Differential diagnosis of serous ovarian tumors using mass spectrometry-based serum lipid profiling: a pilot study
Background: Due to lacking effective methods for early diagnosis, ovarian cancer (OC) is the leading cause of death from gynecologic malignancies.Iurova M.V., Chagovets V.V., Frankevich V.E., Starodubtseva N.L., Khabas G.N., Pavlovich S.V.
Aim: To make a differential diagnosis of ovarian serous malignant and borderline tumors and investigate changes in serum lipidome after treatment using high-performance liquid chromatography with mass spectrometry (HPLC-MS).
Materials and methods: The study included 53 patients with high-grade serous ovarian carcinoma (HGSOC) and serous borderline tumors (SBT) who underwent surgery at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia and 10 patients in control groups. Lipids were extracted from the serum using a modified Folch method and analyzed by HPLC-MS. Statistical analysis was performed by the OPLS multivariate analysis. The statistical significance of between-group differences was tested with a nonparametric Mann-Whitney test with Benjamini–Hochberg correction.
Results: Based on statistically significant differences in serum lipid profiles (phosphatidylcholines, glycerolipids, etc.), differential diagnostic models were developed clustering patients with early and advanced stages of HGSOC, SBT, and control groups (model parameters: R2≥0.5, Q2≥0.4). No systemic changes in the lipidome were found after non-radical surgery. Changes in the lipid profiles were observed after NACT. An increase in serum lysophosphatidylcholine derivatives [OxLPC(22:2(OO))] and glycerophospholipids [PEtOH(20:1_20:1)-H] was proportional to the likelihood of HGSOC recurrence or progression within a year after combined treatment.
Conclusion: The study findings suggest that mass spectrometry-based serum lipid profiling can improve serous ovarian tumors' identification and differential diagnosis in various clinical situations.
Keywords
Ovarian cancer (OC) is the most lethal of all gynecological malignancies (20.9 per 100,000 population) [1]. It has been estimated that up to 70% of patients with ovarian neoplasms have high-grade serous histotypes [2]. The active detection of ovarian cancer has increased over ten years from 9.2 to 19.1 per 100,000 population [3, 4]. However, in 2018, only 37.4% and 62.5% of patients underwent radical surgery and combination therapy, respectively. [3]. According to various sources, from 3 to 14% of patients diagnosed with malignant ovarian neoplasms are women of reproductive age, and more than 80% of them have advanced-stage disease [5, 6]. While population screening is not economically feasible, identifying earlier stages of the disease (stages I-II according to the international classification of the International Federation of Gynecology and Obstetrics – FIGO) is associated with better outcomes. However, because of significant difficulties due to the asymptomatic or low symptom levels, ovarian cancer is diagnosed at late stages. The survival rate of these patients does not exceed 44%, which is almost twice lower than 90% five-year survival after radical treatment of the disease detected at an early stage [1, 7, 8]. Unfortunately, there are no adequate methods for high-precision early and preoperative differential diagnosis of ovarian cancer to date. Thus, there is a great need for developing new and improve existing non-invasive diagnostic modalities.
Modern analytical methods, such as high-performance liquid chromatography with mass spectrometric detection (HPLC-MS), provide detailed information on the molecular composition of biological samples, which can be used to detect a tumor and assess its malignancy during the preoperative period.
The processes associated with epithelial ovarian carcinogenesis lead to changes in the metabolome, including blood lipid profile [1, 9]. The most studied are lysophospholipids (lysophosphatidylethanolamine, lysophosphatidylinositol) [1].
Several authors have shown the relationship between the molecular mechanisms of carcinogenesis and changes in lipidome, which may have a role in the diagnosis and prediction of high-grade ovarian cancer course and outcomes. The tumor suppressor gene p56 is mutated in>95% of high-grade serous ovarian cancers [10]. It has recently been reported that the protein encoded by p53 interacts with sterol regulatory element-binding proteins (SREBPs) and guanidinoacetate N-methyltransferase (GAMT), resulting in the elevated expression of enzymes involved in fatty acid and cholesterol biosynthesis and the inhibition of fatty acid oxidation leading to lipid anabolism and accelerated tumor growth and progression [11, 12].
HPLC-MS analysis of blood plasma lipidome may be used for the diagnosis [13] and allows for monitoring treatment effectiveness [9]. It may be used to predict the risks of disease recurrence and progression at the preoperative stage since 25% of all patients are platinum-refractory [14].
The present study aimed to make a differential diagnosis of ovarian serous malignant and borderline tumors and investigate changes in serum lipidome after treatment using high-performance liquid chromatography with mass spectrometry.
Materials and methods
The study included 53 patients with high-grade serous ovarian carcinoma (HGSOC) and serous borderline tumors (SBT) who underwent surgery at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia and ten patients in the control group. The diagnostic group consisted of without prior pelvic surgery (n =33), the monitoring group consisted of patients after three courses of paclitaxel + carboplatin neoadjuvant chemotherapy (NACT, n=7). This group also included 13 patients with prior non-radical surgical treatment (biopsy or resection of the ovary, organ-sparing surgery to preserve reproductive potential in the absence of verification of the malignant process, refusing to get a solitary ovary removed), who were referred for a repeat surgical intervention (Fig. 1).
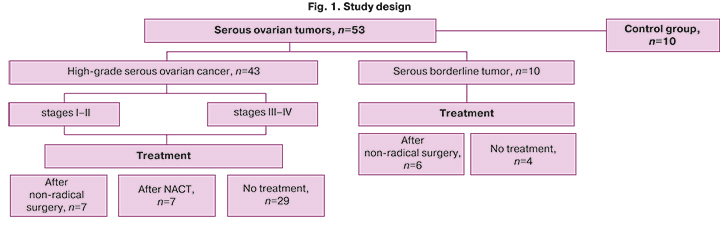
Exclusion criteria were primary multiple neoplastic processes, endometriotic serous ovarian tumors other than borderline and high-grade tumors (clear cell, germ cell, hepatoid, mucinous and benign tumors, low-grade tumors), fallopian tube cancer or primary peritoneal cancer, the presence of a BRCA mutation, comorbidities (diabetes mellitus, acute or chronic infectious and inflammatory process), pregnancy at the time of blood sampling. The control group included women of reproductive age without comorbidities.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P. Before enrollment, all participants provided signed informed consent.
Lipids were extracted from the serum using a modified Folch method [15]. The molecular composition of the samples was determined by HPLC-MS on a Dionex UltiMate 3000 liquid chromatograph (Thermo Scientific, Germany) connected to a Maxis Impact qTOF mass analyzer (Bruker Daltonics, Germany). Separation was carried out by reversed-phase chromatography on a Zorbax C18 column (150x2.1 mm, 5 μm, Agilent, USA). A linear gradient was used from 30 to 90% eluent B (acetonitrile/isopropanol/water solution, 90/8/2 v/v/v, with the addition of 0.1% formic acid and 10 mmol/L ammonium formate) for 20 minutes. As eluent A, an acetonitrile/water solution (60/40, v/v) was used with the addition of 0.1% formic acid and 10 mmol/L ammonium formate. The elution flow rate was 35 μl/min, and 3 μl of the sample was injected for analysis. Mass spectra were obtained in the positive ion mode in the m/z range 100–1700 with the following settings: capillary voltage 4.1 kV, atomizing gas pressure 0.7 bar, drying gas flow rate 6 l/min, drying gas temperature 200oC. Tandem mass spectrometry was performed in a dependent scan mode with a window width of 5 Da to identify lipids.
The original files obtained as a result of HPLC-MS analysis were converted using the msConvert program from the Proteowizard 3.0.9987 package [16] into the open MzXml format, containing information on the mass spectrum at any time, and into the ms2 format, including information on tandem mass spectra at a given point in time. We also used the MzMine program [17] to isolate peaks, normalize the total ion current, and create a table containing information on ion mass, the area of its chromatographic peak, and the release time. Lipids were identified using LipidMatch scripts [18]. The lipid nomenclature corresponds to LipidMaps [19].
Statistical analysis
Statistical analysis was performed using scripts written in the R language version 3.3.3 [20] and the RStudio 1.383 [21].
The multivariate mass spectrometric data analysis was performed by the orthogonal partial least squares discriminant analysis (OPLS-DA) [22] using the "ropls" library [23]. OPLS-DA is a modification of the principal component analysis designed for the creation of trained models. With the help of OPLS-DA, models were created to classify samples according to a particular group. Identified lipids were considered as independent variables in the models. The patient's allocation to one of the study groups was used as a dependent variable. The quality of the developed models was determined by constructing the ROC curve, determining the area under the ROC curve, and calculating the sensitivity and specificity. The quality of OPLS-DA models was also assessed by their ability to describe the variance of the analyzed data (R2) and predict possible new data (Q2). The Q2 parameter was calculated by 7-fold cross-validation. At Q2≥0.4, the model can assign the analyzed samples to a particular clinical subgroup. In addition, OPLS-DA was used to identify lipids that are most relevant for classification. This was done by analyzing the variable importance in the projection (VIP). Lipids with VIP> 1 were classified as potential lipid markers [24]. The statistical significance of between-group differences in lipid levels was tested with Mann–Whitney test with the Benjamini–Hochberg correction. Quantitative variables are presented as the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format. The critical level of significance when interpreting the results of statistical analysis was considered at p<0.05.
Analysis results were visualized using scatter plots for a large dataset (volcano diagram), a type of scatterplot that shows statistical significance versus magnitude of change (fold change) for differences in lipid levels in groups, as well as using a score graph to represent the quality of OPLS-DA models and their ability to group samples by analyzed clinical signs. The points on the scatter plots correspond to individual lipids. The y-axis is the negative decimal logarithm of the p-value with Benjamin-Hochberg correction. The x-axis is the base 2 logarithm of the fold change in the level of the corresponding lipid between groups (log2 FC).
Results
Blood serum samples were taken from 63 patients, in which a total of 345 lipids were identified.
I. Malignant and borderline serous ovarian tumors
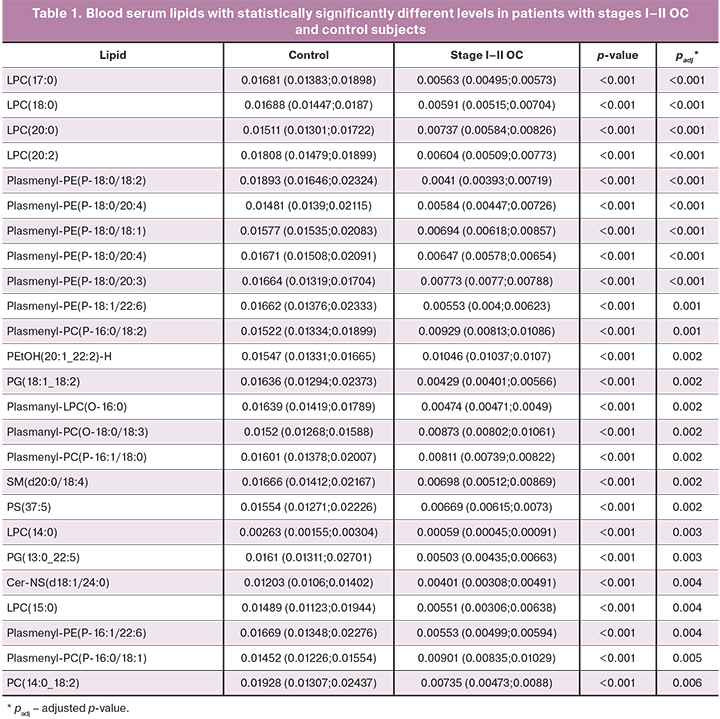
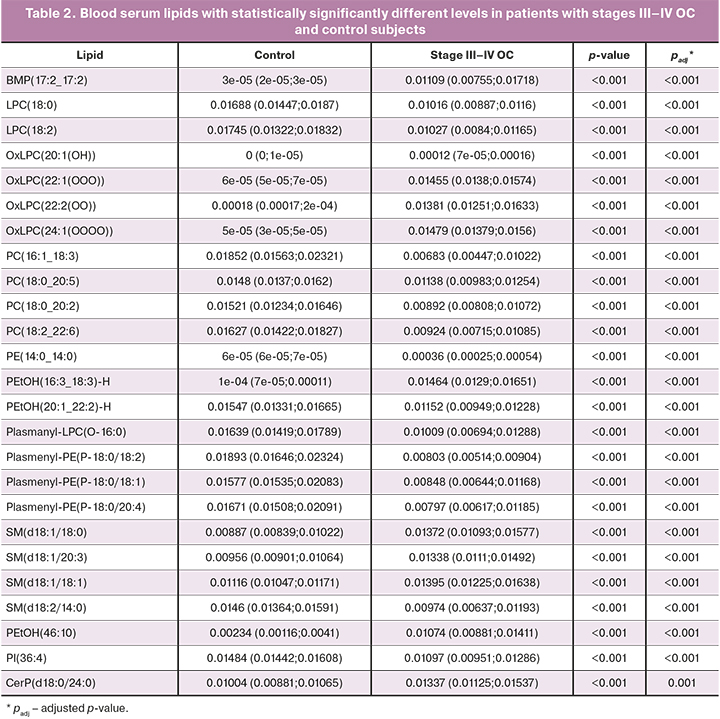
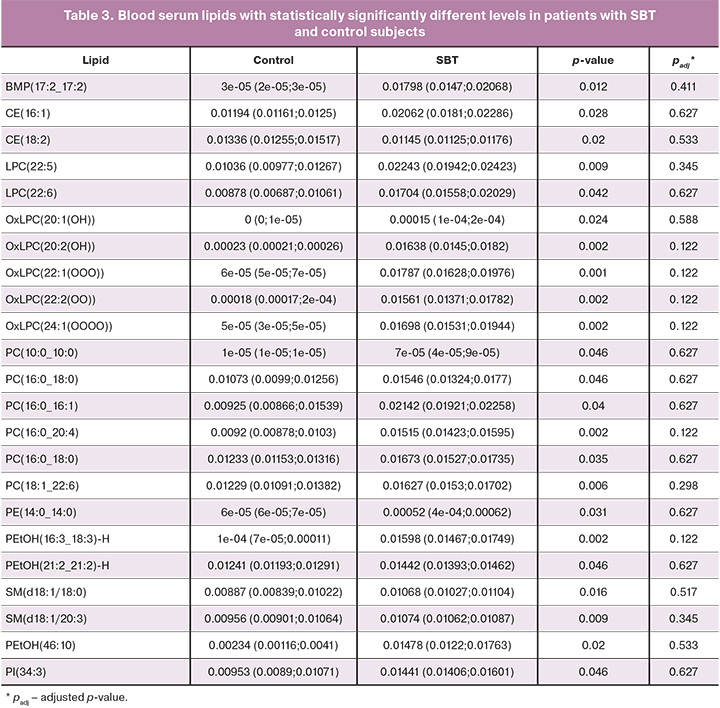
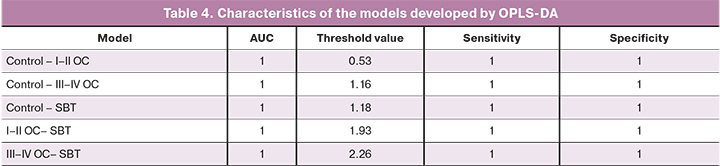
There were no statistically significant differences in blood serum lipid profiles of patients without prior surgery and chemotherapy and early (I–II, n=5) and advanced (III–IV, n=23) stages of ovarian cancer and borderline tumors (n=4), as well as patients of the control group (n=13), for which (Tables 1–3) discriminant OPLS models were developed (Table 4).
Serum levels of 103 lipids in samples from patients with early stages of the disease were 2–4 times statistically significantly lower than that in control patients (Fig. 2).
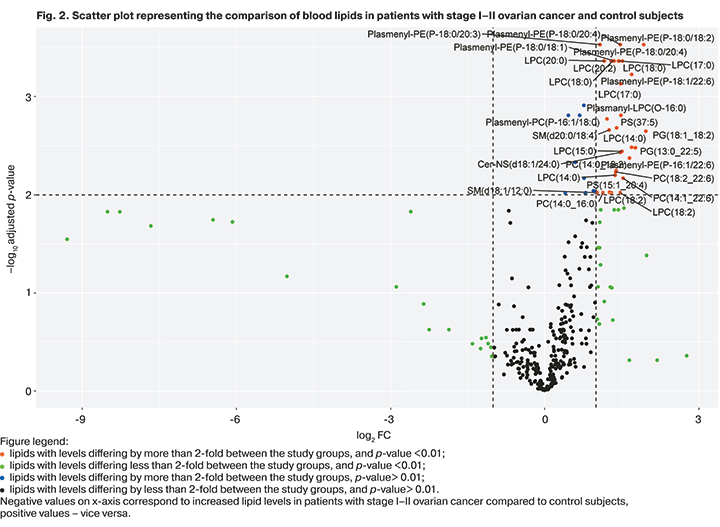
As a result of OPLS of mass spectrometric profiling in the positive ion mode, 108 out of 345 identified lipids were the most significant, making it possible to cluster the analyzed clinical groups (model characteristics: R2=0.97, Q2=0.86).
Blood serum OPLS lipidomics analysis of patients with advanced stages of the disease and patients of the control group also showed statistically significant differences, and clustering of these groups was obtained (model characteristics: R2=0.87, Q2=0.8).
The distribution of characteristic lipids in patients with advanced stages of the disease was as follows: out of 148 statistically significantly altered serum lipids, 40 were increased (approximately 2.0–2.25 times), and 108 decreased (about 2.0–77.4 times).
When comparing the blood lipid profiles of healthy volunteers and patients with borderline ovarian tumors with prior surgery for their ovarian neoplasms (n=4), 109 specific lipids were identified, 25 of which were significant in the construction of models. At the same time, 23 of 25 were statistically significantly increased by about 32.5–64 times, 2 of 25 were decreased (Fig. 3).
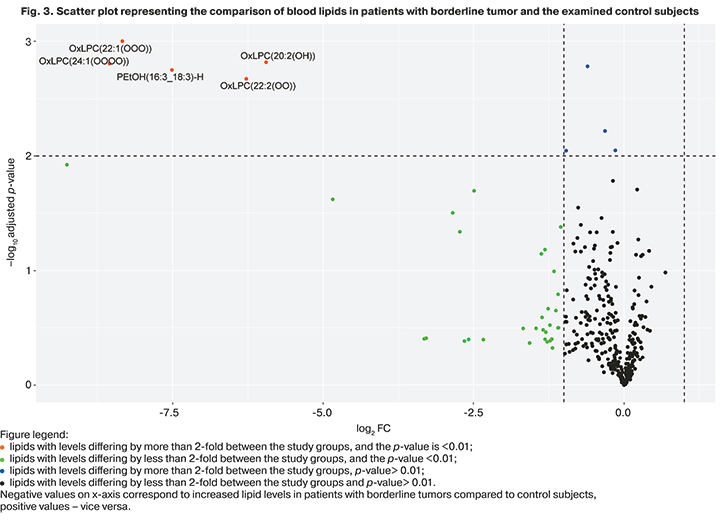
Despite the differences between the groups identified using the Mann–Whitney test, the parameters of the OPLS model did not reach the classification significance (R2=0.91, Q2=0.36), which may be due to the small sample of patients who made up this group.
The blood profile of patients with borderline tumors differed from that of high-grade serous ovarian cancer, both early and advanced.
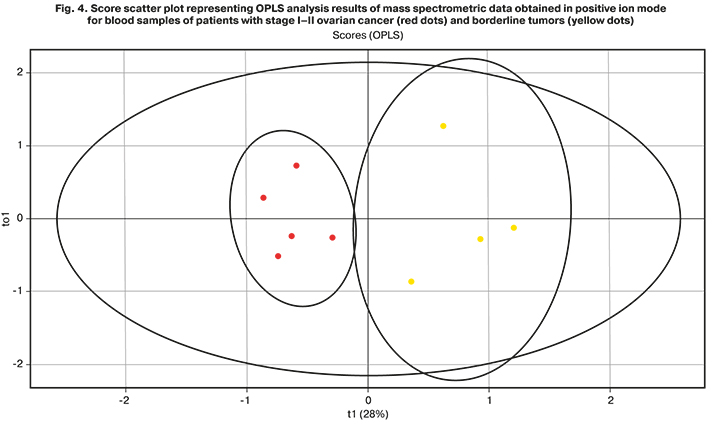
Pairwise comparison of lipid profiles of patients with stages I–II ovarian cancer and borderline tumors allowed for obtaining data on the accuracy of the constructed model (R2=0.88, Q2=0.66; Fig. 4). The most significant contribution to creating the model was made by 111 lipids, and in borderline tumors, a significant increase in 52 lipids was noted.
The model characterizing the differences between patients with late III–IV stages of ovarian cancer and borderline tumors (R2=0.79, Q2=0.27) was built based on 114 lipids. A significant increase in 41 lipids and a decrease in 7 were recorded.
Despite the differences between the groups identified using the Mann–Whitney test, the accuracy of the OPLS model did not reach the classification significance, which may be due to the small sample of patients with borderline ovarian tumors, as well as the prevalence of stage III disease among them (in three out of five patients).
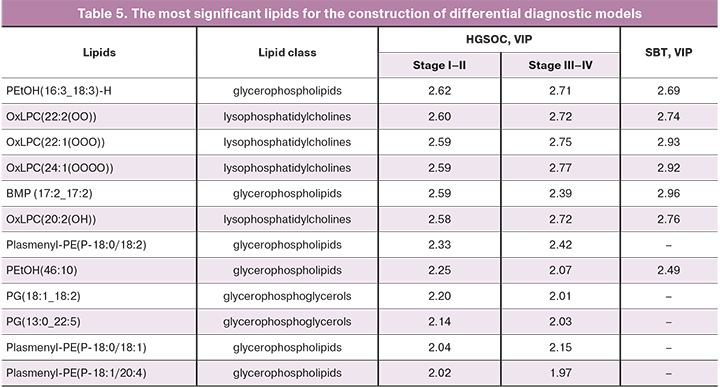
Patients with high-grade I–IV serous ovarian tumors had maximum VIP values for 12 lipids, 7 of which were necessary to build models that differentiate serum samples from patients with borderline tumors from the control group (Table 5).
II. Lipidome changes during treatment
1. Blood samples were examined from patients who underwent primary and repeat (after non-radical surgery) surgical treatment for HGSOC (n=29 and n=7, respectively) and SBT (n=4 and n=6, respectively).
The OPLS analysis showed no differences between the groups of surgical treatment.
The absence of significant changes in the blood lipidome after non-radical surgery for HGSOC and SBT can be explained by the short time between the first and second surgery (on average less than three months).
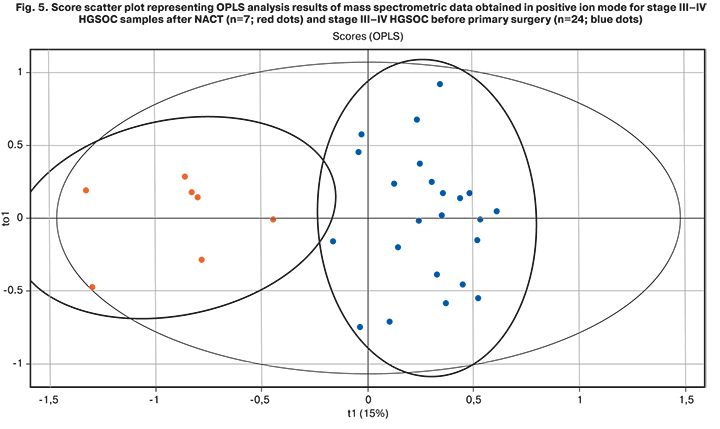
2. Blood samples from patients with stage III–IV HGSOC, who underwent three courses of NACT before surgery, were examined. The lipid profile of these samples (n=7) was compared with the profile of patients with stage III–IV HGSOC who underwent surgical treatment at the first stage (n=24). The OPLS analysis showed statistically significant differences between blood serum lipid profiles of patients with stages III-IV HGSOC after NACT and patients in the primary surgical treatment group (R2>0.5, Q2=0.4; Fig. 5).
3. After 12 months of follow-up of patients with stages IIb–IVb HGSOC who underwent combination therapy (surgery + chemotherapy), early outcomes were recorded: stabilization of the disease was achieved in 16 patients, and nine patients had a relapse or disease progression.
A statistical model was created to assess changes in the blood lipidome before surgery and the registered outcome. The resulting model is characterized by a low coefficient of estimated parameters (R2=0.62, Q2= -0.49).
A targeted examination of lipids, which demonstrated the statistical significance of differences in the analyzed groups (n=122), provided data on the increase in OxLPC (22: 2 (OO)) and PEtOH (20:1_20:1) -H in the group of patients with adverse outcomes.
It can be noted that there was a tendency to the disease recurrence in patients with HGSOC with increased levels of blood lipids. The possibility of predicting the clinical treatment outcome by changes in the serum lipidome detected before treatment initiation might be a promising preventive target requiring further research.
Discussion
HPLC-MS analysis of lipid extracts enabled comparison of the relative values of different lipid classes, including phosphatidylcholines, phosphatidylethanolamines, ceramides, di- and triglycerides, etc. OPLS-DA analysis of the obtained mass spectrometric data revealed differences in blood serum lipid profiles in the study groups.
The study included patients without comorbidities requiring long-term pharmacotherapy or tumors at the time of the research or earlier in the anamnesis. According to several authors [9], each oncological process is accompanied by a specific characteristic change in the lipid profile. Therefore, it was impossible to study the combinations of a heterogeneous "chimeric" set, especially when working with small samples of patients. Due to the presence of confounders, 27 patients were not included in conducted research.
The first hypothesis of this study was the assumption that there are differences in the serum lipid profile of healthy volunteers and patients with borderline tumors and different stages of HGSOC. This statement has been confirmed in many studies. M.F. Buas et al. reported a case-control study analyzing 50 blood plasma samples from patients who underwent surgical treatment for serous cancer or benign serous ovarian tumors (control group). Samples were examined using HPLC-MS. It was found that 34 of the 372 metabolites detected were statistically significantly different between the groups. The authors concluded that these differences in the metabolic profile could improve the accuracy of differential diagnosis of benign and malignant ovarian diseases [25].
R.J. Niemi et al. studied lipidome in early stages of serous ovarian cancer (n=138), borderline (n = 25) and benign (n = 191, control group) ovarian tumors. They analyzed 354 plasma and serum samples of pre-and postmenopausal age women. Changes in 39 lipids were identical regardless of the stage of the disease (early or late) and the age of the patients. Moreover, using 23 lipids as an example, a similar tendency of changes was shown when comparing fluids in tumors of different localizations and histological types [26]. The obtained data indicate some uniformity of changes. Still, at the same time, the authors suggested that lipidome analysis increases the diagnostic value of testing for CA-125 in early stages of ovarian cancer and also does not exclude the possibility of lipids acting as a metabolically active therapeutic target, which, however, needs further research. In the presented study, the blood serum of women of reproductive age was analyzed. In the group of patients with stages I–II, the sensitivity of determining the ROMA index reached 77.5%. For patients with III–IV stages of ovarian cancer it was 99.9% (ROMA=83.36%; CA-125=856.43 U/ml, HE4=398.42 pmol/l). The high informativeness of the created statistical models confirms the possibility of using the discovered list of lipids to increase the sensitivity and specificity of routine non-invasive preoperative diagnostics.
It's also crucial to identify patients with advanced serous ovarian cancer, accounting for more than 70% of all ovarian cancers. P. Knapp et al. investigated blood plasma concentrations of sphingolipids (C16-Cer, C18: 1-Cer and C18-Cer) in 74 patients with stage III–IV high-grade ovarian cancer using HPLC-MS. When the established thresholds were exceeded, the probability of a malignant tumor type was higher (C16-Cer>311.88 ng/100 μL (AUC: 0.76, p=0.0261); C18: 1-Cer>4.75 ng/100 μl (AUC: 0.77, p=0.0160) and C18-Cer>100.76 ng/100 μl (AUC: 0.77, p=0.0136)) than in the control group. A similar trend was observed in the study of tumor tissue samples: 5 lipids were significantly increased in the study group samples (C16-Cer, C18: 1-Cer, C18-Cer, C24: 1-Cer, C24-Cer). In contrast to our study, the study by P. Knapp et al. included only patients with advanced-stage cancers, which may be a limitation in the differential diagnosis [27].
Our study presents data comparing blood lipid profiles of patients with borderline ovarian tumors and patients with HGSOC and healthy volunteers. Considering the small sample in this fragment of the study, as well as the contradictory results of statistical analysis (the samples go beyond the Hotteling ellipses on the visual diagram, the indicators of the estimated parameters (R2=0.91, Q2=0.36)) only allow us to outline trends in lipid metabolism in the presence of a borderline ovarian tumor. This can potentially affect the quality of preoperative diagnosis of this special group of patients. At the time of diagnosis, most women were of reproductive age (according to the STRAW + 10 criteria) and were interested in maintaining fertility. Accordingly, in each case, the strategy and feasibility of organ-sparing surgery should be agreed upon with the patient at the preoperative planning stage. To make an informed decision, it is also necessary to discuss actively developing assisted reproductive technologies [28]. Along with this, the improvement of diagnostic accuracy at the preoperative stage requires further research.
The assumption about the possibility of identifying predictors of adverse HGSOC outcome was studied using the example of patients who received combination therapy and were followed at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia within a year after treatment completion. Despite the absence of significant differences during OPLS analysis, data on substantial changes in several lipids were obtained. In the future, it is planned to study their value as biomarkers.
Conclusion
The presented study investigated changes in serum lipidome by the HPLC-MS in various clinical situations associated with ovarian serous malignant and borderline tumors.
Blood serum lipid profiles of patients with early and advanced ovarian cancer were statistically significantly different from patients with borderline tumors and the control subjects. The greatest significance in the construction of differential diagnostic models, clustering patients with varying stages of HGSOC and SBT, was noted for phosphatidylcholines and glycerolipids. These data can improve the preoperative differential diagnosis of these two types of serous ovarian tumors.
There were no statistically significant differences in systemic lipidome between patients undergoing non-radical surgery for ovarian tumors (HGSOC and SBT) and that of patients without prior surgery. This may be due to a short period of time between two surgical interventions or actual lack of effect.
Differences in lipid profiles were observed between patients with advanced stages of HGSOC after NACT and patients who underwent cytoreductive surgery at the first stage, indicating the effect of chemotherapy on changes in blood lipidome. In the future, it is planned to conduct a similar study on related samples of patients.
To investigate the predictive value of changes in the lipid profile, the outcomes of patients were analyzed within one year after completing combination therapy. The increase in serum derivatives of lysophosphatidylcholines [OxLPC(22:2(OO))] and glycerophospholipids [PEtOH(20:1_20:1)-H] was proportional to the likelihood of adverse early cancer outcomes (relapse or progression of the disease).
Therefore, the presented data indicate the clinical and scientific value of lipidome research for improving the diagnosis and management of patients with malignant neoplasms of the ovaries.
The study findings suggest that serum lipid profiling can improve differential diagnosis and management of patients with serous ovarian tumors.
Limitations
In several cases, differences between the study groups were detected using the Wilcoxon rank-sum test in small patient samples, and the estimated parameters of the OPLS multivariate analysis did not reach the threshold values. In these cases, it is necessary to conduct sufficiently powered studies with larger samples.
References
- Gaul D.A., Mezencev R., Long T.Q., Jones C.M., Benigno B.B., Gray A. et al. Highly-accurate metabolomic detection of early-stage ovarian cancer. Sci. Rep. 2015; 5: 16351. https://dx.doi.org/10.1038/srep16351.
- Amoroso M.R., Matassa D.S., Agliarulo I., Avolio R., Maddalena F., Condelli V. et al. Stress-adaptive response in ovarian cancer drug resistance: Role of TRAP1 in oxidative metabolism-driven inflammation. Adv. Protein Chem. Struct. Biol. 2017; 108: 163-98. https://dx.doi.org/10.1016/bs.apcsb.2017.01.004.
- Каприн А.Д., Старинский В.В., Шахзадова А.О., ред. Состояние онкологической помощи населению России в 2019 году. М.: МНИОИ им. П.А. Герцена − филиал ФГБУ «НМИЦ радиологии» Минздрава России; 2020. 239с. [Kaprin A.D., Starinskiy V.V., Shakhzadova A.O., ed. The state of cancer care for the population of Russia in 2019. M.; 2020. 239 p. (in Russian)].
- Ueland F.R., Li A.J. Serum biomarkers for evaluation of an adnexal mass for epithelial carcinoma of the ovary, fallopian tube, or peritoneum. URL: http://www.uptodate.com/contents/serum-biomarkers-for-evaluation-of-an-adnexal-mass-for-epithelial-carcinoma-of-the-ovary-fallopian-tube-or-peritoneum.
- Tomao F., Di Pinto A., Sassu C.M., Bardhi E., Di Donato V., Muzii L. et al. Fertility preservation in ovarian tumours. Ecancermedicalscience. 2018; 12: 885. https://dx.doi.org/10.3332/ecancer.2018.885.
- Ashraf M.A., Dasari P. Outcome of fertility-preserving surgery for ovarian malignancy in young women. Case Rep. 2018; 1(1): 51-4.
- Bekelman D.B., Hooker S., Nowels C.T., Main D.S., Meek P., McBryde C. et al. Feasibility and acceptability of a collaborative care intervention to improve symptoms and quality of life in chronic heart failure: mixed methods pilot trial. J. Palliat. Med. 2014; 17(2): 145-51.
- Warren L.A., Shih A., Renteira S.M., Seckin T., Blau B., Simpfendorfer K. et al. Analysis of menstrual effluent: Diagnostic potential for endometriosis. Mol. Med. 2018; 24(1): 1-12.
- Ghahremanfard F., Mirmohammadkhani M., Shahnazari B., Gholami G., Mehdizadeh J. The valuable role of measuring serum lipid profile in cancer progression. Oman Med. J. 2015; 30(5): 353-7 https://dx.doi.org/10.5001/omj.2015.71.
- Havrilesky L., Darcy K.M., Hamdan H., Priore R.L., Leon J., Bell J. et al. Prognostic significance of p53 mutation and p53 overexpression in advanced epithelial ovarian cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2003; 21(20): 3814-25. https://dx.doi.org/10.1200/JCO.2003.11.052.
- Cha Y.J., Koo J.S. Roles of omental and bone marrow adipocytes in tumor biology. Adipocyte. 2019; 8(1): 304-17.
- Hu J., Liu Z., Wang X. Does TP53 mutation promote ovarian cancer metastasis to omentum by regulating lipid metabolism? Med. Hypotheses. 2013; 81(4): 515-20. https://dx.doi.org/10.1016/j.mehy.2013.06.009.
- Jelonek K., Roś M., Pietrowska M., Widlak P. Cancer biomarkers and mass spectrometry-based analyses of phospholipids in body fluids. Clin. Lipidol. 2013; 8(1): 137-50. https://dx.doi.org/10.2217/clp.12.79.
- Li J., Xie H., Li A., Cheng J., Yang K., Wang J. et al. Distinct plasma lipids profiles of recurrent ovarian cancer by liquid chromatography-mass spectrometry. Oncotarget. 2017; 8(29): 46834-45. https://dx.doi.org/10.18632/oncotarget.11603.
- Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957; 226(1): 497-509.
- Chambers M.C., Maclean B., Burke R., Amodei D., Ruderman D.L., Neumann S. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012; 30(10): 918-20. https://dx.doi.org/10.1038/nbt.2377.
- Pluskal T., Castillo S., Villar-Briones A., Oresic M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 2010; 11: 395. https://dx.doi.org/10.1186/1471-2105-11-395.
- Koelmel J.P., Kroeger N.M., Ulmer C.Z., Bowden J.A., Patterson R.E., Cochran J.A. et al. LipidMatch: An automated workflow for rule-based lipid identification using untargeted high-resolution tandem mass spectrometry data. BMC Bioinformatics. 2017; 18(1): 331. https://dx.doi.org/10.1186/s12859-017-1744-3.
- Sud M., Fahy E., Cotter D., Brown A., Dennis E.A., Glass C.K. et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007; 35(Database issue): D527-32. https://dx.doi.org/10.1093/nar/gkl838.
- R : A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.
- R team. R Studio: Integrated Development for R. Boston, MA: RStudio, Inc.; 2016.
- Trygg J., Wold S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002; 16(3): 119-28.
- Thevenot E.A., Roux A., Xu Y., Ezan E., Junot C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015; 14(8): 3322-35. https://dx.doi.org/10.1021/acs.jproteome.5b00354.
- Wold S., Sjöström M., Eriksson L. PLS-regression: a basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001; 58(2): 109-30.
- Buas M.F., Gu H., Djukovic D., Zhu J., Drescher C.W., Urban N. et al. Identification of novel candidate plasma metabolite biomarkers for distinguishing serous ovarian carcinoma and benign serous ovarian tumors. Gynecol. Oncol. 2016; 140(1): 138-44. https://dx.doi.org/10.1016/j.ygyno.2015.10.021.
- Niemi R.J., Braicu E.I., Kulbe H,. Koistinen K.M., Sehouli J., Puistola U. et al. Ovarian tumours of different histologic type and clinical stage induce similar changes in lipid metabolism. Br. J. Cancer. 2018; 119(7): 847-54. https://dx.doi.org/10.1038/s41416-018-0270-z.
- Knapp P., Bodnar L., Błachnio-Zabielska A., Świderska M., Chabowski A. Plasma and ovarian tissue sphingolipids profiling in patients with advanced ovarian cancer. Gynecol. Oncol. 2017; 147(1): 139-44. https://dx.doi.org/10.1016/j.ygyno.2017.07.143.
- Kedem A., Yerushalmi G.M., Brengauz M., Raanani H., Orvieto R., Hourvitz A., Meirow D. Outcome of immature oocytes collection of 119 cancer patients during ovarian tissue harvesting for fertility preservation. J. Assist. Reprod. Genet. 2018; 35(5): 851-6. https://dx.doi.org/10.1007/s10815-018-1153-1.
Received 19.02.2021
Accepted 29.07.2021
About the Authors
Mariia V. Iurova, Specialist, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Ph.D. Student at the Chair of Obstetrics, Gynecology, Perinatology and Reproductology, the Faculty of Postgraduate Professional Training of Physicians, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University),+7(495)609-14-00, m_yurova@oparina4.ru, https://orcid.org/0000-0002-0179-7635, 117997, Russia, Moscow, Ac. Oparina, 4.
Vitaliy V. Chagovets, Ph.D., Senior Researcher at the Laboratory of Proteomics and Metabolomics in Human Reproduction, Department of Systems Biology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(926)562-65-90, vvchagovets@gmail.com, https://orcid.org/0000-0002-5120-376X, 117997, Russia, Moscow, Ac. Oparina, 4.
Vladimir E. Frankevich, Ph.D., Head of Department of Systems Biology in Reproduction, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-07-88
ex. 2198, v_frankevich@oparina4.ru, https://orcid.org/0000-0002-9780-4579, 117997, Russia, Moscow, Ac. Oparina, 4.
Nataliia L. Starodubtseva, Ph.D., Head of Laboratory of Proteomics and Metabolomics of Human Reproduction, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)463-98-67, n_starodubtseva@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina, 4.
Grigory N. Khabas, Ph.D., surgeon, oncologist, obstetrician-gynecologist, Head of the Department of Innovative Oncology and Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-78-33, g_khabas@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina, 4.
Stanislav V. Pavlovich, Ph.D., Academic Secretary, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, Faculty of Postgraduate Professional Training of Physicians, I.M. Sechenov First Moscow State Medical University, Ministry of Health
of Russia (Sechenov University), +7(495)438-20-88, s_pavlovich@oparina4.ru, https://orcid.org/0000-0002-1313-7079. 117997, Russia, Moscow, Ac. Oparina, 4.
Authors’ contributions: Iurova M.V. – material collection, creating and maintaining of database, statistical analysis and interpretation of clinical data, design of the study, literature analysis, manuscript drafting and editing, preparing documentation; Chagovets V.V. – design of the study, manuscript editing, mass spectrometric analysis, construction of the OPLS-model, statistical analysis; Frankevich V.E. – design of the study, statistical and bioinformatic analysis; Starodubtseva N.L. – design of the study, preparation of the serum/plasma lipid extract by the modified Folch method, mass spectrometric analysis of samples, preparation of documentation; Khabas G.N. – surgical treatment of patients with oncological pathology, clinical interpretation of the obtained data, manuscript drafting and editing; Pavlovich S.V. – design of the study, manuscript editing, clinical interpretation of the obtained data, preparation of documentation.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was supported by the Russian Science Foundation (RSF) grant «Development of a method for differential diagnosis of ovarian masses by high resolution mass spectrometry», № 20-65-46014.
Acknowledgement: All the materials used in the present study were processed and stored in the biobank of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Iurova M.V., Chagovets V.V., Frankevich V.E., Starodubtseva N.L., Khabas G.N., Pavlovich S.V. Differential diagnosis of serous ovarian tumors using mass spectrometry-based serum lipid profiling: a pilot study.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 9: 107-119 (in Russian)
https://dx.doi.org/10.18565/aig.2021.9.107-119



