Personalised medicine in action: lipidomic markers for predicting premature rupture of membranes
Baisova A.R., Amiraslanov E.Yu., Frankevich V.E., Chagovets V.V., Tokareva A.O., Tyutyunnik V.L.
Objective: Assessment of lipid profile in pregnant women to identify biomarkers that have prognostic potential in premature rupture of membranes (PROM).
Materials and methods: The prospective case-control study included 110 pregnant women. The group with preterm labor and PROM was comprised of 30 women, and the control group included 80 women. Plasma lipid extracts were analyzed using high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS). Lipids were identified using LipidMatch R-script based on exact mass using the LIPID MAPS Structure Database (LMSD) and tandem mass spectrometry (MS/MS).
Results: A total of 141 maternal plasma lipids including cholesteryl esters, ceramides, monogalactosyldiacylglycerols, lysophosphatidylcholines, ether-linked phosphatidylcholines, phosphatidylinositols, phosphatidylglycerols, oxidized lipids, and triacylglycerols were detected in positive and negative ion modes. Comparative analysis of plasma in patients in the control group and in the group with premature rupture of membranes before 37 weeks of gestation showed statistically significant changes (p<0.05) in 34 lipids. Based on analysis of different expression of lipids that demonstrated statistically significant correlation with clinical data according to the Spearman’s correlation, the model was developed to predict PROM before 37 weeks of gestation with sensitivity of 97.3% and specificity of 97.4%. The area under the curve (AUC) was 0.994 and the cut-off value was 0.5.
Conclusion: Metabolomic plasma profile changes in lipids, such as ceramides, monogalactosyldiacylglycerols, lysophosphatidylcholines, ether-linked phosphatidylcholines, phosphatidylinositols, phosphatidylglycerols, oxidized lipids and triacylglycerols correlate with the risk of developing PROM. Lysophosphatidylcholines (LPC, LPE) can be considered to be early markers of proinflammatory activation. The prognostic model based on analysis of plasma lipid profile opens perspectives in the improvement of preventive measures against PROM, and also contributes to the improvement of perinatal outcomes both in mothers and newborns.
Authors' contributions: Baisova A.R. – development of the study design, obtaining data for analysis, collecting biological materials for the study, drafting the manuscript, Amiraslanov E.Yu. – development of the study design, review of publications on the topic of the article, analysis of the obtained data, writing the text of the manuscript; Chagovets V.V. – conducting metabolomic analysis using mass spectrometry, statistical analysis; Tokareva A.O. – statistical data analysis; Amiraslanov E.Yu., Frankevich V.E., Chagovets V.V., Tyutyunnik V.L. – manuscript editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was supported by Russian Science Foundation grant No. 24-64-00006 (https://rscf.ru/project/24-64-00006/).
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Protocol No. 11 of November 11, 2021).
Patient Consent for Publication: The patients have signed informed consent for participation in the study and publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Baisova A.R., Amiraslanov E.Yu., Frankevich V.E., Chagovets V.V., Tokareva A.O., Tyutyunnik V.L. Personalised medicine in action: lipidomic markers for predicting premature rupture of membranes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (1): 55-65 (in Russian)
https://dx.doi.org/10.18565/aig.2024.334
Keywords
Premature rupture of membranes (PROM) is an important obstetric problem that complicates 3–4% of all pregnancies and is the cause of 40–50% of preterm labor (PL) [1]. PROM occurs when the fetal membranes break before labor begins, and can cause serious complications both for the mother and the newborn [2, 3]. PROM contributes to the increase in maternal morbidity and perinatal mortality, and according to various authors reaches 4.87–5.08% [4].
PROM is a complex condition with the multifaceted etiology, including inflammatory and non-inflammatory causes. Inflammation plays a critical role in PROM, regardless of gestational age, including maternal intravascular inflammation and localized inflammation of the amniotic membrane [5, 6]. Bacterial products, proinflammatory cytokines and matrix metalloproteinases contribute to changes in the morphology and integrity of the amniotic membrane and the development of intra-amniotic inflammation [7]. Based on current studies, there is a direct link between certain lipid biomarkers and inflammatory cytokine levels. For example, elevated triglyceride levels or the ratio between certain fatty acids, such as omega-6 (linoleic acid, arachidonic acid) and omega-3 (alpha-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid acid), may indicate active inflammatory processes [8, 9]. Arachidonic acid, that can be derived from linoleic acid, is a precursor for several classes of inflammatory mediators known as eicosanoids (prostaglandins, thromboxanes, and leukotrienes), which play a key role in inflammatory processes by regulating inflammation, pain, and immune response. [8–10].
Detection of these indicators and their metabolites in plasma and amniotic fluid will help to identify women at high risk for developing PROM [11]. Lipids can also act as signaling molecules, activating various cellular pathways and initiating a cascade of inflammatory responses that lead to PROM [9, 12–15].
Intraamniotic infection is present in 25–40% of PL, activating inflammatory pathways. Inflammation can be caused both by pathogen-associated and damage-associated molecular patterns, that leads to increased oxidative stress and tissue damage. While PROM is associated with long-term oxidative stress and significant structural tissue damage, PL is characterized by acute exposure and pronounced antioxidant status [16]. The earlier the gestational age at the time of PROM, the higher the intensity of the intra-amniotic inflammatory response is in women with intra-amniotic infection. At the same time, the intensity of systemic inflammatory response in mother was not associated with gestational age, when PROM occurred. [17].
Fetal response and intra-amniotic inflammatory response are more pronounced in PL than in PROM, despite the fact that intra-amniotic infection is less detected, that may be due to diagnostic difficulties [18]. This unexpected finding suggests fundamental differences in the pathogenesis of these conditions. Treatment of PROM, especially in the second trimester of pregnancy, remains challenging due to a high risk of infectious complications, preterm labor and premature birth. Management of such patients includes careful monitoring of pregnant woman and fetal condition, antibiotics prescription and treatment with corticosteroids to improve neonatal outcomes [7].
Non-inflammatory causes include mechanical factors and oxidative stress-induced cellular senescence [5]. The sterile inflammatory response is induced in fetal membranes through progressive cellular senescence and changes that can destabilize membrane function and propagate inflammatory signals into maternal tissues via extracellular vesicles [5, 19]. Understanding diverse pathways is essential for developing effective strategies to prevent and treat PROM. Patient management strategy depends on gestational age and requires making a balanced decision in favor of continuing pregnancy or accelerated delivery [20]. Despite the fact that the issue of PROM has been sufficiently studied, there are still unresolved issues that can create difficulties in diagnosis and treatment [3]. Thus, there is an urgent need for further research aimed at improving screening tools and better understanding of the pathophysiological mechanisms underlying PROM [1].
The objective of the study was assessment of the lipid profile in pregnant women to identify biomarkers that have prognostic potential in PROM.
Materials and methods
The prospective case-control study was conducted. Group I (the main group) included 30 patients who had PROM at 320–366 weeks of pregnancy. Group II included 80 patients with intact fetal membranes in full-term pregnancy. Inclusion criteria were: voluntary informed consent, singleton pregnancy, patients’ age 18–45 years, gestational age ≥32 weeks. Non-inclusion criteria were severe somatic diseases, lipid metabolism disorders, diabetes mellitus, arterial hypertension, multiple pregnancy, fetal chromosomal abnormalities, congenital malformations, maternal cancer, hypertensive disorders in pregnancy, gestational diabetes mellitus. The diagnosis of PROM was made based on the criteria specified by clinical guidelines [21].
Treatment of patients and follow-up wa provided in accordance with the Order of the Ministry of Health of the Russian Federation of October 20, 2020 N 1130n "On approval of the Procedure for the provision of medical care in obstetrics and gynecology” and domestic clinical guidance.
Plasma samples were collected from the patients in the main group upon admission to the Academician V.I. Kulakov National Medical Research Center for Obstetrics and Gynecology of the Ministry of Health of Russia. Plasma samples from the patients in the control group were collected at ambulatory care facilities at 32–34 weeks of pregnancy.
Plasma samples processing
Modified Folch's method extraction was used for lipid extraction.
Microcentrifuge tube 2 ml was filled with 40 μl of plasma, 480 μl of chloroform/methanol (2:1) and 150 μl of purified water. The mixture was vortexed for 10 min, then centrifuged at -5°C at 15.000 rpm for 10 min. The lower organic layer was collected in another tube. For re-extraction, 250 µl of chloroform/methanol solution was added to the sample, mixed and centrifuged again as described above. From the upper aqueous methanol layer 50 µl was collected for further analysis. The organic layer was dried under nitrogen flow at room temperature. The remaining dry residue was then dissolved in 200 µl of isopropanol/acetonitrile (1:1) for semi-quantitative analysis. The volume of 1 µl was injected into the chromatography system. The upper aqueous-methanol layer was similarly treated and dissolved in 300 µl of methanol/water (1:1) for analysis.
Lipid extracts were analyzed using Dionex UltiMate 3000 liquid chromatograph with the Maxis Impact qTOF mass spectrometer. Separation was done using Zorbax C18 column (150×2.1 mm), gradient: 15%–45% B for 2 min, then 45%– 99% B for 15 min. Lipid identification was done using tandem mass spectrometry and LipidMatch software according to the LipidMaps nomenclature.
Statistical analysis
Statistical data processing was performed using IBM SPSS Statistics for Windows, Version 21.0.
The parameters of normal distribution are represented as the mean (M) and standard deviation (SD). Abnormal distribution is represented as median (Me), the upper and lower quartiles (Q1; Q3). The normality of distribution was evaluated using the Shapiro-Wilk test. Comparison of quantitative data between the groups, when distribution differed from normal, was performed using the Mann-Whitney U-test with Bonferroni correction.
The odds ratio (OR) was used as a quantitative measure of effect in the comparative analysis of relative indicators. The values of OR were calculated to measure the association for contingency tables. To project the obtained values of OR for the sample population, the upper and lower bounds of the 95% confidence interval were determined.
Pearson's chi-squared test (χ2) was used to compare the percentages in analyzing multi-dimensional contingency tables. The differences were considered to be statistically significant at p<0.05
Spearman's rank correlation test was used to detect dependency between different factors.
Logistic regression models were developed to assess the ability to predict PROM based on maternal plasma lipid concentrations. A 95% CI was defined for a model. The quality of the developed models was determined by constructing the ROC curve, assessment of the area under the ROC curve, and calculation of sensitivity and specificity.
Results
The mean age of pregnant women enrolled in the study varied from 20 to 45 years and was 32/0 (5.2) years. In the main group, premature rupture of membranes occurred in women aged 35–39 years (p=0.014). Assessment of anthropometric parameters showed no statistically significant differences between the groups. Body mass index (BMI) in patients in the pain group was 26.5 (4.7) kg/m2 and 26.0 (3.2) kg/m2 in the control group (p=0.396) (Table 1).
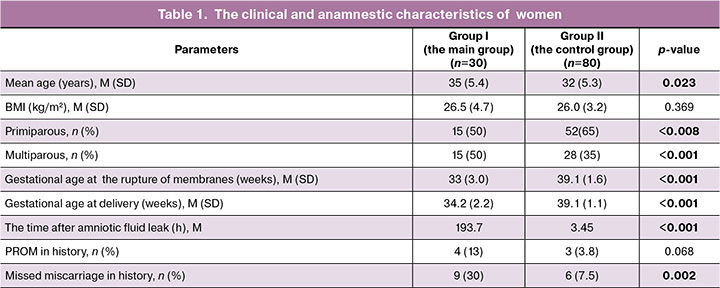
Analysis of the prevalence of infectious, inflammatory and extragenital diseases showed no statistically significant differences between the groups.
Half of patients in the group of PL and PROM were primiparous women – 15/30 (50%), versus 52/80 (65%) in the control group (p=0.008). There was no statistically significant difference in the groups with regard to multiparous women (p<0.153) (Table 1).
Assessment of specific features of reproductive history in the main group showed statistically higher frequency of missed miscarriages – 9/30 (30%, p=0.002). There was no statistically significant difference in the frequency of PROM in history among women in this group (p=0.068) (Table 1).
In the structure of extragenital diseases in the main group, the frequency of chronic pyelonephritis and chronic gastritis was higher. There were no statistically significant differences with regard to other disorders. In the structure of gynecological diseases in the main group, vulvovaginal candidiasis, ovarian cysts, external genital endometriosis, uterine fibroids, polycystic ovary syndrome were identified as statistically significant. The data are represented in Table 2.
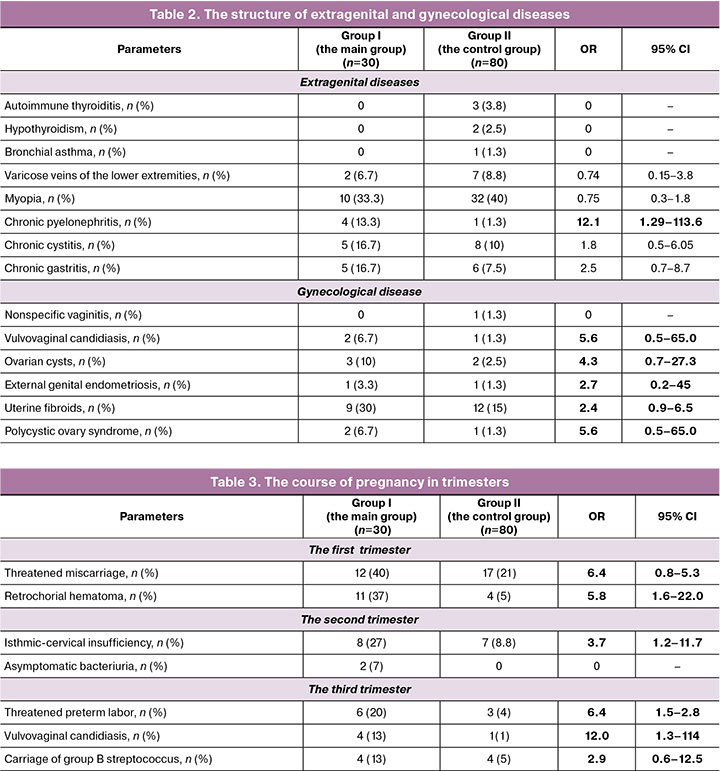
Analysis of the course of pregnancy in trimesters in the main group showed that there was a high risk of threatened miscarriage and retrochorial hematoma in the first trimester. The frequency of isthmic-cervical insufficiency was significantly higher in the second trimester. Increased risk of preterm delivery, the presence of vulvovaginal candidiasis and carriage of group B streptococcus was in the third trimester (Table 3).
Predominantly in all groups, the women had vaginal delivery. However, in the main group, the rate of operative birth interventions was 43% (13/30). It is important to note that there was a high rate of emergency surgeries – 90% (27/30), that exceeded the rate of elective surgeries.
The results of lipidomic analysis
A total of 141 maternal plasma lipids were detected in positive and negative ion modes, and were classified as cholesterol esters, ceramides, monogalactosyldiacylglycerols, lysophosphatidylcholines, ether-linked phosphatidylcholines, phosphatidylinositols, phosphatidylglycerols, oxidized lipids, and triacylglycerols. Comparative analysis of plasma lipid concentrations in patients in the control group and in the group with premature rupture of membranes before 37 weeks of gestation showed statistically significant changes in 34 lipids. The results are represented in Table 4. The only exception was reduced PC lipid (16:0 16:1) concentration. Increased concentrations of other lipids were in the group of women with PROM.
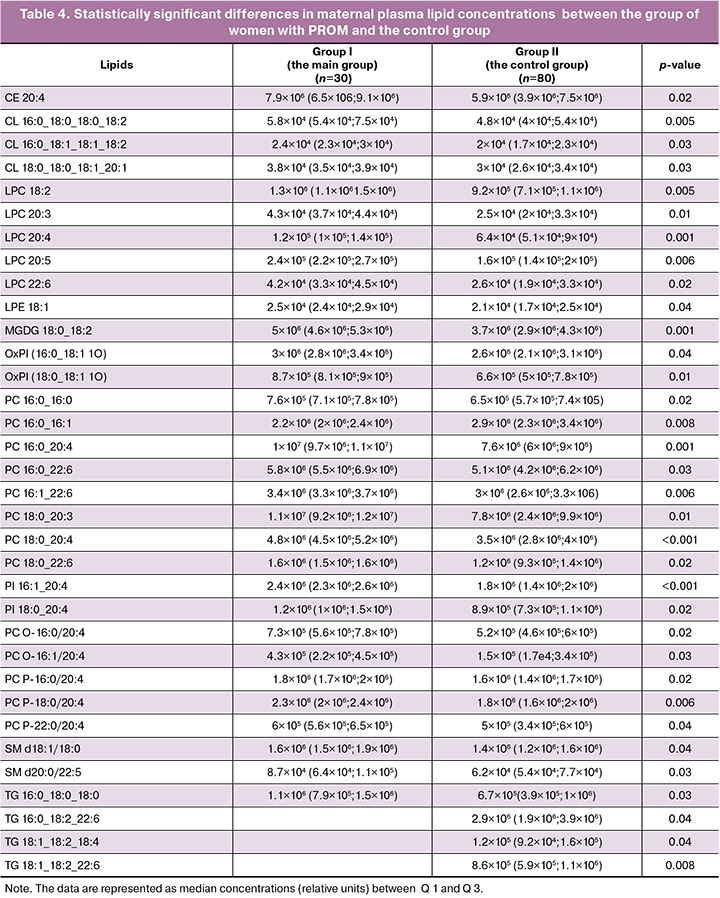
Spearman's rank correlation test was used to assess the relationship between lipid concentrations and the clinical parameters: age, BMI, high risk of threatened miscarriage in the first and second trimesters, isthmic-cervical insufficiency, threatened preterm labor, vulvovaginal candidiasis in the third trimester, and carriage of group B streptococcus. The selected lipids belonged to the classes of phosphatidylcholines, lysophosphatidylcholines, and phosphatidylinositols, and were chosen for the development of prognostic models for PROM (Table 5).
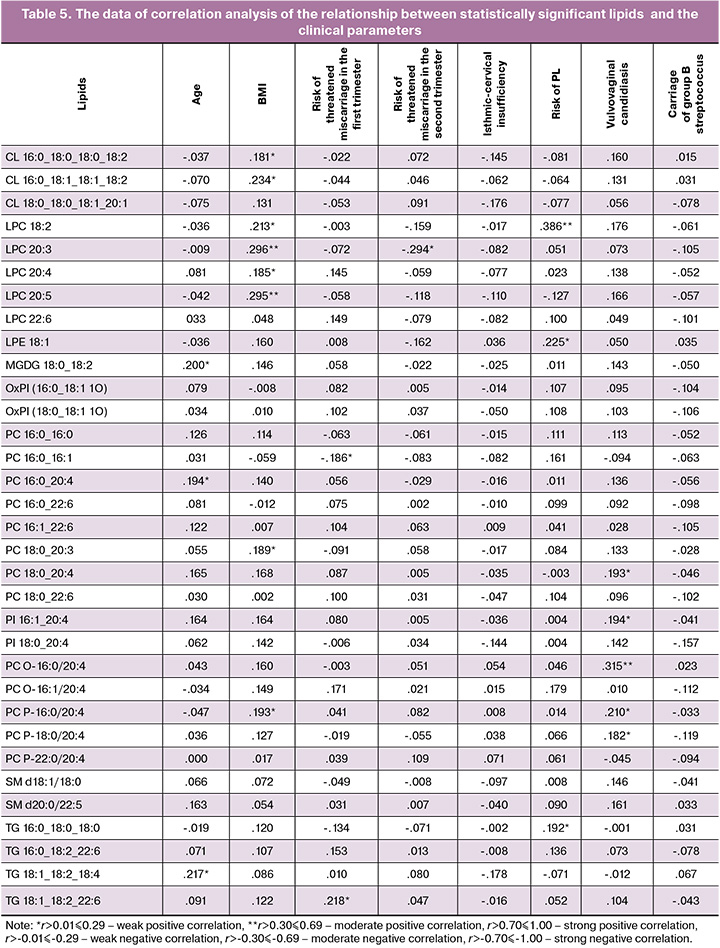
Based on the obtained results of the lipid profile and the data in medical history, a model for prediction of PROM before 37 weeks of pregnancy was developed using logistic regression:
P=1/(1+e-z), where е is the base of the natural logarithm equal to 2.71828182845904;
z = -18,80 + 11,59×Х1 - 1,038×Х2 - 26,5×X3×10-5 + 50,8×X4×10-5 - 27,3×X5×10-5 + 11,0×Х6×10-5,
where X1 is threatened miscarriage in the first trimester (I – yes, 0 – no); X2 is threatened miscarriage in the second trimester; X3 is maternal plasma concentration of LPC20:3; X4 is maternal plasma concentration of LPC20:4; X5 is maternal plasma concentration of LPE18:1, X6 is maternal plasma concentration of LPC18:2.
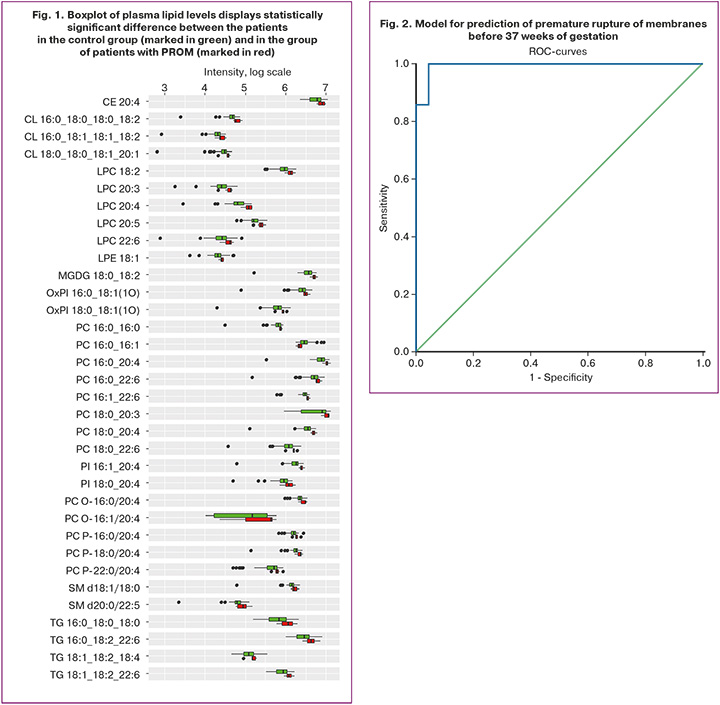
The constructed regression model for prediction of PROM was statistically significant (p<0.001). ROC curve was derived by assessment of dependence of the probability of predicting PROM on P-value in logistic regression using ROC analysis. ROC curve is displayed in Figure 2.
The area under the curve was 0.994 with 95% CI: 0.98–1.0 (Table 6). The obtained model was statistically significant (p<0.001). Cut-off of 0.5 for P-value corresponded to the maximum value of the Youden’s index. PROM was diagnosed when P-value was higher or equal to 0.5. In this study, prediction model 1 was developed with sensitivity and specificity of 97.3% and 97.4%, respectively.

Discussion
Omics technologies include genomics, transcriptomics, proteomics and metabolomics. Each of them provides valuable translational studies of biological processes. Metabolomics is a system approach to pathogenic processes and investigates the number and diversity of endogenous metabolites caused by pathophysiological changes, external stimuli and changes in the body, that can more accurately reflect their condition [22]. The metabolomic approach makes it possible to assess metabolic pathways and correlate biochemical changes associated with pathophysiology of disease, providing top-down gene expression data and higher sensitivity for disease phenotype [23–26]. Identification of the end product of the body's metabolism – the metabolome can help to assess the underlying conditions and factors associated with occurrence, severity or prognosis of diseases with complex determinants [26].
PROM is one of the most common factors leading to preterm birth [27]. Preterm birth that happens before 37 weeks of gestation affects 5–9% of all pregnancies in the developed countries, and is the main cause of perinatal mortality. Spontaneous preterm birth following preterm labor accounts for 31–50% of all preterm births, and 30% of births are complicated by PROM [28], but the underlying pathophysiology remains poorly understood [29].
Our study was aimed to identify lipidomic pathways involved in the pathophysiology of PL for finding prognostic markers that can help prevent this complication [30, 31].
The results of this study demonstrated significant changes in maternal plasma lipid profile in patients with PROM. In particular, this included the following classes of lipids: ceramides, monogalactosyldiacylglycerols, lysophosphatidylcholines, ether-linked phosphatidylcholines, phosphatidylinositols, phosphatidylglycerols, oxidized lipids, and triacylglycerols.
These classes of lipids are involved in different metabolic pathways and pathogenetic processes. Most of them are part of the structure of bilayer lipid membranes, providing structural integrity and functionality of cellular and subcellular membranes. Imbalanced ratio and concentrations of such lipids can affect the strength of membrane structures, permeability and ability to cell signaling.
Based on the results of research conducted over the past two decades, it was discovered that lysophospholipids are not only structural components of cell membranes, but also biologically active molecules that influence a wide range of processes such as carcinogenesis, neurogenesis, immunity, vascular development or regulation of metabolic diseases [32].
The results our study showed that lipids with anti-inflammatory activity have higher plasma concentrations in patients with PROM.
Under stressful conditions, such as the presence of infections or the development of inflammatory processes, the body is able to change compensatory metabolic pathways. Increased concentrations of some lipids can be explained by their compensatory increase for the synthesis of important molecules, such as antioxidants or structural components of cells.
In the context of determining the potential role of lipids in prediction of PROM, we selected the class of lysophosphatidylcholines, which belong to the subclass of phospholipids that play an important role in biological membranes and are involved in various cellular processes [32, 33]. Changes in concentration or composition of LPC, LPE may be associated with various pathological conditions, including inflammatory processes and cellular aging [32, 33].
The obtained data demonstrate a high potential of the lipidomic analysis in search of prognostic markers for diagnosing PROM, that is confirmed by the data reported by foreign authors.
The recent study by Hong S.H. et al. (2023), that was based on lipidomic analysis, identified potential biomarkers for predicting PL. Their study identified different classes of lipids associated with PL, including phospholipids and sphingolipids. Reduction of sphingomyelin level in cervicovaginal fluid was associated with PL [34]. Plasma extracellular vesicles showed to have promising results. In addition, a lipid panel for five lipids had high prognostic accuracy for PL [35]. Maternal plasma analysis identified plasminylphosphatidylethanolamines and free fatty acids as risk factors for spontaneous preterm birth [31]. Another study found reduced plasma levels of glycerophospholipids and sphingolipids in women with spontaneous preterm birth. [36]. The obtained results indicate that lipidomic profiling of different biological fluids can provide valuable insights into the mechanisms of PROM and potential prognostic biomarkers. However, further validation in larger cohorts is needed to determine clinical utility of these lipid signatures for predicting PROM.
Conclusion
Based on the obtained data it can be concluded that changes in plasma lipid profile, especially in classes of lipids, such as ceramides, monogalactosyldiacylglycerols, lysophosphatidylcholines, ether-linked phosphatidylcholines, phosphatidylinositols, phosphatidylglycerols, oxidized lipids and triacylglycerols are associated with the risk of developing PROM.
Lysophosphatidylcholines (LPC, LPE) can be considered to be early markers of proinflammatory activation. The prognostic model based on analysis of plasma lipid profile opens perspectives in the improvement of preventive measures against PROM, and also contributes to the improvement of perinatal outcomes both in mothers and newborns.
References
- Menon R., Richardson L.S. Preterm prelabor rupture of the membranes: A disease of the fetal membranes. Semin. Perinatol. 2017; 41(7): 409-19. https://dx.doi.org/10.1053/j.semperi.2017.07.012.
- ACOG Practice Bulletin No. 188: Prelabor rupture of membranes. Obstet. Gynecol. 2018; 131(1): e1-e14. https://dx.doi.org/10.1097/AOG.0000000000002455.
- Shazly S.A., Ahmed I.A., Radwan A.A., Abd-Elkariem A.Y., El-Dien N.B., Ragab E.Y. et al.; Middle-East Obstetrics and Gynecology Graduate Education (MOGGE) Foundation Practice Group. Middle-East OBGYN Graduate Education (MOGGE) Foundation Practice Guidelines: Prelabor rupture of membranes; Practice guideline No. 01-O-19. J. Glob. Health. 2020; 10(1): 010325. https://dx.doi.org/10.7189/jogh.10.010325.
- Patel A., Sirohiwal D., Malik R., Singh P., Patel S., Gandhi K. Maternal and perinatal outcome in preterm premature rupture of membrane. Int. J. Health Sci. Res. 2016; 6(2): 89-94.
- Menon R., Behnia F., Polettini J., Richardson L.S. Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin. Immunopathol. 2020; 42(4): 431-50. https://dx.doi.org/10.1007/s00281-020-00808-x.
- Баисова А.Р., Амирасланов Э.Ю., Франкевич В.Е., Чаговец В.В., Тютюнник В.Л. Современные представления об этиологии и патогенезе преждевременного разрыва плодных оболочек. Акушерство и гинекология. 2023; 10: 21-7. [Baisova A.R., Amiraslanov E.Yu., Frankevich V.E., Chagovets V.V., Tyutyunnik V.L. Modern concepts on the etiology and pathogenesis of premature rupture of membranes. Obstetrics and Gynecology. 2023; (10): 21-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.199.
- Tchirikov M., Schlabritz-Loutsevitch N., Maher J., Buchmann J., Naberezhnev ., Winarno A.S. et al. Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. J. Perinat. Med. 2018; 46(5): 465-88. )]. https://dx.doi.org/10.1515/jpm-2017-0027.
- Slotkowski R., VanOrmer M., Akbar A., Hahka T., Thompson M., Rapoza R. et al. Bioactive metabolites of OMEGA-6 and OMEGA-3 fatty acids are associated with inflammatory cytokine concentrations in maternal and infant plasma at the time of delivery. Clin. Nutr. ESPEN. 2024; 60: 223-33. https://dx.doi.org/10.1016/j.clnesp.2024.02.006.
- Mauro A.K., Rengarajan A., Albright C., Boeldt D.S. Fatty acids in normal and pathological pregnancies. Mol. Cell. Endocrinol. 2022; 539: 111466. https://dx.doi.org/10.1016/J.MCE.2021.111466.
- Eick S.M., Geiger S.D., Alshawabkeh A., Aung M., Barrett E.S., Bush N. et al. Urinary oxidative stress biomarkers are associated with preterm birth: an Environmental Influences on Child Health Outcomes program study. Am. J. Obstet. Gynecol. 2023; 228(5): 576.e1-576.e22. https://dx.doi.org/10.1016/j.ajog.2022.11.1282.
- Горина К.А., Ходжаева З.С., Чаговец В.В., Стародубцева Н.Л., Франкевич В.Е., Припутневич Т.В. Особенности профиля органических кислот амниотической и цервико-вагинальной жидкостей беременных высокого риска преждевременных родов. Акушерство и гинекология. 2022; 3: 39-48. [Gorina K.A., Khodzhaeva Z.S., Chagovets V.V., Starodubtseva N.L., Frankevich V.E., Priputnevich T.V. Characteristics of the organic acid profile of amniotic and cervicovaginal fluids in pregnant women at high risk for spontaneous preterm birth. Obstetrics and Gynecology. 2022; (3): 39-48. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.3.39-48.
- Yan H., Zhu L., Zhang Z., Li H., Li P., Wang Y. et al. HMGB1-RAGE signaling pathway in pPROM. Taiwan J. Obstet. Gynecol. 2018; 57(2): 211-6. https://dx.doi.org/10.1016/j.tjog.2018.02.008.
- Bredeson S., Papaconstantinou J., Deford J.H., Kechichian T., Syed T.A., Saade G.R. et al. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One. 2014; 9(12): e113799. https://dx.doi.org/10.1371/journal.pone.0113799.
- Bouvier D., Giguère Y., Blanchon L., Bujold E., Pereira B., Bernard N. et al. Study of sRAGE, HMGB1, AGE, and S100A8/A9 concentrations in plasma and in serum-extracted extracellular vesicles of pregnant women with preterm premature rupture of membranes. Front. Physiol. 2020; 11: 609. https://dx.doi.org/10.3389/fphys.2020.00609.
- Plazyo O., Romero R., Unkel R., Balancio A., Mial T.N., Xu Y. et al. HMGB1 induces an inflammatory response in the chorioamniotic membranes that is partially mediated by the inflammasome. Biol. Reprod. 2016; 95(6): 130. https://dx.doi.org/10.1095/biolreprod.116.144139.
- Prearo Moço N., Ribeiro de Andrade Ramos B., de Castro Silva M., Polettini J., Menon R., Guimarães da Silva M. Spontaneous prematurity, innate immune system, and oxidative stress at the maternal-fetal interface: an overview. In: Translational Studies on Inflammation. IntechOpen; 2020. https://dx.doi.org/10.5772/intechopen.88379.
- Oh K.J., Romero R., Park J.Y., Hong J.S., Yoon B.H. The earlier the gestational age, the greater the intensity of the intra-amniotic inflammatory response in women with preterm premature rupture of membranes and amniotic fluid infection by Ureaplasma species. J. Perinat. Med. 2019; 47(5): 516-27. https://dx.doi.org/10.1515/jpm-2019-0003.
- Park C.W., Yoon B.H., Park J.S., Jun J.K. A fetal and an intra-amniotic inflammatory response is more severe in preterm labor than in preterm PROM in the context of funisitis: unexpected observation in human gestations. PLoS One. 2013; 8(5): e62521. https://dx.doi.org/10.1371/journal.pone.0062521.
- Низяева Н.В., Карапетян А.О., Гапаева М.Д., Синицына В.А., Баев О.Р. Структурные особенности плодных оболочек при преждевременных родах. Акушерство и гинекология; 2019; 8: 63-9. [Nizyaeva N.V., Karapetyan A.O., Gapaeva M.D., Sinitsyna V.A., Baev O.R. Structural features of fetal membranes in preterm labor. Obstetrics and Gynecology. 2019; (8): 63-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.8.63-69.
- Prelabor Rupture of Membranes. Obstet. Gynecol. 2020; 135(3): 739-43. https://dx.doi.org/10.1097/AOG.0000000000003701.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Преждевременные роды. М.; 2024. 65 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Preterm birth. Moscow; 2024. 65 p. (in Russian)].
- Rinschen M.M., Ivanisevic J., Giera M., Siuzdak G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell. Biol. 2019; 20(6): 353-67. https://dx.doi.org/10.1038/s41580-019-0108-4.
- Romero R., Mazaki-Tovi S., Vaisbuch E., Kusanovic J.P., Chaiworapongsa T., Gomez R. et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J. Matern. Fetal Neonatal. Med. 2010; 23(12): 1344-59. https://dx.doi.org/10.3109/14767058.2010.482618.
- Dharuri H., Demirkan A., van Klinken J.B., Mook-Kanamori D.O., van Duijn C.M., 't Hoen P.A. et al. Genetics of the human metabolome, what is next? Biochim. Biophys. Acta. 2014; 1842(10): 1923-31. https://dx.doi.org/10.1016/j.bbadis.2014.05.030.
- Fanos V., Atzori L., Makarenko K., Melis G.B., Ferrazzi E. Metabolomics application in maternal-fetal medicine. Biomed. Res. Int. 2013; 2013: 720514. https://dx.doi.org/10.1155/2013/720514.
- Putri S.P., Nakayama Y., Matsuda F., Uchikata T., Kobayashi S., Matsubara A. et al. Current metabolomics: Practical applications. J. Biosci. Bioeng. 2013; 115(6): 579-89. https://dx.doi.org/10.1016/j.jbiosc.2012.12.007.
- Rayburn W.F. Premature rupture of membranes: the most common factor leading to preterm birth. Obstet. Gynecol. Clin. North Am. 2020; 47(4): xi-xii. https://dx.doi.org/10.1016/j.ogc.2020.09.003.
- Brown R.G., Al-Memar M., Marchesi J.R., Lee Y.S., Smith A., Chan D. et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl. Res. 2019; 207: 30-43. https://dx.doi.org/10.1016/j.trsl.2018.12.005.
- Castro D., Norwitz E.R. Preterm premature rupture of membranes. Obstet. Gynecol. Online first. 2021. https://dx.doi.org/10.2310/OBG.19056.
- Yang K., Han X. Lipidomics: techniques, applications, and outcomes related to biomedical sciences. Trends Biochem. Sci. 2016; 41(11): 954-69. https://dx.doi.org/10.1016/j.tibs.2016.08.010.
- Aung M.T., Ashrap P., Watkins D.J., Mukherjee B., Rosario Z., Vélez-Vega C.M. et al. Maternal lipidomic signatures in relation to spontaneous preterm birth and large-for-gestational age neonates. Sci. Rep. 2021; 11(1): 8115. https://dx.doi.org/10.1038/s41598-021-87472-9.
- Grzelczyk A., Gendaszewska-Darmach E. Novel bioactive glycerol-based lysophospholipids: New data – New insight into their function. Biochimie. 2013; 95(4): 667-79. https://dx.doi.org/10.1016/j.biochi.2012.10.009.
- Makide K., Kitamura H., Sato Y., Okutani M., Aoki J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat. 2009; 89(3-4): 135-9. https://dx.doi.org/10.1016/j.prostaglandins.2009.04.009.
- Hong S.H., Lee J.Y., Seo S., Shin B., Jeong C.H., Bae E. et al. Lipidomic analysis of cervicovaginal fluid for elucidating prognostic biomarkers and relevant phospholipid and sphingolipid pathways in preterm birth. Metabolites. 2023; 13(2): 177. https://dx.doi.org/10.3390/metabo13020177.
- Zhao Q., Ma Z., Wang X., Liang M., Wang W., Su F. et al. Lipidomic biomarkers of extracellular vesicles for the prediction of preterm birth in the early second trimester. J. Proteome Res. 2020; 19(10): 4104-13. https://dx.doi.org/10.1021/acs.jproteome.0c00525.
- Morillon A.C., Yakkundi S., Thomas G., Gethings L.A., Langridge J.I., Baker P.N. et al. Association between phospholipid metabolism in plasma and spontaneous preterm birth: a discovery lipidomic analysis in the cork pregnancy cohort. Metabolomics. 2020; 16(2): 19. https://dx.doi.org/10.1007/s11306-020-1639-6.
Received 27.12.2024
Accepted 21.01.2025
About the Authors
Almira R. Baisova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, almira.baisova@mail.ru, https://orcid.org/0009-0004-4546-2388Elrad Yu. Amiraslanov, PhD, Head of the Department of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, eldis@mail.ru, https://orcid.org/0000-0001-5601-1241
Vladimir E Frankevich, Dr. Sci. (in Physics and Mathematics), Head of Department of Systems Biology in Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Leading Researcher at the Laboratory
of Translational Medicine, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moskovsky tract, 2, v_frankevich@oparina4.ru,
https://orcid.org/0000-0002-9780-4579
Vitaliy V. Chagovets, PhD, Senior Researcher at the Proteomics of Human Reproduction Department, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, v_chagovets@oparina4.ru,
https://orcid.org/0000-0002-5120-376X
Alisa O. Tokareva, PhD, Specialist at the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, alisa.tokareva@phystech.edu, https://orcid.org/0000-0001-5918-9045
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099



