Бондаренко К.Р., Гущин А.Е., Румянцева Т.А., Долгова Т.В., Кузнецов П.А., Джохадзе Л.С. Результаты исследования цервико-вагинальной микробиоты методом ПЦР в реальном времени у беременных с угрожающими преждевременными родами. Акушерство и гинекология. 2018; 11: 50-9. [Dobrokhotova Yu.E., Bondarenko K.R., Gushchin A.E., Rumyantseva T.A., Dolgova T.V., Kuznetsov P.A., Dzhokhadze L.S. The results of the examination of cervical-vaginal microbiota in pregnant women with threatened preterm birth using a real-time polymerase chain reaction. Obstetrics and Gynecology. 2018; (11): 50-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.11.50-59.
- Oh K.J., Romero R., Park J.Y., Lee J., Conde-Agudelo A., Hong J.S., Yoon B.H. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am. J. Obstet. Gynecol. 2019; 221(2): 140.e1-140.e18. https://dx.doi.org/10.1016/j.ajog.2019.03.017.
- Tchirikov M., Schlabritz-Loutsevitch N., Maher J., Buchmann J., Naberezhnev Y., Winarno A.S., Seliger G. Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. J. Perinat. Med. 2018; 46(5): 465-88. https://dx.doi.org/10.1515/jpm-2017-0027.
- Низяева Н.В., Карапетян А.О., Гапаева М.Д., Приходько А.М., Синицына В.А., Баев О.Р. Повышение ангиогенеза как фактора, способствующего разрыву плодных оболочек при физиологическом течении беременности и при преждевременных родах. Гены и клетки. 2019; 14(3): 164-5. [Nizyaeva N.V., Karapetyan A.O., Gapaeva M.D., Prikhod'ko A.M., Sinitsyna V.A., Baev O.R. Povyshenie angiogeneza kak faktora, sposobstvuyushchego razryvu plodnykh obolochek pri fiziologicheskom techenii beremennosti i pri prezhdevremennykh rodakh. Genes & Cells. 2019; 14(3): 164-5. (in Russian)]. https://dx.doi.org/10.23868/gc123216.
- Hadley E.E., Sheller-Miller S., Saade G., Salomon C., Mesiano S., Taylor R.N., Taylor B.D., Menon R. Amnion epithelial cell-derived exosomes induce inflammatory changes in uterine cells. Am. J. Obstet. Gynecol. 2018; 219(5): 478.e1-478.e21. https://dx.doi.org/10.1016/j.ajog.2018.08.021.
- Николаева А.М., Бабушкина Н.П., Рябов В.В. Некоторые про- и противовоспалительные цитокины, полиморфные варианты их генов и постинфарктное ремоделирование сердца. Российский кардиологический журнал. 2020; 25(10): 4007. [Nikolaeva A.M., Babushkina N.P., Ryabov V.V. Some pro- and anti-inflammatory cytokines, their genetic polymorphism and postinfarct cardiac remodeling. Russian Journal of Cardiology. 2020; 25(10): 4007. (in Russian)]. https://dx.doi.org/10.15829/1560-4071-2020-4007.
- Zakar T., Paul J.W. Fetal membrane epigenetics. Front. Physiol. 2020; 11: 588539. https://dx.doi.org/10.3389/fphys.2020.588539.
- Janzen C., Sen S., Lei M.Y., Gagliardi de Assumpcao M., Challis J., Chaudhuri G. The role of epithelial to mesenchymal transition in human amniotic membrane rupture. J. Clin. Endocrinol. Metab. 2017; 102(4): 1261-9. https://dx.doi.org/10.1210/jc.2016-3150.
- Richardson L.S., Taylor R.N., Menon R. Reversible EMT and MET mediate amnion remodeling during pregnancy and labor. Sci. Signal. 2020; 13(618): eaay1486. https://dx.doi.org/10.1126/scisignal.aay1486.
- Sharma A., Kumar D., Moore R.M., Deshmukh A., Mercer B.M., Mansour J.M., Moore J.J. Granulocyte macrophage colony stimulating factor (GM-CSF), the critical intermediate of inflammation-induced fetal membrane weakening, primarily exerts its weakening effect on the choriodecidua rather than the amnion. Placenta. 2020; 89: 1-7. https://dx.doi.org/10.1016/j.placenta.2019.10.003.
- Murray E.J., Gumusoglu S.B., Santillan D.A., Santillan M.K. Manipulating CD4+ T cell pathways to prevent preeclampsia. Front. Bioeng. Biotechnol. 2022; 9: 811417. https://dx.doi.org/10.3389/fbioe.2021.811417.
- Piccinni M.P., Raghupathy R., Saito S., Szekeres-Bartho J. Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front. Immunol. 2021; 12: 717808. https://dx.doi.org/10.3389/fimmu.2021.717808.
- Motedayyen H., Fathi F., Fasihi-Ramandi M., Ali Taheri R. The effect of lipopolysaccharide on anti-inflammatory and pro-inflammatory cytokines production of human amniotic epithelial cells. Reprod. Biol. 2018; 18(4): 404-9. https://dx.doi.org/10.1016/j.repbio.2018.09.005.
- Zhu J., Ma C., Zhu L., Li J., Peng F., Huang L., Luan X. A role for the NLRC4 inflammasome in premature rupture of membrane. PLoS One. 2020; 15(8): e0237847. https://dx.doi.org/10.1371/journal.pone.0237847.
Received 15.12.2023
Accepted 06.03.2024
About the Authors
Maria A. Kaganova, PhD, Associate Professor at the Department of Obstetrics and Gynecology, Institute of Vocational Education, Samara State Medical University,Ministry of Health of Russia, 443100, Russia, Samara, Polevaya str., 80, +7(846)207-19-68, mkaganova@yandex.ru, https://orcid.org/0000-0001-5879-418x
Natalia V. Spiridonova, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Institute of Vocational Education, Samara State Medical University, Ministry of Health of Russia, 443100, Russia, Samara, Polevaya str., 80, +7(846)207-19-68, nvspiridonova@mail.ru, eLibrary SPIN: 3079-3658;
https://orcid.org/0000-0003-3390-8034; Web of Science S-6918-2016; Scopus 56089251400.
Corresponding author: Maria A. Kaganova mkaganova@yandex.ru



