Changes in serum lipid profile during the menopausal transition
Objective: To compare metabolic profiles of women in the early and late phase of menopausal transition.Komedina V.I., Yureneva S.V., Chagovets V.V., Starodubtseva N.L.
Materials and methods: The study involved 125 women experiencing menopause transition. Baseline evaluation included carbohydrate and lipid metabolism parameters, uric acid, C-reactive protein (CRP), leptin, and adiponectin. The serum lipidome was analyzed by high performance chromatography with tandem mass spectrometry (HPLC-MS). Body composition was measured with dual-energy X-ray absorptiometry.
Results: Women in the early and late phase of the menopausal transition had no significant differences in body composition, carbohydrate and lipid metabolism, levels of uric acid, CRP, leptin, and adiponectin levels. However, they had statistically significant differences in the levels of 14 lipids determined by HPLC-MS. Women in the late phase of menopausal transition had higher levels of ceramide Cer(d18:1/22:0), phospholipids OxLPC(24:1(OOOO)), OxPC(18:0_18:4(Ke,OH)), OxPC(20:4_14:0(COOH)), PC(18:1_18:1), PC(18:0_20:2), PEtOH(18:0_24:0), PI(18:1_18:2), and sphingomyelin SM(d26:0/16:1). These substances had a positive correlation with FSH, LDL, atherogenicity coefficient, glucose, insulin, HOMA index, glycated hemoglobin, and blood pressure.
Conclusion: In women experiencing early and late phase of menopausal transition, HPLC-MS allowed the identification of differences in lipid profiles undetectable by traditional biochemical methods. Changes in lipidome may be an initial step in the pathogenesis of cardiometabolic disorders in women undergoing menopause transition. These findings can be used for further study of various diseases associated with menopause.
Keywords
The menopausal transition, regardless of chronological aging, is associated with adverse changes in the lipid profile that increase women's cardiovascular disease (CVD) in women [1, 2].
In the SWAN (Study of Women’s Health Across the Nation) study, women’s total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B increased within the period from 1 year before and 1 year after the final menstrual period, relative to the years before or after this 2-year interval [1, 3]. The severity of atherosclerotic changes in the carotid arteries in postmenopause is proportional to the increase in the atherogenicity of the blood lipid spectrum during the menopausal transition [3]. Vascular remodeling occurs already at the stage of menopausal transition. In its late phase, there is a significant increase in the thickness of the intima-media complex and the diameter of the carotid arteries compared to the early phase of the menopausal transition and premenopause [1].
Menopause is associated with a change in the key antiatherogenic function of high-density lipoproteins (HDL) - reverse transport of cholesterol from peripheral tissues to the liver, which leads to a decrease in the protective properties of HDL. A higher level of HDL, associated in premenopause with less severe vessels atherosclerosis, in postmenopause is associated with more severe atherosclerotic lesions [1].
Menopausal transition in women is associated with an increase in blood pressure (BP), glucose, insulin, triglycerides (TG), which is mainly due to chronological aging [1, 3]. However, regardless of age, there is an increase in the prevalence and severity of the metabolic syndrome (increase in the number of its components) associated with the menopausal transition [1, 3].
Menopausal transition is a period of formation of risk factors for cardiometabolic diseases, and all available modern methods should be used to study the underlying pathophysiological processes. The study of lipid profiles using mass spectrometry, which provides information on hundreds of lipids, has great potential to expand preventive and therapeutic options for menopause-related diseases [4]. Lipids, as components of cell membranes and acting as secondary messengers, are involved in almost all processes that occur in the human body [4]. To date, the role of various lipids as biomarkers of preeclampsia, cervical cancer, obesity, atherosclerosis, insulin resistance (IR), inflammation, type 2 diabetes mellitus (type 2 DM), CVD and neurodegenerative diseases has been described [4–7].
This study aimed to investigate the metabolic profile in women in the early and late phases of the menopausal transition using traditional biochemical methods, high performance chromatography with tandem mass spectrometry (HPLC-MS), and body composition to gain insight into the mechanisms underlying metabolic disorders and their role in the development of cardiometabolic diseases.
Materials and methods
A single-center observational study was conducted in 125 women aged 42–52 years experiencing menopausal transition (reproductive aging stage -2; -1 according to STRAW +10 [1]), who were managed at the Department of Gynecologic Endocrinology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. The noninclusion criteria were body weight deficiency (body mass index (BMI) less than 18.5 kg/m²), obesity (BMI greater than or equal to 30 kg/m²), pregnancy, breastfeeding, severe somatic diseases, therapy with drugs containing sex hormones or affecting metabolism less than 6 months before the study, the history of polycystic ovary syndrome.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P. All participants provided their signed informed consent to participate in the study.
Anthropometric parameters included body weight, height, waist circumference (WC). BMI was measured as weight (kg)/height (m2). Body composition was assessed by dual energy X-ray absorptiometry (DERA) on a Lunar model 8743 (GE Medical Systems, USA) using CoreScan software (GE Healthcare, USA) [8].
Blood pressure was measured using an automatic tonometer (Omron M2 Basic, Japan) and determined as the average value of two measurements.
Fasting blood samples were taken from study participants on the 2nd-4th day of the menstrual cycle. Serum concentrations of sex hormones (follicle-stimulating hormone (FSH), estradiol, and total testosterone), sex hormone-binding globulin (SHBG), and insulin were determined by electrochemiluminescent method on a Cobas e411 automatic immunoassay (Roche Diagnostics GmbH, Germany). Free testosterone index (FTI) was calculated using the following formula:
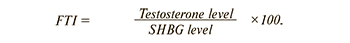
Leptin and adiponectin levels were determined in serum by enzyme immunoassay using DBC (Canada) and Mediagnost (Germany) commercial kits, respectively. The concentration of C-reactive protein (CRP) was determined by an immunoturbidimetric high-sensitivity method.
The plasma levels of TC, TG, LDL-C, HDL-C, apolipoprotein A1, apolipoprotein B, uric acid in blood serum and glucose, glycated hemoglobin (HbA1c) were determined by standard biochemical methods. Calculation of the atherogenicity coefficient (AC) was performed according to the following formula:
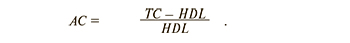
To assess insulin sensitivity, HOMA-IR was calculated according to the formula:
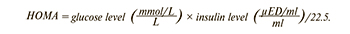
A HOMA index ≥2.7 was a criterion for IR [9]. Metabolic syndrome (MS) was diagnosed based on the 2006 IDF criteria including abdominal obesity (OT ≥80 cm) and two of the following features: TG≥1.7 mmol/l, HDL-C<1.29 mmol/l (or dyslipidemia therapy), systolic blood pressure ≥130 mm Hg /diastolic blood pressure ≥85 mm Hg (or antihypertensive therapy), fasting plasma glucose ≥5.6 mmol/l [10].
The serum lipid composition was analyzed by electrospray ionization on a Dionex UltiMate 3000 liquid chromatograph (Thermo Scientific, Germany) coupled to a Maxis Impact qTOF mass analyzer with an ERI ion source (Bruker Daltonics, Germany). The samples were separated by reverse-phase chromatography on a Zorbax C18 column (150×2.1 mm, 5 μm, Agilent, USA) with a linear gradient from 30% to 90% of eluent B (acetonitrile/isopropanol/water solution, 90/8/2 vol./vol, with 0.1% formic acid and 10 mmol/L ammonium formate added) for 20 min. Acetonitrile/water solution (60/40, vol./vol.) with the addition of 0.1% formic acid and 10 mmol/L ammonium formate was used as eluent A. The elution flow rate was 40 μl/min, and the injected sample volume was 3 μl. Mass spectra were collected in positive ion mode in the m/z 100–1700 range with the following parameters: capillary voltage 4.1 kV, atomizing gas pressure 0.7 bar, flow rate and drying gas temperature 6 l/min and 200°C. To identify lipids, we performed tandem mass spectrometry in a dependent scanning mode with 5 Da window width. Lipid levels were determined by a semiquantitative method, presented in conventional units.
Statistical analysis
Statistical analysis was performed using Statistica 13.5.0 software. The normality of the distribution was tested by the Shapiro–Wilk test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, medians (Me) with interquartile range (Q1; Q3) were reported. Categorical variables were presented as counts and percentages. Differences between normally distributed continuous variables were assessed using the Student’s t-test for two independent samples. Variables that did not meet normality assumptions were compared with a nonparametric Mann–Whitney test. Categorical variables were compared using Fisher’s exact test. The critical level of significance when testing statistical hypotheses was considered at p<0.05. Correlation analysis was performed using the nonparametric Spearman correlation test.
Results
The study participants were categorized by their stage of reproductive aging into Group 1, including 62 women in the early phase of menopausal transition [(-2 in STRAW +10), mean age 45.8 (2.36) years] and Group 2 included 63 women in the late phase of menopausal transition [(-1 in STRAW +10), mean age 48.1 (2.58) years]. Comparison of clinical, anamnestic and hormonal characteristics showed differences in age and FSH levels, which were higher in the late phase, and in estradiol levels, which were higher in the early phase, p<0.001 (Table 1).
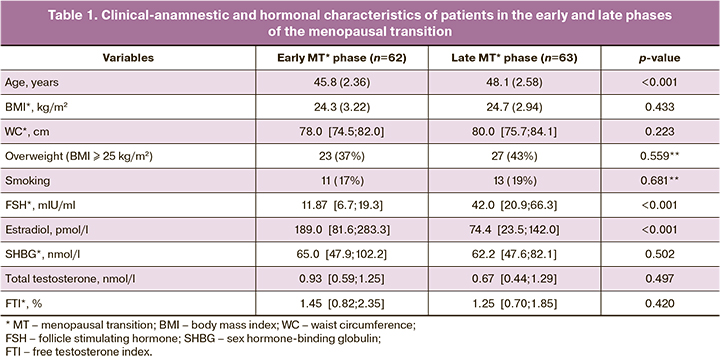
No significant differences were found between the groups in body composition (Table 2). Biochemical parameters of carbohydrate and lipid metabolism, levels of uric acid, high-sensitivity CRP, leptin, adiponectin, as well as the incidence of arterial hypertension and metabolic disorders (dyslipidemia, BP, MI) did not differ significantly between the groups. Patients in the late phase of the menopausal transition had higher systolic BP levels than those in the early phase, p=0.029 (Table 2).
The serum lipid profile of the patients was then examined by HPLC-MS. There were statistically significant differences in 14 lipid levels, p<0.05 (Table 3) in women in the early and late stages of the menopausal transition.
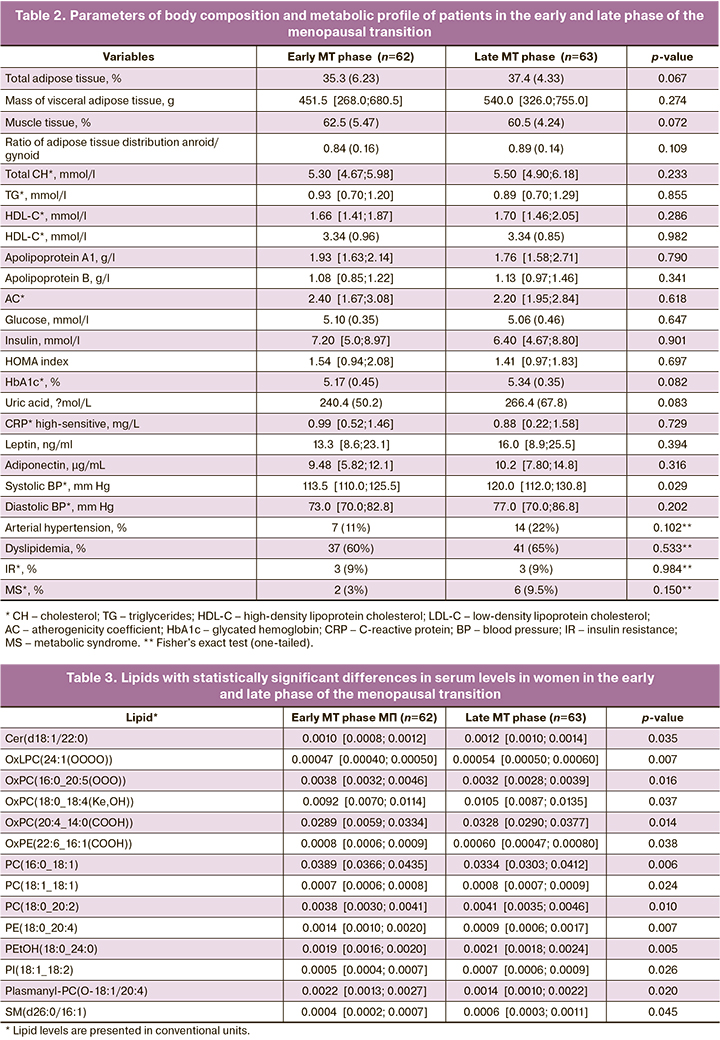
Women in the late phase of the menopausal transition had higher levels of Cer ceramide Cer(d18:1/22: 0), OxLPC oxidized phospholipids (2: 1 (OOOO)), OxPC (18: 0_18: 4 (Ke, OH)), OxPC(20: 4_14: 0 (COOH)), PC phospholipids (18: 1_18: 1), PC(18: 0_20: 2), PEtOH(18: 0_24: 0), PI(18: 1_18: 2) and sphingomyelin SM(d26:0/16:1), which were positively correlated with FSH, LDL-C, AC, glucose, insulin, HOMA index, HbA1c, and systolic and diastolic BP (Figure). The strongest association was found between Cer(d18:1/22:0) and insulin (r=0.469, 95% CI: 0.207–0.668, p=0.001), OxPC(18:0_18:4(Ke,OH)) and HOMA index (r=0.490, 95% CI: 0.219–0.691, p=0.049), PC(18:0_20:2) and systolic BP (r=0.434, 95% CI: 0.158–0.648, p=0.003), SM(d26:0/16:1) and glucose (r=0.421, 95% CI: 0.184–0.611, p=0.001).
Women in the early phase of the menopausal transition had higher levels of oxidized phospholipids OxPC(16:0_20:5 (OOO)), OxPE(22.6_16:1 (COOH)) and phospholipids PC(16:0_18:1), PE(18:0_20:4), Plasmanyl-PC(18: 1: 1: 4). According to the results of the correlation analysis, Plasmanyl-PC(O-18:1/20:4) had a negative association with the level of HbA1c (r =-0.337, 95% CI: -0.574–-0.048, p=0.024) and systolic BP (r=-0.306, 95% CI -0.544–0.002, p=0.043). The results of the correlation analysis are shown in the figure.
AC – atherogenicity coefficient; LDL – low-density lipoproteins; HOMA-IR – HOMA insulin resistance index; HbA1c – glycated hemoglobin; FSH – follicle-stimulating hormone; Syst. BP – systolic blood pressure; Diast. BP – diastolic blood pressure; Vis. Fat – mass of visceral fat.
Discussion
Cer (18: 1: 2: 0) Ceramide levels were higher in women in the late phase of the menopausal transition, with a positive correlation with LDL-C, CA, insulin, and the HOMA index. Ceramides are sphingolipids that, in addition to their structural functions in cell membranes, play the role of secondary messengers in intracellular and intercellular signaling pathways. Acting as secondary messengers, ceramides increase the expression of cytokines (tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), CRP, the production of reactive oxygen species, leading to the development of atherosclerosis [11]. In addition, ceramides play a key role in the induction of pancreatic β-cell apoptosis, IR, and decreased insulin gene expression [5]. According to studies, higher Cer levels are associated with greater severity of coronary artery stenosis and increased risk of ischemic stroke in patients with coronary heart disease, as well as increased risk of type 2 DM [6,12,13]. Cer levels have been found to be elevated 5 years before the diagnosis of type 2 DM, allowing it to be used as an early biomarker of this disease [14].
Women experiencing the late phase of the menopausal transition had higher levels of sphingomyelin SM(d26:0/16:1), a sphingolipid that has a positive relationship with fasting glucose levels. There are reports in the literature on the role of sphingomyelins in the development of IR [15]. Excess visceral fat has been found to be associated with an increase in sphingomyelin concentrations. When sphingomyelin synthase 2, responsible for sphingomyelin synthesis, was blocked, mice showed a decrease in blood sphingomyelin levels and increased insulin sensitivity [15]. The creation of drugs that regulate sphingolipid metabolism is a new direction in the treatment of obesity and type 2 diabetes [15]. In blood plasma, 87% of all sphingomyelins are in lipoproteins. Very low-density lipoproteins (VLDL) contain 63–75% and HDL contain 25–35% of plasma sphingomyelins [16]. To date, data have been accumulated on the heterogeneity of HDL in density and size, which is due to the different content of protein apolipoproteins and lipids (including sphingomyelins) in their composition. We distinguish HDL2, which is rich in lipids and is characterized by a slightly larger size and lower density, and HDL3, which is rich in protein and has a smaller size and higher density. HDL2 contains more sphingomyelin relative to other lipid compounds than HDL3, resulting in changes in the structure of HDL particles and the ability to reverse cholesterol transport. HDL3 has been shown to have higher antiatherogenic activity (ability to reverse cholesterol transport), antioxidant effect on LDL oxidation, as well as antithrombotic, anti-inflammatory and antiapoptotic activity compared to HDL2 [16]. LDL in atherosclerotic plaques is characterized by a high sphingomyelin content. Thus, dysregulation of sphingomyelin synthesis and transport is a link in the pathogenesis of CVD [16].
Oxidized phospholipids, which include OxLPC(24:1(OOOO)), OxPC(18:0_18:4(Ke,OH)), OxPC(20:4_14:0(COOH)) were determined in higher concentration in the late phase of menopausal transition, and OxPC(16:0_20:5(OOO)), OxPE(22:6_16:1(COOH)) were determined in higher concentration in the early phase. Oxidation of phospholipids occurs with the participation of reactive oxygen and nitrogen forms, which can have an endogenous (mitochondrial respiratory chain, myeloperoxidase, etc.) or exogenous (air pollution, smoking, etc.) origin. In recent years, oxidized phospholipids have been assigned an increasingly important role in both normal and pathological processes. Oxidized phospholipids are involved in the regulation of inflammation, thrombosis, angiogenesis, endothelial barrier function, immune tolerance, and other important processes. Numerous studies have demonstrated that oxidized phospholipids induce proinflammatory and prothrombotic effects in endothelial cells, vascular smooth muscle cells, leukocytes and platelets [17–19]. Additionally, oxidized phospholipids induce vasa vasorum growth and stimulate phenotypic modulation of smooth muscle cells, which are an important step in the development of atherosclerotic lesions. These results suggest a role of oxidized phospholipids in the pathogenesis of atherosclerosis and its complications. However, oxidized phospholipids can induce pleiotropic effects. In parallel with the induction of inflammatory mediators and cell adhesion molecules, they activate anti-inflammatory pathways and inhibit acute inflammation in experimental animal models. Based on these data, scientists believe that oxidized phospholipids play a role in the transition from an acute to chronic inflammatory state and may be biomarkers of atherosclerosis, DM, cancer, and neurodegenerative diseases [17–19].
Phospholipids, which included PC(18:1_18:1), PC(18:0_20:2), PEtOH(18:0_24:0), PI(18:1_18:2), had higher concentration in the late phase of the menopausal transition. PC(16:0_18:1), PE(18:0_20:4), Plasmanyl-PC(O-18:1/20:4) were determined at higher concentration in the early phase. Phosphatidylcholines (PC) are the most abundant components of cell membranes. In addition to their structural function, phosphatidylcholines interact with peroxisome proliferator-activated receptors (PPARs). PPARs are a group of cell nucleus receptors that function as a transcription factor and regulate gene expression [20]. PC(16:0_18:1), whose levels are higher in women in the early phase of the menopausal transition, is an endogenous ligand of the nuclear PPARa receptor in hepatocytes, a transcription factor that regulates the expression of many genes responsible for lipid metabolism. According to the Chakravarthy M.V. study, injection of PC(16:0_18:1) into the liver portal vein in mice for several days contributed to the reduction of the severity of liver steatosis [20]. Another important function of phosphatidylcholines, which together with apolipoprotein B participate in the secretion of LDL-C, is currently known. Deficiency of phosphatidylcholines, as well as changes in their composition with a decrease in polyunsaturated fatty acids (PUFAs) leads to impaired secretion of VLDL, which results in excessive accumulation of triacylglycerols in the liver with the development of steatosis [7]. It can be assumed that lower concentrations of PC(16:0_18:1) in women in the late phase of the menopausal transition may contribute to the development of hepatic steatosis.
Phosphatidylcholines and phosphatidylethanolamines (PE) are the most abundant phospholipids of mitochondrial membranes and play a critical role in their function. Impaired synthesis of these phospholipids can lead to mitochondrial dysfunction, which is associated with CVD, metabolic syndrome, DM, neurodegenerative diseases, and progression of malignancies [7]. Lower concentrations of phosphatidylethanolamine PE(18:0_20:4) have been determined in the late phase of the menopausal transition. Recent studies have reported the role of phosphatidylethanolamines in the development of Alzheimer's and Parkinson's disease [21]. Patients with Alzheimer's disease have lower serum concentrations of phosphatidylethanolamines compared to healthy individuals. In addition, in patients with mild cognitive impairment, lower concentrations of phosphatidylethanolamines are a marker of accelerated progression into Alzheimer's disease [22]. There is evidence that low levels of phosphatidylethanolamines result in increased accumulation of alpha-synuclein, which plays a critical role in the pathogenesis of Parkinson's disease [21].
Conclusion
The use of omics revealed heterogeneity in the lipid profile in terms of phospholipid and sphingolipid content in women in the early and late phase of the menopausal transition who did not differ significantly in body composition and metabolic parameters. Considering the results of correlation analysis of lipids with metabolic parameters and literature data on their role in various pathophysiological processes, one may assume that changes in phospholipid and sphingolipid metabolism have a pathogenetic role in cardiometabolic disorders in women during menopausal transition. Information on hundreds of lipid species obtained by mass spectrometry expands the possibilities for a detailed investigation of the pathogenesis of various diseases associated with menopause and the identification of therapeutic targets.
References
- El Khoudary S.R., Aggarwal B., Beckie T.M., Hodis H.N., Johnson A.E., Langer R.D. et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: A Scientific Statement from the American Heart Association. Circulation. 2020; 142(25): 506-32. https://dx.doi.org/10.1161/CIR.0000000000000912.
- Юренева С.В., Комедина В.И., Кузнецов С.Ю. Диагностические возможности антропометрических показателей для оценки ожирения у женщин в период менопаузального перехода. Акушерство и гинекология. 2022; 2: 72-9. [Yureneva S.V., Komedina V.I., Kuznetsov S.Yu. Diagnostic accuracy of anthropometric measures for assessing obesity in women during the menopausal transition. Akusherstvo i Ginekologiia/Obstetrics and Gynecology. 2022; 2: 72-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.2.72-79.
- Thurston R.C., Karvonen-Gutierrez C.A., Derby C.A., El Khoudary S.R., Kravitz H.M., Manson J.E. Menopause versus chronologic aging: their roles in women’s health. Menopause. 2018; 25(8): 849-54. https://doi.org/10.1097/GME.0000000000001143.
- Юренева С.В., Комедина В.И., Чаговец В.В., Стародубцева Н.Л. Роль липидов, определяемых методом масс-спектрометрии, в развитии кардиометаболических заболеваний у женщин в период менопаузы. Акушерство и гинекология. 2020; 12: 76-80. [Yureneva S.V., Komedina V.I., Chagovets V.V., Starodubtseva N.L. The role of lipids determined by mass spectrometry in the development of cardiometabolic diseases in women during menopause. Akusherstvo i Ginekologiia/Obstetrics and Gynecology. 2020; 12: 76-80. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.76-80.
- Neeland I.J., Singh S., McGuire D.K., Vega G.L., Roddy T., Reilly D.F. et al. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: the Dallas Heart Study. Diabetologia. 2018; 61(12): 2570-9. https://dx.doi.org/10.1007/s00125-018-4720-1.
- Gui Y.-K., Li Q., Liu L., Zeng P., Ren R.F., Guo Z.F. et al. Plasma levels of ceramides relate to ischemic stroke risk and clinical severity. Brain Res. Bull. 2020; 158: 122-7. https://dx.doi.org/10.1016/J.BRAINRESBULL.2020.03.009.
- Van der Veen J.N., Kennelly J.P., Wan S., Vance J.E., Vance D.E., Jacobs R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017; 1859 (9, Pt B): 1558-72. https://dx.doi.org/10.1016/j.bbamem.2017.04.006.
- Messina C., Albano D., Gitto S., Tofanelli L., Bazzocchi A., Ulivieri F.M. et al. Body composition with dual energy X-ray absorptiometry: From basics to new tools. Quant. Imaging Med. Surg. 2020; 10(8): 1687-98. https://dx.doi.org/10.21037/QIMS.2020.03.02.
- Tang Q., Li X., Song P., Xu L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: developments in research and prospects for the future. Drug Discov. Ther. 2015; 9(6): 380-5. https://dx.doi.org/10.5582/ddt.2015.01207.
- Bovolini A., Garcia J., Andrade M.A., Duarte J.A. Metabolic syndrome pathophysiology and redisposing factors. Int. J. Sports Med. 2021; 42(3):199-214. https://dx.doi.org/10.1055/a-1263-0898.
- Choi R.H., Tatum S.M., Symons J.D., Summers S.A., Holland W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nature reviews. Cardiology. 2021;18(10): 701-11. https://dx.doi.org/10.1038/s41569-021-00536-1.
- Mantovani A., Bonapace S., Lunardi G., Canali G., Dugo C., Vinco G. et al. Associations between specific plasma ceramides and severity of coronary-artery stenosis assessed by coronary angiography. Diabet. Metab. 2020; 46(2): 150-7. https://dx.doi.org/10.1016/j.diabet.2019.07.006.
- Fretts A.M., Jensen P.N., Hoofnagle A., McKnight B., Howard B.V., Umans J. et al. Plasma ceramide species are associated with diabetes risk in participants of the Strong Heart Study. J. Nutr. 2020; 150(5): 1214-22. https://dx.doi.org/10.1093/jn/nxz259.
- Wigger L., Cruciani-Guglielmacci C., Nicolas A., Denom J., Fernandez N., Fumeron F. et al. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 2017; 18(9): 2269-79. https://dx.doi.org/10.1016/j.celrep.2017.02.019.
- Im S.-S., Park H.Y., Shon J.C., Chung I.-S., Cho H.C., Liu K.-H. et al. Plasma sphingomyelins increase in pre-diabetic Korean men with abdominal obesity. PLoS ONE. 2019; 14: e0213285. https://dx.doi.org/10.1371/journal.pone.0213285.
- Kikas P., Chalikias G. Cardiovascular implications of sphingomyelin presence in biological membranes. Eur. Cardiol. Rev. 2018; 13: 42. https://dx.doi.org/10.15420/ecr.2017:20:3.
- Bochkov V., Gesslbauer B., Mauerhofer C., Philippova M., Erne P., Oskolkova O.V. Pleiotropic effects of oxidized phospholipids. Free Rad. Biol. Med. 2017; 111: 6-24. https://dx.doi.org/10.1016/j.freeradbiomed.2016.12.034.
- López-López Á., Godzien J., Soldevilla B., Gradillas A., López-Gonzálvez Á., Lens-Pardo A. et al. Oxidized lipids in the metabolic profiling of neuroendocrine tumors – Analytical challenges and biological implications. J. Chromatogr. A. 2020; 1625: 461233. https://dx.doi.org/10.1016/j.chroma.2020.461233.
- Nie J., Yang J., Wei Y., Wei X. The role of oxidized phospholipids in the development of disease. Mol. Aspects Med. 2020; 76: 100909. https://dx.doi.org/10.1016/J.MAM.2020.100909.
- Furse S., de Kroon A.I.P.M. Phosphatidylcholine’s functions beyond that of a membrane brick. Mol. Membr. Biol. 2015; 32(4): 117-9. https://dx.doi.org/10.3109/09687688.2015.1066894.
- Calzada E., Onguka O., Claypool S.M. Phosphatidylethanolamine metabolism in health and disease. Int. Rev. Cell Mol. Biol. 2016; 321: 29-88. https://dx.doi.org/10.1016/bs.ircmb.2015.10.001.
- Llano D.A., Devanarayan V. Serum phosphatidylethanolamine and lysophosphatidylethanolamine levels differentiate Alzheimer’s disease from controls and predict progression from mild cognitive impairment. J. Alzheimers Dis. 2021; 80(1): 311-9. https://dx.doi.org/10.3233/JAD-201420.
Received 07.04.2022
Accepted 26.05.2022
About the Authors
Veronika I. Komedina, Ph.D. Student at the Department of Gynecologic Endocrinology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, komedina.veronika@gmail.com, https://orcid.org/0000-0002-9084-5044,117997, Russian Federation, Moscow, Oparin str., 4.
Svetlana V. Yureneva, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Department of Vocational Education, Leading Researcher at the Department of Gynecologic Endocrinology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, syureneva@gmail.com, 117997, Russian Federation, Moscow, Oparin str., 4.
Vitaliy V. Chagovets, Ph.D., Senior Researcher, Laboratory of Proteomics and Metabolomics of Human Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, vvchagovets@gmail.com, https://orcid.org/0000-0002-5120-376X, 117997, Russian Federation, Moscow, Oparin str., 4.
Natalia L. Starodubtseva, Ph.D, Head of the Laboratory of Proteomics and Metabolomics of Human Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, n_starodubtseva@oparina4.ru,
https://orcid.org/0000-0001-6650-5915,117997, Russian Federation, Moscow, Oparin str., 4.
Corresponding author: Veronika I. Komedina, komedina.veronika@gmail.com
Authors' contributions: Yureneva S.V., Chagovets V.V., Starodubtseva N.L. – conception and design of the study, research material analysis; Komedina V.I. – collection and analysis of research material, statistical analysis, manuscript drafting; Chagovets V.V. – conducting HPLC-MS studies, bioinformatic analysis; Yureneva S.V., Chagovets V.V. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Komedina V.I., Yureneva S.V., Chagovets V.V., Starodubtseva N.L.
Changes in serum lipid profile during the menopausal transition.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 6: 90-97 (in Russian)
https://dx.doi.org/10.18565/aig.2022.6.90-97



