Plasma proteome cluster analysis in pregnant women with preeclampsia
Objective: To investigate the molecular biology and development of preeclampsia through proteomic profiling of the blood plasma of pregnant women using cluster analysis of proteins.Nikitina N.A., Sidorova I.S., Ziganshin R.Kh., Kir'yanova M.A., Ageev M.B.
Materials and methods: The study comprised 27 pregnant women, including 15 patients with healthy pregnancies (control group, median gestational age 39.5 (39.5; 40.0) weeks) and 12 patients with severe preeclampsia (study group, median gestational age 32.1 (29;35) weeks). The baseline evaluations included clinical, laboratory, and instrumental methods. The proteomic profile of blood plasma was determined using ultra-high-resolution liquid chromatography mass spectrometry. Protein cluster analysis was performed using the DAVID online tool.
Results: Plasma proteomic analysis identified approximately 1500 proteins in each sample. Differential differences were found for 317 proteins in pregnant women with preeclampsia, and changes in 113 of them were statistically significant (70 proteins with overexpression and 43 proteins with reduced expression). Cluster analysis of plasma proteins differentiated in preeclampsia allowed the identification of nine of the largest clusters, indicating a significant role of abnormalities in the complement and coagulation systems, inflammatory and immune responses, metabolic disorders, and changes in cellular processes (particularly endoplasmic reticulum function) in the pathogenesis of preeclampsia. Data are available via ProteomeXchange with the identifier PXD036175.
Conclusion: The proteomic profile of maternal blood in preeclampsia differs significantly from that in uncomplicated pregnancies and is characterized by the variability of changes reflecting multiple and multidirectional disturbances of biological processes and molecular functions.
Authors' contributions: Sidorova I.S., Nikitina N.A. – conception and design of the study; Ziganshin R.Kh., Kir'yanova M.A., Ageev M.B. – data collection and analysis; Ziganshin R.Kh. – blood plasma proteome determination using chromato‑mass spectrometry; Nikitina N.A., Ziganshin R.Kh. – manuscript drafting; Sidorova I.S. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the I.M. Sechenov First MSMU (Sechenov University) (Ref. No. 22‑21 of 09.12.2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Nikitina N.A., Sidorova I.S., Ziganshin R.Kh., Kir'yanova M.A., Ageev M.B. Plasma proteome cluster analysis in pregnant women with preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (5): 37-49 (in Russian)
https://dx.doi.org/10.18565/aig.2023.15
Keywords
The results of scientific research in recent decades have significantly improved our understanding of the mechanisms underlying preeclampsia pathogenesis. However, there is still no clear understanding of the etiology of this pregnancy complication or the main factors that trigger its development. So far, it has not been possible to explain such features of preeclampsia as the occurrence of clinical symptoms only in the second half of pregnancy (in the perinatal period, after 22 weeks of gestation, when the fetus can survive outside the uterus) and its development only in humans [1]. There is still no effective preventive strategy and delivery remains the only treatment.
Due to the versatility and wide variability of preeclampsia in terms of maternal genetic predisposition, association with a number of diseases (diabetes mellitus, chronic arterial hypertension, metabolic syndrome, etc.), a variety of clinical manifestations, and omics technologies, it is an ideal tool for studying its molecular mechanisms, which allow receiving and processing a colossal amount of data (“Big Data”) [2].
Omix technologies include high-throughput qualitative and quantitative analysis of genes (genomics), RNA (transcriptomics), proteins (proteomics), and metabolites (metabolomics) using methods such as liquid chromatography coupled with mass spectrometry (LC–MS), new-generation sequencing (NGS), and chip arrays [3].
In recent decades, proteomics methods have been significantly improved for the identification and quantification of proteins in various biological fluids and tissues, as well as for the analysis of their post-translational modifications. Modern LC–MS platforms enable the analysis of thousands of proteins in just 1–2 h [3]. The sources of biological material can be serum/blood plasma, urine, placenta, cerebrospinal fluid, and others [4].
Quantitative proteomic analysis of blood plasma or serum to identify biomarkers associated with preeclampsia has long been an important task in omics studies [5]. On one hand, blood is an easily accessible source of biological material; on the other hand, it is the most representative medium because it contains proteins secreted by all tissues, including the placenta and decidua. Simultaneously, the plasma/serum proteome is complex and has a large dynamic range. 99% of all plasma proteins are the 20 most common proteins (albumin, antibodies, apolipoproteins, complement proteins, and others), making it more difficult to identify and quantify less common proteins [6].
Studies have shown that the proteomic profile of maternal blood can reflect the growth and development processes of the fetus, the state of the immune system, the functioning of systems that support the gestational process, and predict complications (in particular, preeclampsia) and pregnancy outcomes [7–9].
Although the maternal blood proteome is poorly understood over the course of a healthy pregnancy, comparative proteomic analysis of plasma in normal and preeclampsia pregnancies may be useful for identifying protein factors and biological processes associated with abnormal pregnancy and fetal distress [10].
In addition, in Russia, there are practically no studies devoted to the analysis of the proteome of biological fluids and tissues in pregnant women in general, and in preeclampsia in particular. In domestic literature, there are single publications on this problem [11, 12].
This study aimed to investigate the molecular biology of preeclampsia development based on a comparative proteomic analysis of the blood plasma of healthy pregnant women and pregnant women with preeclampsia using high-performance liquid chromatography mass spectrometry and cluster analysis of proteins that are differentially expressed in women who develop preeclampsia during pregnancy.
Materials and methods
The study included 27 pregnant women, including 15 healthy pregnant women (control group, median gestational age 39.5 (39.5; 40.0) weeks) and 12 patients with severe preeclampsia (study group, median gestational age 32.1 (29;35) weeks). Identification of infections and inflammatory processes at any location, as well as fetal malformations and chromosomal and genetic abnormalities, served as criteria for excluding patients from the study. All women in the study and control groups underwent general clinical, laboratory, and instrumental investigations (ultrasound examination of the fetus and placenta, Doppler, and cardiotocography), and the proteomic profile of blood plasma was determined using high-performance liquid chromatography-mass spectrometry.
Blood samples (3–4 ml) for proteomic evaluation were collected in EDTA-containing vacuum tubes from patients on admission to the maternity hospital. The blood was then centrifuged for 10–12 minutes at 3000 rpm, and the collected plasma was placed in two Eppendorf tubes, labelled, frozen, and stored at -20°C.
Chromato-mass-spectrometric analysis
Samples were loaded onto a laboratory-made guard column 50×0.1 mm packed with Inertsil ODS3 3 μm sorbent (GL Sciences) in a solution containing 2% acetonitrile, 98% H2O, and 0.1% TFA at a flow rate of 4 μl/min and separated at room temperature on a fused silica column (300×0.5 mm) with an emitter, made on a P2000 Laser Puller (Sutter, USA) and packed in the laboratory with Reprosil PUR C18AQ 1.9 sorbent (Dr. Maisch). Reverse-phase chromatography was performed using an Ultimate 3000 Nano LC System chromatograph (Thermo Fisher Scientific) connected to a Q Exactive Plus Orbitrap mass spectrometer (Thermo Fisher Scientific) via a nanoelectrospray source (Thermo Fisher Scientific). For chromatographic separation of the peptides, a system of solvents A (99.9% water, 0.1% formic acid) and B (19.9% water, 0.1% formic acid, 80% acetonitrile) was used. Peptides were eluted from the column with a linear gradient: 3–35% B in 105 min; 35–55% B in 18 min, 55–99% B in 0.1 min, 99% B in 10 min, 99–3% B in 0.1 min at a flow rate of 500 nl/min. After each analysis, the column was equilibrated with 3% solution of B for 10 min. Mass spectrometric analysis was performed in DDA mode (TopN=10) with the following instrument settings: MS1 scan: resolution 70000, scan range 200–1600 m/z, maximum ion injection time 35 ms, AGC level 3×106; MS2 scan: resolution 17500, HCD fragmentation with energy 30%, maximum ion injection time 80 ms, and AGC level 1×105.
For the analysis of proteins present in plasma at low concentrations, they were pre-enriched using the Proteominer small capacity kit (Bio-Rad Laboratories; USA), the isolation procedure was carried out according to the manufacturer's recommendations.
Statistical analysis
Statistical analysis was performed using SPSS (version 10.0.7) and Statistica (version 10.0, StatSoft Inc., USA) for Windows. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. Quantitative variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Frequencies (n) and percentages (%) were reported for categorical variables. Categorical variables were compared using Fisher's two-tailed exact test for small samples and a Z-test with correction for endpoints (in the case of a comparison of 0% or 100%). Differences between groups were assessed using the Student’s t-test when comparing continuous variables showing normal distribution and equality of variance (Levene's test). Otherwise, the nonparametric Mann–Whitney U test was used if the normality assumption was not met.
Analysis of the obtained arrays of chromato-mass spectrometric data was carried out using computer programs MaxQuant 2.0.3.1 (MQ) [https://www.maxquant.org] and Perseus 2.0.3.1. [https://maxquant.net/perseus/]. Tandem mass spectra were correlated with the human protein sequence database using Swiss-Prot (www.uniprot.org). To identify statistically significant differences in the content of identified proteins between the groups, we used a t-test for independent samples. The critical level of significance was considered using the Benjamini–Hochberg correction for a false discovery rate (FDR)-adjusted p-value of <0.05. The protein was considered differentially expressed between the preeclamptic and control groups at p<0.05 (t-test). Hierarchical protein cluster analysis, in which the Euclidian Distance was taken as a measure for determining the distance between two points on the plane formed by the x and y coordinate axes, was carried out using the DAVID online service [https://david.ncifcrf.gov/].
The critical level of significance when testing the statistical hypotheses was considered at p<0.05.
All proteomic mass spectrometry data obtained were submitted to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset ID PXD009325 and 10.6019/PXD036175.
Results
The clinical characteristics and perinatal outcomes of the study participants are shown in Table 1. The study and control groups were comparable in age, social status, unhealthy habits, and parity.
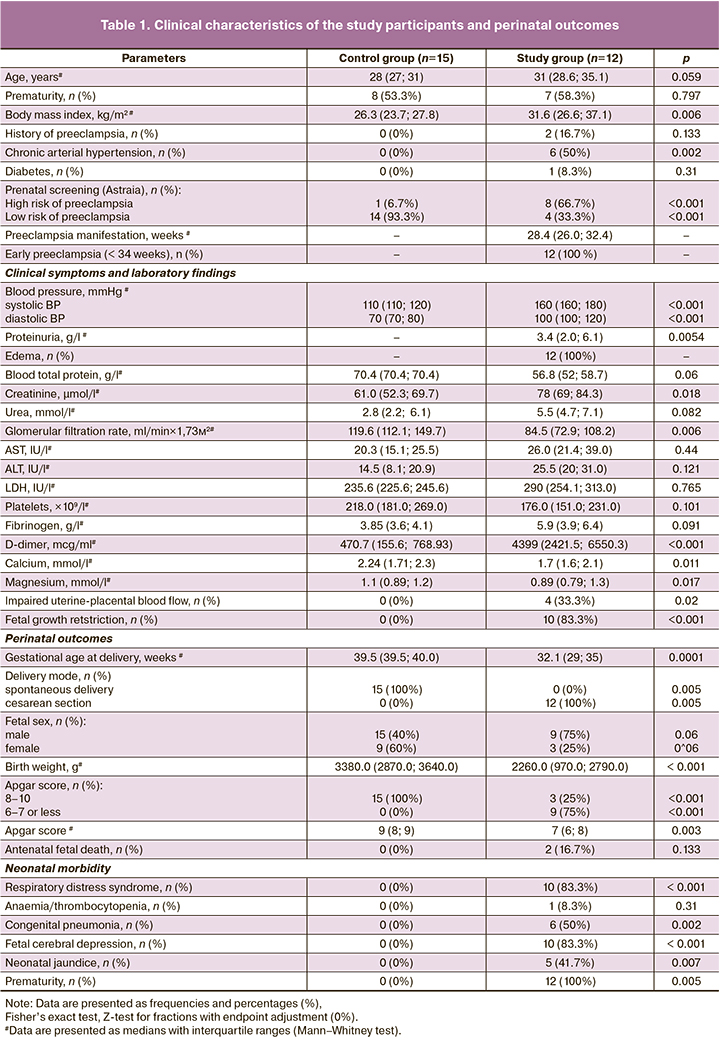
Proteomic examination of the blood plasma allowed the identification of approximately 1500 proteins in each sample. Further analysis showed that the proteomic profile of the plasma of pregnant women with preeclampsia showed marked differences from that of the control group in a total of 317 proteins. The changes in 113 of these proteins in the study group were statistically significant:70 proteins were overexpressed and 43 were underexpressed. The point distribution of the mass spectrometry dataset is shown in a scatter plot (Fig. 1).
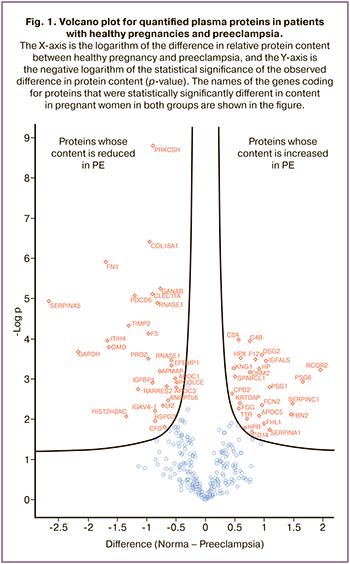
difference in relative protein content between healthy pregnancy and preeclampsia, and the Y-axis is the negative logarithm of the statistical significance of the observed difference in protein content (p-value). The names of the genes coding for proteins that were statistically significantly different in content in pregnant women in both groups are shown in the figure.
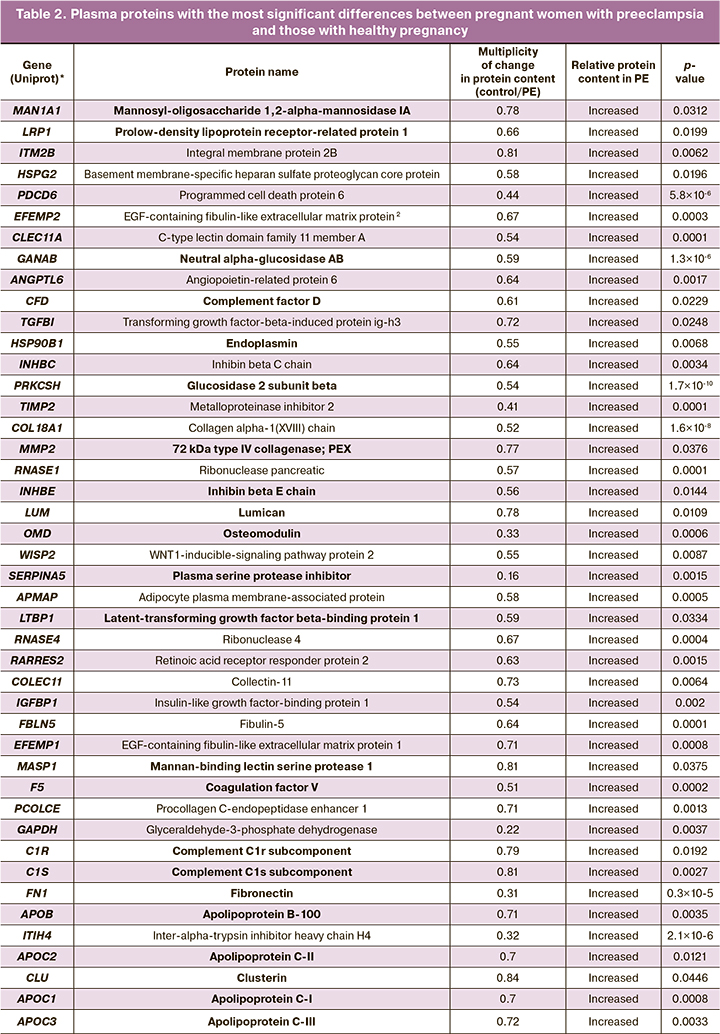
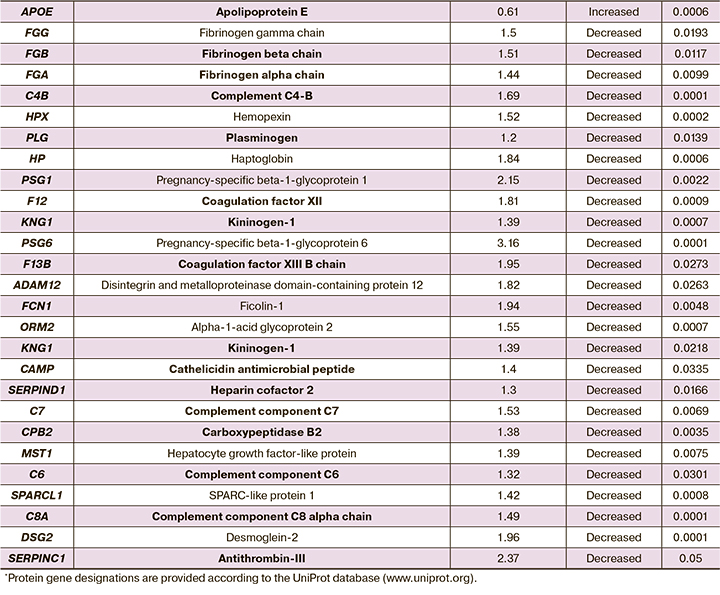
The most important differentially expressed plasma proteins in preeclampsia are listed in Table 2.
Hierarchical cluster analysis of the lists of identified proteins was performed using the online resource DAVID, which is an accessible and widely used tool for the cluster analysis of arrays of identified proteins and genes. DAVID was used to identify key biological processes, molecular functions, cellular components, and interacting proteins/genes in the list of differentially expressed proteins in the plasma of pregnant women with preeclampsia.
The plasma proteins that were significantly different between pregnant women with preeclampsia and healthy pregnant women were divided into two groups: those with increased and decreased expression. The lists of identifiers for each protein in one and the other group were then analyzed using the online resource DAVID using the Gene Ontology [http://geneontology.org/] and KEGG (Kyoto Encyclopedia Genes and Genomes) databases [https://www.genome.jp/]. Proteins differentially represented in the blood plasma of healthy pregnancies and preeclampsia were clustered according to their molecular functions and involvement in particular biological processes. The results of the cluster analysis of plasma proteins indicate a very broad and diverse spectrum of abnormalities that determine the pathophysiology of preeclampsia; more than 60 biological processes are significantly impaired, and almost 50 molecular functions are altered. The most important biological processes involved in pre-eclampsia are shown in Figure 2.
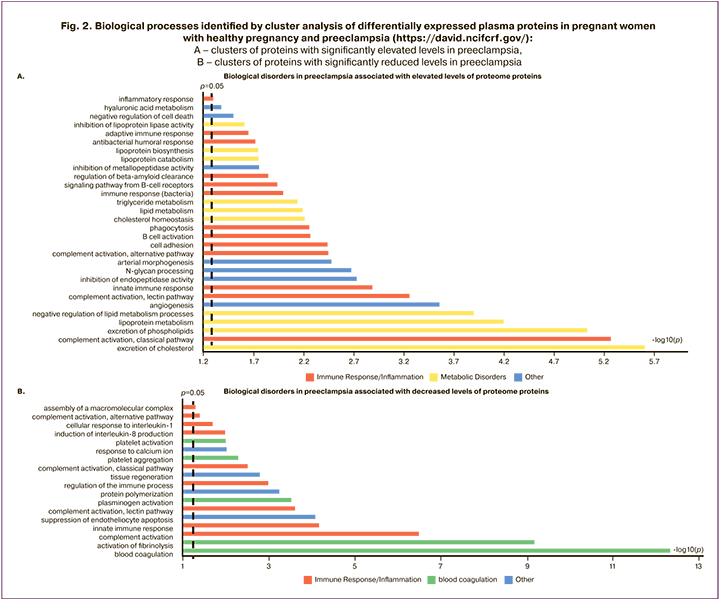
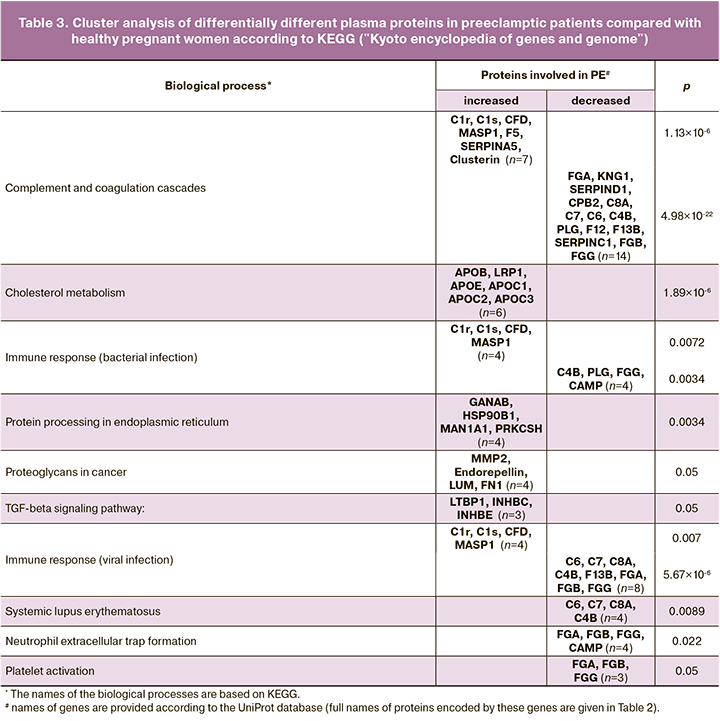
The largest and most significant protein clusters differentiated by differences in preeclampsia patients are shown in Table 3.
Discussion
This study identified features of the maternal blood proteomic profile in preeclampsia, which was significantly different from that in uncomplicated pregnancies. The results demonstrated changes in a wide range of circulating proteins and associated pathophysiological abnormalities in this pregnancy complication.
Proteome protein cluster analysis has demonstrated significant immunological abnormalities in severe preeclampsia, including the activation of the immune response (both innate and adaptive) and the development of autoimmune reactions. Preeclampsia has long been regarded as an excessive maternal inflammatory response to pregnancy, with the development of a systemic proinflammatory response; the most severe damage is identified in the maternal endothelium. However, studies have shown that there is a close relationship between inflammation and the complement system, as well as the coagulation cascade, with the coagulation system influencing inflammatory activity, and vice versa [13].
The results of plasma proteome analysis in preeclampsia patients also showed that the most pronounced changes occurred in the complement system and coagulation cascade. A total of 21 proteins involved in these biological processes were included in this cluster: seven proteins with elevated levels and 14 with reduced levels. In addition, significant activation of platelet function was noted in women with preeclampsia.
The complement system was discovered over 100 years ago by Jules Bordet and was named after its ability to 'complement' the role of antibodies in protecting the body against bacteria [14]. Since then, numerous studies have shown a wide range of biological processes involving complement factors, from immunopathology to hemostasis and fetal development.
Studies have shown that healthy pregnancy is accompanied by moderate activation of the complement system. However, overactivation may be one of the causes of so-called great obstetric syndromes (including preeclampsia) and adverse pregnancy outcomes [15–19].
The three pathways of initiation of the extracellular complement cascade (classical, alternative, and lectin) culminate in a common terminal pathway, including the assembly of the C5b-9 membrane attack complex (MAC) and cell lysis. Analysis of the plasma proteomic profile of pregnant women with preeclampsia showed a significant increase in complement factors C1r, C1s, CFD, and MASP1, and a decrease in C4B, C6, C7, and C8A, suggesting the involvement of all three activation pathways in this pregnancy complication. The levels of many other complement cascade proteins in preeclampsia, including regulatory proteins, also differed from those in the controls; however, the differences were not statistically significant.
C1r and C1s are serine proteases that are activated when C1q binds to the antigen-antibody complex that triggers the classical complement pathway. An increase in their levels is evidence of the activation of this complement activation pathway. One of the intermediate components of the cascade is the formation of C4B, the reduction of which in preeclampsia seems likely to be explained by its substantial and/or prolonged consumption during ongoing complement activation. The same reason leads to a decrease in factors C6, C7, and C8A, whose consumption during IAC formation predominates over their production in the liver tissue.
An increase in complement factor D (CFD) is evidence of the involvement of an alternative pathway for complement activation in preeclampsia, whose unique feature is "auto-activation" by hydrolysis of the disulfide bond of the C3 protein. The alternative pathway is triggered when the C3b protein binds directly to microorganisms, alien materials, or damaged cells. C3 then binds to and changes the conformation of factor B, which in turn is cleaved by CFD to form the C3-convertase alternative pathway and the terminal complex (MASP).
MASP1 is a key link in the lectin pathway of complement activation, which is induced when mannose-binding lectin (MBL) or ficolins bind to monosaccharides on the target membrane, after which MASPs (MBL-associated serine proteases) are activated, cleaving C4 and C2 to form C3-convertase and MAC assembly.
Over-activation of the complement system in preeclampsia eventually leads to depletion of cascade factors and "consumption hypocomplementemia" against a background of failure of the regulatory systems controlling activation. Specifically, we observed a decrease in carboxypeptidase B2 (CPB2), an activation inhibitor of the classical pathway. The increase in clusterin (Clu), which normally inhibits the C5b-8 complex, appears to be compensatory but insufficient.
Our findings are supported by those of a recent prospective study by He Y.D. et al. [20]. The authors studied complement activation in preeclampsia and showed that dysregulation of classical and alternative pathways occurred in the first trimester of pregnancy and persisted throughout gestation. Karumanchi S.A. demonstrated a significant correlation between high levels of placental soluble fms-like tyrosine kinase 1 and complement activation in the placenta of patients with preeclampsia, which contributes to complement-mediated placental damage [21].
In this context, severe preeclampsia must be considered a complement-associated complication of pregnancy. At the same time, modern principles of precision medicine dictate the need for further research into the possibility of targeted therapeutic correction of complement dysfunction (in particular, the use of monoclonal antibodies to factors C5, C3, factor D, MASP-2, selective low molecular weight peptides that bind abnormal complement factors, blockade of the synthesis C5 in the liver, etc.) [22].
The results of our study showed that coagulation and platelet activation are initiated in parallel with complement activation. Significant multidirectional changes in 12 proteins of the coagulation cascade were found in pregnant women with preeclampsia, including an increase in coagulation factor V (F5), a plasma inhibitor of protein C (SERPINA5), a decrease in α-chain fibrinogen, β-chain fibrinogen, and γ-chain fibrinogen (FGA, FGB, and FGG), kininogen-1 (KNG1), heparin cofactor 2 (SERPIND1), plasminogen (PLG), coagulation factors XII and XIII (F12, F13B), and antithrombin-III (SERPINC1).
Recent studies indicate a rather close interaction between the complement system and the coagulation cascade, which manifests in both physiological and pathological conditions, such as the inflammatory response to thrombosis, thrombosis at the site of inflammation, and thrombotic complications in complement dysfunction [23]. Complement activation pathways and coagulation systems are functionally and evolutionarily related, and have a similar organization in the form of a cascade of serine proteases and their regulators.
Thus, thrombin is known to cleave complement factor C5 at the site of coagulation with the formation of C5aT (structurally and functionally similar to C5a) and C5bT, which is involved in subsequent complement activation, leading to the formation of C5bT-9, which has even higher lytic activity than C5b-9. At a much lower rate, thrombin can also generate classical C5a and C5b [24]. Potent anaphylatoxin C5a, in turn, activates the chemotaxis of neutrophils and monocytes to the site of thrombosis and increases the expression of tissue factors on monocytes and endotheliocytes, which additionally stimulates coagulation [25].
Plasma protein C inhibitor (SERPINA5) is a procoagulant and proinflammatory factor that contributes significantly to the development of preeclampsia. An increase in SERPINA5 leads to blockade of anticoagulant-activated protein C, inhibiting its binding to the thrombin/thrombomodulin complex, as well as blockade of other blood coagulation enzymes, including thrombin and factor Xa [https://www.uniprot.org/uniprotkb/P05154/entry].
Coagulation factor V (F5), the central regulator of hemostasis, is a key cofactor in the implementation of factor Xa prothrombinase activity, which leads to the conversion of prothrombin to thrombin. Unidirectionally high levels of F5 and SERPINA5 in preeclampsia maintain a high coagulation potential of the blood coagulation system and are linked to disseminated intravascular coagulation.
As for other factors of the coagulation cascade that are differentiated in preeclampsia, we found a predominant decrease in their plasma levels.
Thus, fibrinogen-α is cleaved by thrombin to monomers, which, together with fibrinogen-β and fibrinogen-γ (FGA, FGB, and FGG), polymerize to form an insoluble fibrin matrix, one of the main components of blood clots; the γ chain also carries the main binding site for the platelet receptor.
Kininogen-1 (KNG1) is a precursor protein for high molecular weight kininogen (HMWK), low molecular weight kininogen (LMWK), and bradykinin. HMWK is involved in the initiation of blood coagulation, activation of the kallikrein-kinin system, and formation of bradykinin. KNG1 is activated during endothelial injury, which is characteristic of preeclampsia, binding to subendothelial proteins and thereby initiating coagulation (internal blood coagulation pathway). Its binding to intact endotheliocytes or platelets has also been previously described. A decrease in KNG1 leads to a decrease in the formation of bradykinin, which is a powerful endothelium-dependent vasodilator as well as a mild diuretic that helps lower blood pressure. Dysfunction of the kallikrein-kinin system appears to be another mechanism that maintains hypertension in preeclampsia patients.
Heparin cofactor II (SERPIND1) is a blood clotting factor that inhibits IIa and is a cofactor for heparin and dermatan sulfate ("minor antithrombin"). This protein shares homology with antithrombin III and other members of the alpha-1 antitrypsin family. Studies have shown that pregnant women normally have elevated levels of the cofactor heparin II, which also exhibits a stronger ability to inhibit thrombin in pregnant women, thereby protecting them from thrombosis [26]. SERPIND1 deficiency leads to increased thrombin formation, hypercoagulability, and arterial thrombosis [27].
Antithrombin III (AT-III, SERPINC1) is a small glycoprotein that inactivates several enzymes in the blood coagulation system. Physiological targets of antithrombin III are proteases of the contact (internal) pathway of activation of the coagulation cascade, namely, activated factors X, IX, XI, XII, and, to a greater extent, factor II (thrombin), as well as the activated form of factor VII of the external pathway of tissue factor. AT-III deficiency is a potential risk factor for serious thromboembolic complications in both the mother and fetus and may also cause the ineffectiveness of anticoagulant therapy with heparin and its low molecular weight analogs [28]. The decrease in plasminogen (PLG) that we found also contributes to thrombosis because PLG is a circulating proenzyme, a precursor of plasmin, which plays an important role in fibrinolysis.
The deficiency of the coagulation factors described above in severe preeclampsia can be explained by three mechanisms.
1) Increased excretion of proteins by the kidneys due to nephrotic syndrome and proteinuria.
2) A decrease in protein production against the background of impaired liver function.
3) Prolonged and accelerated consumption against the background of constant activation of the coagulation cascade (similar to consumption hypocomplementemia).
The blood plasma proteome in preeclampsia also indicates the activation of platelets, which are concentrated in the loci of endothelial damage, with the formation of microthrombi and the development of microangiopathy. Activated platelets, in addition to their central role in maintaining hemostasis, also promote inflammation, participate in atherogenesis, and regulate the immune response [29, 30].
Attempts to therapeutically influence the above pathophysiological mechanisms of development and progression of preeclampsia with low-molecular-weight heparin and aspirin turned out to be ineffective, since they do not affect etiological factors [15, 31].
An important fact was A significant change in the protein cluster was observed during preeclampsia, indicating a violation of the molecular processes in the endoplasmic reticulum (ER), where post-translational modifications of proteins occur (glycosylation, acetylation, formation of disulfide bonds, etc.). In the ER, proteins are folded using luminal chaperones, packaged into transport vesicles, and delivered to the Golgi complex. Misfolded proteins remain in the ER lumen in complex with molecular chaperones and are degraded further. Accumulation of misfolded proteins causes ER stress and the activation of signaling pathways to restore its function. However, in severe situations, protective mechanisms are insufficient and such cells die as a result of apoptosis [32].
The study of the plasma proteome in pregnant women with preeclampsia showed a significant increase, apparently of a compensatory nature, in HSP90B1, the endoplasmin molecular chaperone MAN1A1 (mannosyl-oligosaccharide 1,2-alpha-mannosidase IA), which is involved in protein glycosylation, glycan biosynthesis, and protein processing in the endoplasmic reticulum, as well as catalytic (GANAB) and regulatory (PRKCSH) subunits of glucosidase II involved in the metabolism of glycans.
In pregnant women with preeclampsia, a violation of the mechanism of "neutrophil extracellular trap (NET) formation" was also revealed indicating the ability of neutrophils to form neutrophil extracellular traps (NET), which is closely related to the "NETosis" process. A significant decrease in the proteins of this cluster (FGA, FGB, FGG, and CAMP) was observed. NETosis is a regulated form of neutrophil death that contributes to the body's defence against foreign agents and pathogens. During NETosis, neutrophils release neutrophil extracellular traps that can capture and inactivate bacteria, viruses, and other foreign agents [33]. Studies have shown that the relationship between blood coagulation and the complement system is obvious and indisputable. However, in recent years, NEToz has also been considered the third component of this interaction. Neutrophils and NETs provide another link among acute injury, thrombosis, and inflammation. Additionally, the role of NET in the initiation of autoimmune diseases, diabetes, atherosclerosis, and systemic vasculitis has been actively studied [34].
Recent epidemiological data suggest that preeclampsia is associated with a 2–4-fold increase in the risk of cardiovascular diseases, in particular, chronic hypertension, coronary heart disease, venous thromboembolism, and stroke [35, 36]. There is little evidence regarding metabolic complications associated with preeclampsia. In particular, a systematic review and meta-analysis showed that preeclampsia is associated with a two-fold increase in the risk of developing type II diabetes mellitus within 1–10 years of childbirth [37, 38].
The study of the proteome of women with preeclampsia revealed significant changes in their cardiometabolic profiles. Particularly, pronounced disturbances were found in 23 biological processes involved in fat metabolism (such as lipoprotein and cholesterol metabolism, chylomicron clearance, cholesterol and phospholipid excretion, lipid transport, low and very low-density lipoprotein biosynthesis and assembly, high-density lipoprotein clearance, triglyceride homeostasis, and regulation of lipid metabolism). For example, the largest cholesterol metabolism cluster that we identified included 6 proteins with elevated levels (Apolipoprotein B-100, Apolipoprotein C-I, Apolipoprotein C-II Apolipoprotein C-III, Apolipoprotein E, Pro-low-density lipoprotein receptor-related protein 1).
In addition to pronounced disorders of fat metabolism, pregnant women with preeclampsia also have other metabolic and cellular disorders (pathological orientation of the processes of angiogenesis, apoptosis (including endothelial cells), cytolysis, proteolysis, proteoglycan metabolism, tissue regeneration, TGF-ß signaling pathways and insulin-like growth factor, etc.)
According to the authors investigating the blood proteome in women with a history of preeclampsia, they still have signs of dysregulation of processes such as inflammation, immune response, blood coagulation, and metabolism even one year after childbirth, which emphasizes the need for long-term monitoring of their health [37].
Thus, the blood plasma proteomic profile in pregnant women with severe preeclampsia is characterized by a wide variability in the changes in biological processes that determine the pathogenesis and clinical manifestations of this pregnancy complication. Compared to healthy pregnant women, there are significant disorders associated with the hemostasis system, inflammatory and immune reactions, and metabolic, cellular, and intracellular processes.
The question of the preceding state of the pathological processes that we discovered also remains open, which requires the study of the proteomic blood profile in women before pregnancy, in the first half, and before the onset of symptoms of preeclampsia. In addition, since preeclampsia is now considered a placental syndrome, a study of the correlation between protein expression in the placenta and plasma may provide valuable information.
Modern proteomic technologies have great potential for studying the pathophysiology of preeclampsia; however, they have some limitations. Protein analysis using mass spectrometry requires many resources, which limits its practical applications. Difficulties remain in the discovery of low-content proteins that may have diagnostic and prognostic potential. We also recognize the relatively small sample size of patients included in the study and slightly different gestational ages in the study and control groups (however, all within the third trimester), which was dictated by the need to simultaneously study the placental proteome. It is also necessary to study the proteome in different preeclampsia phenotypes, which will allow the development of therapeutic and preventive strategies. Combining the results of proteomics with other branches of systems biology, such as metabolomics and genomics, is also important for a complete understanding of the molecular mechanisms of preeclampsia.
Conclusion
The proteomic profile of maternal blood in severe preeclampsia differs significantly from that in uncomplicated pregnancies, characterized by variability in changes reflecting multiple and multidirectional disturbances in biological processes and molecular functions in the pathogenesis of preeclampsia.
References
1. Сидорова И.С., Никитина Н.А. Обоснование современной концепции развития преэклампсии. Акушерство и гинекология. 2019; 4: 26‑33. [Sidorova I.S., Nikitina N.A. Validation of the modern concept of the development of preeclampsia. Obstetrics and Gynecology. 2019; (4): 26‑33. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.4.26‑33.
2. Benny P.A., Alakwaa F.M., Schlueter R.J., Lassiter C.B., Garmire L.X. A review of omics approaches to study preeclampsia. Placenta. 2020; 92: 17‑27. https://dx.doi.org/ 10.1016/j.placenta.2020.01.008.
3. Navajas R., Corrales F., Paradela A. Quantitative proteomics‑based analyses performed on pre‑eclampsia samples in the 2004‑2020 period: a systematic review. Clin. Proteomics. 2021; 18(1): 6. https://dx.doi.org/10.1186/ s12014‑021‑09313‑1.
4. Sun Y.V., Hu Y.J. Integrative analysis of multi‑omics data for discovery and functional studies of complex human diseases. Adv. Genet. 2016; 93: 147‑90. https://dx.doi.org/10.1016/bs.adgen.2015.11.004.
5. Никитина Н.А., Сидорова И.С., Агеев М.Б., Тимофеев С.А., Кирьянова М.А., Морозова Е.А. Новые технологии в решении проблем преэклампсии. Акушерство и гинекология. 2022; 10: 5‑13. [Nikitina N.A., Sidorova I.S., Ageev M.B., Timofeev S.A., Kiryanova M.A., Morozova E.A. New technologies in solving the problems of preeclampsia. Obstetrics and Gynecology. 2022; (10): 5‑13. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.10.5‑13.
6. Deutsch E.W., Omenn G.S., Sun Z., Maes M., Pernemalm M., Palaniappan K.K. et al. Advances and utility of the human plasma proteome. J. Proteome Res. 2021; 20(12): 5241‑63. https://dx.doi.org/10.1021/acs.jproteome.1c00657.
7. Romero R., Erez O., Maymon E., Chaemsaithong P., Xu Z., Pacora P. et al. The maternal plasma proteome changes as a function of gestational age in normal pregnancy: a longitudinal study. Am. J. Obstet. Gynecol. 2017; 217(1): 67.e1‑67. e21. https://dx.doi.org/10.1016/j.ajog.2017.02.037.
8. Aghaeepour N., Lehallier B., Baca Q., Ganio E.A., Wong R.J., Ghaemi M.S. et al. A proteomic clock of human pregnancy. Am. J. Obstet. Gynecol. 2018; 218(3): 347.e1‑347.e14. https://dx.doi.org/10.1016/j.ajog.2017.12.208.
9. Tarca A.L., Romero R., Benshalom-Tirosh N., Than N.G., Gudicha D.W., Done B. et al. The prediction of early preeclampsia: Results from a longitudinal proteomics study. PLoS One. 2019; 14(6): e0217273. https://dx.doi.org/10.1371/ journal.pone.0217273.
10. Hedman A.M., Lundholm C., Andolf E., Pershagen G., Fall T., Almqvist C. Longitudinal plasma inflammatory proteome profiling during pregnancy in the Born into Life study. Sci. Rep. 2020; 10(1): 17819. https://dx.doi.org/10.1038/ s41598‑020‑74722‑5.
11. Стародубцева Н.Л., Бугрова А.Е., Кононихин А.С., Вавина О.В., Широкова В.А., Наумов В.А., Гаранина И.А., Лагутин В.В., Попов И.А., Логинова Н.С., Ходжаева З.С., Франкевич В.Е., Николаев Е.Н., Сухих Г.Т. Возможность прогнозирования и ранней диагностики преэклампсии по пептидному профилю мочи. Акушерство и гинекология. 2015; 6: 46‑52. [Starodubtseva N.L., Bugrova A.E., Kononikhin A.S., Vavina O.V., Shirokova V.A., Naumov V.A., Garanina I.A., Lagutin V.V., Popov I.A., Loginova N.S., Khodzhaeva Z.S., Frankevich V.E., Nikolaev E.N., Sukhikh G.T. Possibility for the prediction and early diagnosis of preeclampsia from the urinary peptide profile. Obstetrics and Gynecology. 2015; (6): 46‑52. (in Russian)].
12. Прокопенко В.М. Применение протеомного анализа в акушерстве (первые результаты исследований). Российский вестник акушера‑гинеколога. 2016; 16(1): 28‑32. [Prokopenko V.M. Use of proteomic analysis in obstetrics: first results of investigations. Russian Bulletin of Obstetrician‑Gynecologist. 2016; 16(1): 28‑32. (in Russian)]. https://dx.doi.org/10.17116/ rosakush201616128‑32.
13. Michalczyk M., Celewicz A., Celewicz M., Woźniakowska-Gondek P., Rzepka R. The Role of Inflammation in the Pathogenesis of Preeclampsia. Mediators Inflamm. 2020; 2020: 3864941. https://dx.doi.org/10.1155/2020/3864941.
14. Cavaillon J.M., Sansonetti P., Goldman M. 100th Anniversary of Jules Bordet's Nobel Prize: tribute to a founding father of immunology. Front. Immunol. 2019; 10: 2114. https://dx.doi.org/10.3389/fimmu.2019.02114.
15. Youssef L., Miranda J., Blasco M., Paules C., Crovetto F., Palomo M. et al. Complement and coagulation cascades activation is the main pathophysiological pathway in early‑onset severe preeclampsia revealed by maternal proteomics. Sci. Rep. 2021; 11(1): 3048. https://dx.doi.org/10.1038/s41598‑021‑82733‑z.
16. Regal J.F., Gilbert J.S., Burwick R.M. The complement system and adverse pregnancy outcomes. Mol. Immunol. 2015; 67(1): 56‑70. https://dx.doi.org/10.1016/j.molimm.2015.02.030.
17. Сидорова И.С., Никитина Н.А., Унанян А.Л., Агеев М.Б., Кокин А.А. Система комплемента при физиологической беременности. Акушерство и гинекология. 2021; 6: 14‑20. [Sidorova I.S., Nikitina N.A., Unanyan A.L., Ageev M.B., Kokin A.A. The complement system during physiological pregnancy. Obstetrics and Gynecology. 2021; (6): 14‑20. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.6.14‑20.
18. Сидорова И.С., Никитина Н.А., Унанян А.Л., Агеев М.Б., Кокин А.А. Система комплемента при беременности, осложненной преэклампсией. Акушерство и гинекология. 2021; 8: 5‑12. [Sidorova I.S., Nikitina N.A., Unanyan A.L., Ageev M.B., Kokin A.A. The complement system in preeclampsia‑complicated pregnancy. Obstetrics and Gynecology. 2021; (8): 5‑12. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.8.5‑12.
19. Сидорова И.С., Никитина Н.А., Агеев М.Б., Кокин А.А., Кирьянова М.А. Дисрегуляция системы комплемента при развитии преэклампсии. Акушерство и гинекология. 2022; 2: 46‑58. [Sidorova I.S., Nikitina N.A., Ageev M.B., Kokin A.A., Kir'yanova M.A. Complement system dysregulation in patients with preeclampsia. Obstetrics and Gynecology. 2022; (2): 46‑58. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.2.46‑58.
20. He Y.D., Xu B.N., Wang M.L., Wang Y.Q., Yu F., Chen Q. et al. Dysregulation of complement system during pregnancy in patients with preeclampsia: A prospective study. Mol. Immunol. 2020; 122: 69‑79. https://dx.doi.org/10.1016/j.molimm.2020.03.021.
21. Yonekura Collier A.R., Zsengeller Z., Pernicone E., Salahuddin S., Khankin E.V., Karumanchi S.A. Placental sFLT1 is associated with complement activation and syncytiotrophoblast damage in preeclampsia. Hypertens Pregnancy. 2019; 38(3): 193‑9. https://dx.doi.org/10.1080/10641955.2019.1640725.
22. Jia C., Tan Y., Zhao M. The complement system and autoimmune diseases. Chronic Dis. Transl. Med. 2022; 8(3): 184‑90. https://dx.doi.org/10.1002/ cdt3.24.
23. Oncul S., Afshar-Kharghan V. The interaction between the complement system and hemostatic factors. Curr. Opin. Hematol. 2020; 27(5): 341‑52. https://dx.doi.org/10.1097/MOH.0000000000000605.
24. Krisinger M.J., Goebeler V., Lu Z., Meixner S.C., Myles T., Pryzdial E.L. et al. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood. 2012; 120(8): 1717‑25. https://dx.doi.org/10.1182/blood‑2012‑02‑412080.
25. Ritis K., Doumas M., Mastellos D., Micheli A., Giaglis S., Magotti P. et al. A novel C5a receptor‑tissue factor cross‑talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006; 177(7): 4794‑802. https://dx.doi.org/10.4049/jimmunol.177.7.4794.
26. Huntington J.A. Chemistry and biology of heparin and heparan sulfate. Elsevier; 2005: 367‑98. https://dx.doi.org/10.1016/B978‑008044859‑6/50014‑9.
27. Bano S., Fatima S., Ahamad S., Ansari S., Gupta D., Tabish M. Identification and characterization of a novel isoform of heparin cofactor II in human liver. IUBMB Life. 2020; 72(10): 2180‑93. https://dx.doi.org/10.1002/ iub.2361.
28. Čápová I., Salaj P., Hrachovinová I. Hereditary antithrombin deficiency in pregnancy ‑ severe thrombophilic disorder as a danger for mother and foetus. Ceska Gynekol. 2021; 86(3): 175‑82. https://dx.doi.org/10.48095/ cccg2021175.
29. Thomas M.R., Storey R.F. The role of platelets in inflammation. Thromb. Haemost. 2015; 114(3): 449‑58. https://dx.doi.org/10.1160/TH14‑12‑1067.
30. Kim S.J., Davis R.P., Jenne C.N. Platelets as modulators of inflammation. Semin. Thromb. Hemost. 2018; 44(2): 91‑101.https://dx.doi.org/10.1055/ s‑0037‑1607432.
31. Groom K.M., David A.L. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am. J. Obstet. Gynecol. 2018; 218(Suppl. 2): S829‑40. https://dx.doi.org/10.1016/j.ajog.2017.11.565.
32. Database «Kyoto Encyclopedia of Genes and Genome» (KEGG). Available at: https://www.genome.jp/kegg‑bin/show_pathway?hsa04141.
33. de Bont C.M., Boelens W.C., Pruijn G.J.M. NETosis, complement, and coagulation: a triangular relationship. Cell. Mol. Immunol. 2019; 16(1): 19‑27. https://dx.doi.org/10.1038/s41423‑018‑0024‑0.
34. Jorch S.K., Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017; 23(3): 279‑87. https://dx.doi.org/ 10.1038/nm.4294.
35. Garovic V.D., White W.M., Vaughan L., Saiki M., Parashuram S., Garcia- Valencia O. et al. Incidence and long‑term outcomes of hypertensive disorders of pregnancy. J. Am. Coll. Cardiol. 2020; 75(18): 2323‑34. https://dx.doi.org/6/ j.jacc.2020.03.028.
36. Li Y.Y., Cao J., Li J.L., Zhu J.Y., Li Y.M., Wang D.P. et al. Screening high‑risk population of persistent postpartum hypertension in women with preeclampsia using latent class cluster analysis. BMC Pregnancy Childbirth. 2022; 22(1): 687. https://dx.doi.org/10.1186/s12884‑022‑05003‑4.
37. Manousopoulou A., Abad F.S., Garay-Baquero D.J., Birch B.R., van Rijn B.B., Lwaleed B.A. et al. Increased plasma CD14 levels 1 year postpartum in women with pre‑eclampsia during pregnancy: a case‑control plasma proteomics study. Nutr. Diabetes. 2020; 10(1): 2. https://dx.doi.org/10.1038/s41387‑019‑0105‑x.
38. Wu P., Kwok C.S., Haththotuwa R., Kotronias R.A., Babu A., Fryer A.A. et al. Pre‑eclampsia is associated with a twofold increase in diabetes: a systematic review and meta‑analysis. Diabetologia. 2016; 59(12): 2518‑26. https://dx.doi.org/10.1007/s00125‑016‑4098‑x.
Received 25.01.2023
Accepted 04.05.2023
About the Authors
Natalya A. Nikitina, Dr. Med. Sci., Professor, I.M. Sechenov First MSMU, N.V. Sklifosovsky Institute of Clinical Medicine, Department of Obstetrics and Gynaecology No. 1, natnikitina@list.ru, 119991, Russia, Moscow, Trubetskaya str., 8, bld. 2.Iraida S. Sidorova, Dr. Med. Sci., Professor, Academician of RAS, Merited Scholar of the Russian Federation, I.M. Sechenov First MSMU, N.V. Sklifosovsky Institute of Clinical Medicine, Department of Obstetrics and Gynaecology No. 1, sidorovais@yandex.ru, 119991, Russia, Moscow, Trubetskaya str., 8, bld. 2.
Rustam Kh. Ziganshin, PhD, Senior Researcher, Head of the Group of Mass Spectrometry, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the RAS, rustam. ziganshin@gmail.com, 117997, Russia, Moscow, Miklukho-Maklaya str., 16/10.
Marina A. Kir'yanova, PhD Student at the Department of Obstetrics and Gynecology No. 1, I.M. Sechenov First MSMU, N.V. Sklifosovsky Institute of Clinical Medicine, kiryanova.marina8@mail.ru, 119991, Russia, Moscow, Trubetskaya str., 8, bld. 2.
Mikhail B. Ageev, PhD, Teaching Assistant at the Department of Obstetrics and Gynecology No. 1, I.M. Sechenov First MSMU, N.V. Sklifosovsky Institute of Clinical Medicine, mikhaageev@yandex.ru, 119991, Russia, Moscow, Trubetskaya str., 8, bld. 2.



