Comparative analysis of blood and follicular fluid lipid profiles in women undergoing infertility treatment with assisted reproductive technologies
Fortygina Yu.A., Makarova N.P., Drapkina Yu.S., Novoselova A.V., Gamisonia A.M., Chagovets V.V., Frankevich V.E., Kalinina E.A.
Background: Follicle fluid (FF) composition can significantly affect oocyte development, fertilization, and early embryo cleavage. Therefore, studying the molecular composition of FF can provide valuable insights into the mechanisms and factors that influence oogenesis. Investigating the lipid profile of FF as an additional marker for assessing oocyte quality has shown promising results. However, FF collection is an invasive procedure; therefore, it is important to explore indirect sources of information about FF composition.
Objective: To compare the lipid profiles of blood plasma and FF in women undergoing infertility treatment using assisted reproductive technology (ART).
Materials and methods: The study involved 40 married couples, aged 24–39 years, with a normal body mass index (up to 25 kg/m2), seeking infertility treatment with ART. Patients underwent ovarian stimulation following a protocol with gonadotropin-releasing hormone antagonists (GnRH antagonists). On the day of the puncture, FF and blood plasma were collected and cryopreserved. Liquid chromatography with mass spectrometry was used to determine the molecular compositions of the samples.
Results: The study determined the lipid composition of FF (175 lipids) and blood plasma (185 lipids). Among these molecules, 70 lipids were identical in both FF and blood plasma. Of these, 42 lipids showed a statistically significant correlation. Additionally, when analyzing the correlation between plasma lipid levels and FF from the left and right ovaries, 25 lipids were identified, with plasma levels significantly correlated with FF from the left ovary, and 40 lipids showed a significant correlation with FF from the right ovary. The levels of all 175 identified lipids showed a statistically significant correlation between the left and right ovarian FF.
Conclusion: These findings suggest that the similarities and differences found between blood plasma and FF lipidomes can be used to develop noninvasive methods for assessing oocyte status and predicting the effectiveness of ART. The study results suggest the potential for personalized infertility treatment and preparation for ART programs, as well as a deeper understanding of the mechanisms underlying impaired oocyte maturation and the causes of low fertilization rates.
Authors' contributions: Makarova N.P., Chagovets V.V., Frankevich V.E., Kalinina E.A. – conception and design of the study; Fortygina Yu.A., Novoselova A.V., Gamisonia A.M., Chagovets V.V. – material collection and processing, statistical analysis; Fortygina Yu.A., Drapkina Yu.S. – drafting of the manuscript; Kalinina E.A., Makarova N.P. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Fortygina Yu.A., Makarova N.P., Drapkina Yu.S., Novoselova A.V., Gamisonia A.M., Chagovets V.V., Frankevich V.E., Kalinina E.A. Comparative analysis of blood and follicular fluid lipid profiles in women undergoing infertility treatment with assisted reproductive technologies.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (4): 93-102 (in Russian)
https://dx.doi.org/10.18565/aig.2024.62
Keywords
Despite the widespread use and development of assisted reproductive technology (ART), the effectiveness of infertility treatment using in vitro fertilization (IVF) remains limited, with a success rate of 30–40% [1]. Approximately 15% of couples undergoing ART, who have a good chance of pregnancy, experience low rates of oocyte fertilization, embryonic arrest at different stages of cleavage, and production of embryos of unsatisfactory quality [2]. Moreover, even when a good- or excellent-quality embryo is transferred in 70% of cases, pregnancy does not occur despite satisfactory endometrial indicators on the day of transfer. The quality of the embryo itself should be emphasized as one of the most common reasons for repeated implantation failures, especially considering that the pregnancy rate increases significantly when a euploid embryo is transferred [3]. Therefore, the most promising scientific research in the field of ART focuses on developing strategies to improve gamete quality and embryo culture conditions. Additionally, it is important to study noninvasive or minimally invasive predictors of gamete quality that can predict both the quality of the resulting embryo before ovarian stimulation and the effectiveness of infertility treatment using ART [4].
Oocyte development, fertilization, and early embryo cleavage are influenced by the composition of follicular fluid (FF), which provides a nutrient-containing microenvironment. FF contains trace elements and molecules that surround and nourish the oocyte in response to changes in the intrafollicular environment [5]. The composition of FF affects oocyte maturation, activates nuclear and cytoplasmic oocyte maturation, and provides energy for meiotic maturation required for pronuclei formation and further developmental potential [6]. The maturing follicle also secretes growth factors to maintain a balance in FF composition. Among these molecules are lipids, which provide energy and are involved in plasma membrane formation, signaling, and protein regulation. High-density lipoproteins in FF may protect oocytes against oocyte fragmentation [7]. Studies have been conducted on the lipid profiles of FF using different stimulation protocols [8, 9]. Research shows that specific lipid composition is positively correlated with embryo implantation in ART programs [10, 6]. Changes in the lipid composition of FF have been observed in endometriosis, polycystic ovary syndrome, and reproductively older patients [11, 12, 5, 13]. Investigating the FF lipid profile as an additional marker to assess oocyte quality is promising and can help to determine the cause of low fertilization rates and unsatisfactory embryo production in patients with multiple unsuccessful IVF attempts. Predicting oocyte quality before ovarian stimulation by studying the lipid profile of blood plasma is of particular interest. Calonge et al. found that the blood lipid profile reflects the FF lipid composition and can serve as a marker of the ovarian response to stimulation [14].
Studies on a comprehensive set of lipids in the tissues and blood plasma are limited. Mass spectrometry (MS) is the main method used to screen lipids and other substances. Given the potential of developing a test system to predict the quality of oocytes during ovarian stimulation by analyzing the lipid profile of blood plasma, this study aimed to analyze the lipid profile of blood plasma in relation to the FF lipidome and identify potential groups of lipids that may correlate with oocyte quality.
Materials and methods
This study involved 40 married couples, aged 24–39 years, with a normal body mass index (up to 25 kg/m2), seeking infertility treatment with ART at the B.V.. Leonov Department of Assisted Technologies for the Treatment of Infertility. Written, voluntary, informed consent was obtained from each couple to participate in this study and process personal data. The criteria for inclusion in the study were infertility caused by tubo-peritoneal factors, male factors (without pronounced pathozoospermia), and preserved ovarian reserve. The exclusion criteria were uterine abnormalities, karyotype abnormalities, use of donor oocytes or sperm, class II–III obesity, severe forms of male infertility, and hereditary hypercholesterolemia.
Patients included in the study underwent ovarian stimulation according to a protocol using a gonadotropin-releasing hormone antagonist from the 2nd or 3rd day of the menstrual cycle. Once the follicle diameter reached at least 17 mm, patients were administered human chorionic gonadotropin as a trigger for final oocyte maturation. 35–36 hours after ovulation induction, ovarian transvaginal puncture (TVP) was performed, followed by oocyte retrieval and quality assessment. The resulting oocytes were fertilized by IVF or intracytoplasmic sperm injection (ICSI). All stages of culture were carried out in multi-gas incubators SOOK (Ireland) in 25 μl drops under oil (Irvine Sc., USA) in the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility. In patients included in the study, fasting blood plasma was collected on the day of TVP before anesthesia. FF was aspirated under a negative pressure of 140–150 mmH2O into preheated sterile tubes, which were given to the embryologist.
Before analyzing the lipid spectrum, FF obtained from both ovaries, as well as blood plasma, was processed and stored in the collection of the Biobank of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Lipid extracts were prepared according to a modified Folch method. Then, 480 μL of chloroform-methanol solution (2:1, v/v) was added to 40 μL of the sample, and the mixture was incubated for 10 min and mixed thoroughly. Then, water was added to the solution. The mixture was then centrifuged at 13,000 × G for 10 min at room temperature. The organic lower layer containing lipids (150 µL) was added to 250 µL of a chloroform-methanol solution (2:1, v/v), mixed again, centrifuged at 13,000 × G for 10 min, and another 300 µL of the lower layer was collected. The organic phase was dried under a stream of nitrogen and dissolved in 200 μL of acetonitrile-2-propanol (1:1, v/v) for subsequent analysis.
The molecular composition of the samples was determined by gas chromatography-mass spectrometry on a Dionex UltiMate 3000 liquid chromatograph (Thermo Scientific, Germany) connected to a Maxis Impact qTOF mass analyzer with an electrospray ionization source (Bruker Daltonics, Germany). Samples were separated by reverse-phase chromatography on a Zorbax C18 column (150×2.1 mm, 5 µm, Agilent, USA) with a gradient from 15% to 45% eluent B for 2 min, and then from 45% to 99% within 15 min. A solution of acetonitrile/water (60/40, v/v) with the addition of 0.1% formic acid and 10 mmol/L ammonium formate was used as eluent A. Eluent B was acetonitrile/isopropanol/water (90/8/2 v/v/v), supplemented with 0.1% formic acid and 10 mmol/L ammonium formate. The elution flow rate was 35 μL/min and the injected sample volume was 0.5 μL. Mass spectra were obtained in positive ion mode in the range m/z 100–1700 with the following settings: capillary voltage 4.1 kV, atomizing gas pressure 0.7 bar, drying gas flow rate 6 l/min, drying gas temperature 200°C. To identify lipids, tandem mass spectrometry was performed in dependent scanning mode with a window width of 5 Da.
For the preliminary processing of chromatography-mass spectrometric data, msConvert programs from Proteowizard 3.0.9987 and MzMine were used. Using msConvert, we converted the source files into the MzXml format, containing information about the mass spectrum at any time, and the ms2 format, containing information about the fragmentation spectrum of an ion at a given time. MzMine was used to extract the peaks, normalize the total ion current, and create a table containing information about the peak ion mass, peak area, and escape time. The lipids were identified using LipidMatch scripts [11]. The nomenclature of lipids corresponds to LipidMap [12].
Statistical analysis
Statistical analysis was performed using scripts written in R [R Core Team, 2018). R: Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/] in RStudio [RStudio Team (2016). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/].
The lipid content of a sample was assessed semi-quantitatively by the relative level of the analyte, which was calculated by dividing the chromatographic peak area of the corresponding analyte by the total peak area of the analytes in a given sample.
Before statistical analysis, lipid levels were transformed to have a mean of 0 and standard deviation of 1 for a better graphical representation of the analyzed data. Conversion formula:
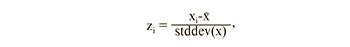
where zi is the standardized value of the parameter, xi is the initial value of the parameter, x ̅ is the mean of the parameter, stddev(x) is population standard deviation.
Comparisons of lipid levels between groups were performed using Kruskal–Wallis one-way analysis of variance by ranks. To identify statistically significant differences, pairwise comparisons of groups were performed using the Mann–Whitney U test with Bonferroni correction. Correlation analysis was performed using nonparametric Spearman’s correlation coefficients. The threshold significance level p was set at 0.05.
Results
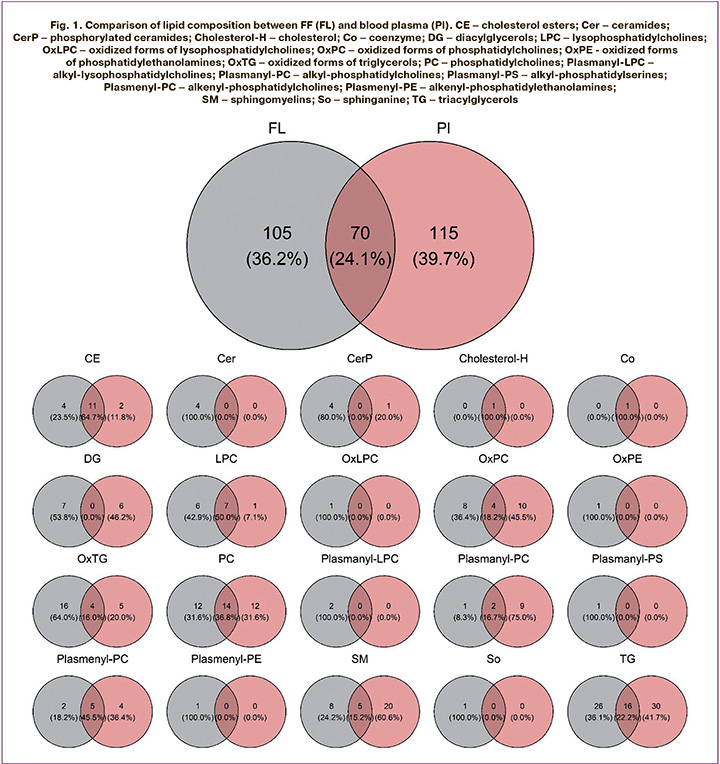
Lipidome analysis identified 175 lipids in FF samples and 185 lipids in plasma samples, belonging to 20 classes. Of all identified lipids, 70 were found in both FF and blood plasma samples (Fig. 1). The largest number of common lipids belong to the classes cholesteryl esters (CE), lysophosphatidylcholines (LPC), phosphatidylcholines (PC), sphingomyelins (SM), and triacylglycerols (TG).
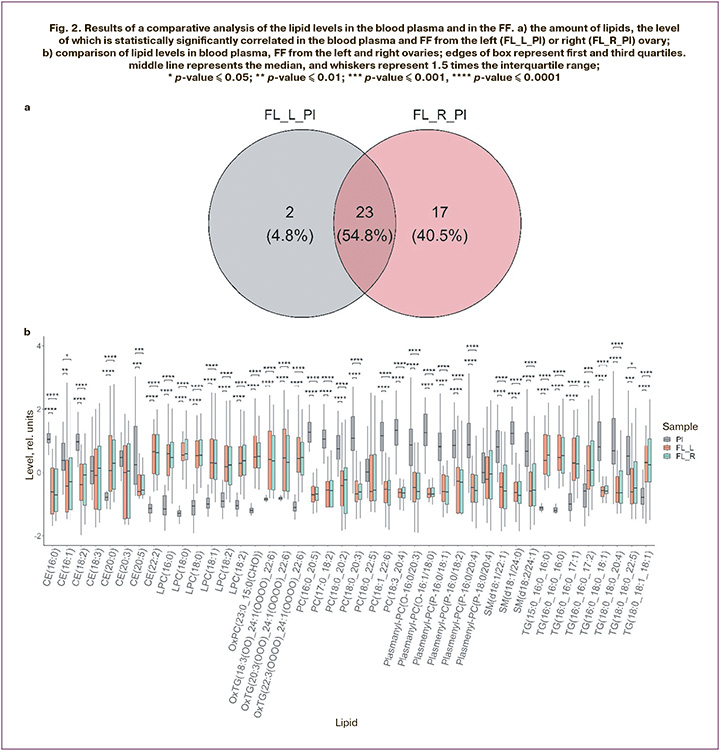
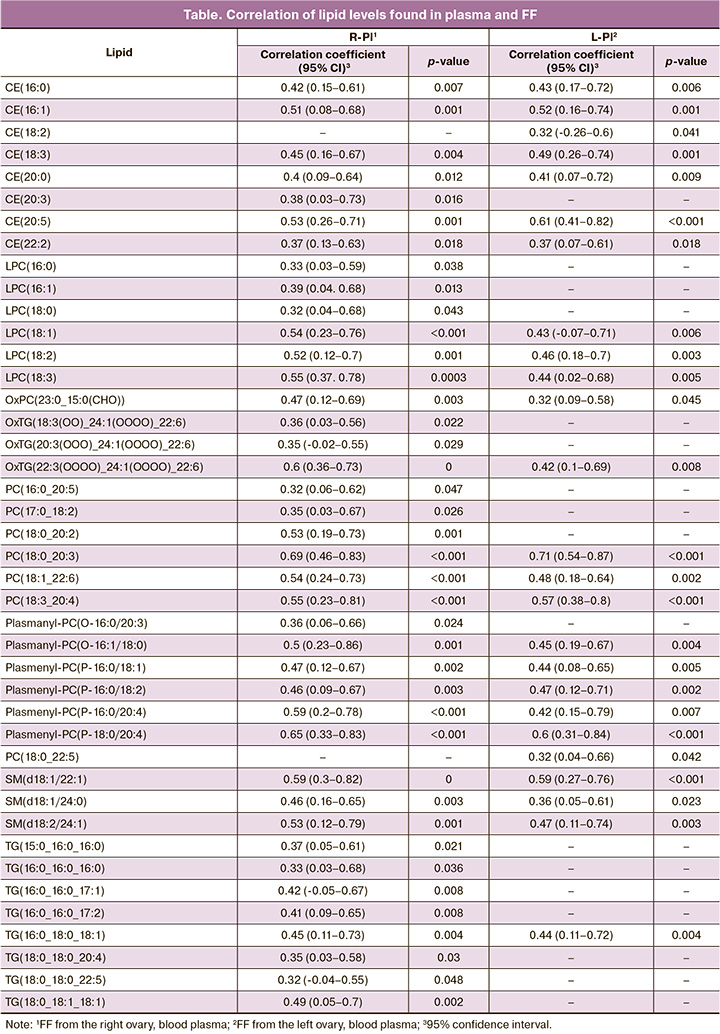
Analysis of the correlation between plasma lipid levels and FF from the left and right ovaries identified 25 lipids whose plasma levels were significantly correlated with FF levels from the left ovary and 40 lipids with a significant plasma-FF correlation from the right ovary (Fig. 2a, table). The correlation between FF levels in the left and right ovaries of all 175 identified lipids was statistically significant. Figure 2b shows the results of the comparison of lipid levels between plasma and FF. According to the results of the Kruskal–Wallis test, statistically significant differences were observed for all lipids shown in Figure 2a, except for CE(18:3), CE(20:3), PC(18:0_22:5), and plasmenyl-PC (P-18:0/20:4). Further pairwise comparison of groups using the Mann–Whitney test with Bonferroni correction showed no statistically significant differences between lipid levels in the FF from the left and right ovaries. At the same time, blood plasma levels of the same lipids were significantly different, despite a significant correlation.
Discussion
The evaluation of the lipidomic profile of various biological fluids, including the lipidome of blood plasma and FF, is a promising direction for further scientific research on the effectiveness of ART and prediction of the outcome of infertility treatment using IVF [15]. Analysis of blood plasma lipid composition by chromatography-mass spectrometry allowed the identification of lipid groups whose levels in blood plasma were significantly correlated with FF. Further study of the profile of these molecules seems promising from the point of view of identifying new non-invasive molecular markers for predicting FF composition and oocyte quality prior to ovarian stimulation.
One of the lipid groups identified in both blood and follicular fluid is phosphatidylcholines, which are the primary phospholipids found in cell membranes. These lipids play important roles in intracellular signaling as well as in metabolic and structural functions [16]. When these lipids are hydrolyzed by phospholipase and phosphatidate phosphohydrolase, they produce fatty acids such as arachidonic acid. If the metabolic system is overactive, fatty acid levels can increase, resulting in the generation of free radicals and oxidative stress [17]. In a 2021 study, it was found that an elevated phosphatidylcholine profile leads to excessive accumulation of arachidonic acid in the follicular fluid of patients in the ART program [18]. This elevated level of arachidonic acid leads to a decrease in embryo quality on the third day after fertilization due to poor oocyte quality. The study utilized a machine learning method, specifically the random forest algorithm, to identify other metabolites in follicular fluid that affect the quality of oocytes and embryos. Among these metabolites, lipids of the sphingomyelin group were identified. This indicates that the accumulated metabolites are released from oocytes into the follicular fluid, and profiling of this biological environment reflects their levels in the cells themselves.
Sphingolipids are vital components of the biological cell membranes. They are a diverse group of bioactive lipids that regulate various cellular processes including proliferation, growth inhibition, apoptosis, differentiation, aging, and cell motility. Research has demonstrated a positive correlation between the levels of four types of sphingolipids in FF and oocyte quality in the ART program [19]. The interaction between sphingomyelin, cholesterol, and glycosphingolipids leads to the formation of plasma membrane rafts, which are involved in cell signaling, lipid and protein sorting, and molecular transport. Ceramide, a specific sphingolipid, serves as a biologically active component of the mitochondrial membrane [20]. Mitochondria are crucial organelles responsible for energy production and play roles in fatty acid oxidation, calcium homeostasis, and apoptosis. Impaired energy production in the cytoplasm of oocytes may be associated with low-quality oocytes and embryos [21]. An experimental study conducted by Eliyahu E. et al. found that adding an enzyme called acid ceramidase, expressed in human and FF cumulus cells, to the culture medium significantly improved embryo quality and increased the live birth rate five-fold [22]. Therefore, genetic disorders of mitochondrial DNA that affect lipid metabolism and related enzymes may play a critical role in oocyte development. Shehadeh A. et al. also demonstrated that changes in the FF lipidomic profile in patients undergoing the ART program were associated with a higher incidence of clinical pregnancy. Decreased plasma triglyceride levels and increased membrane lipid levels (phospholipids and sphingolipids) were statistically linked to an increased incidence of clinical pregnancy. Furthermore, another study revealed that other lipid groups, such as cholesteryl esters, were decreased in FF from patients with positive treatment outcomes [6].
Lipids, specifically cholesterols and their esters, play a crucial role in cell membrane function as well as vitamin D synthesis, which is essential for the human reproductive system [16]. However, recent studies have produced conflicting data on the importance of vitamin D. Some studies have shown that vitamin D metabolites derived from cholesterol derivatives are linked to positive outcomes in assisted reproductive technology (ART) programs [23, 24]. However, other studies have found a negative correlation between the concentration of 25-hydroxyvitamin D in follicular fluid (FF), oocyte fertilization ability, and embryo implantation [25]. Moreover, lower levels of 25-hydroxyvitamin D in FF have been associated with a better response to ovarian stimulation [26]. Therefore, analysis of the lipid profile of FF has the potential to be an informative and promising diagnostic method. However, FF analysis is only possible after ovarian stimulation and transvaginal puncture for oocyte retrieval, making it less suitable for certain situations. From a practical point of view, the most promising task is to predict the FF lipid profile by analyzing the blood plasma of patients undergoing infertility treatment with ART before stimulation. To develop a test system, it is necessary to identify the lipids present in both FF and blood serum. A previous study by Nunez Calonge R. et al. identified seven groups of lipids in FF that can also be determined in blood serum using mass spectrometry [14]. Using a multivariate linear regression model to account for age and body mass index, the study showed that lipid profiles in FF and serum significantly influenced the ovarian response and the number of mature oocytes.
Another noteworthy study conducted in 2021 by Kermack A.J. et al. demonstrated that modifying the diet prior to an in vitro fertilization (IVF) treatment cycle significantly improved oocyte quality. The diet included supplementation with polyunsaturated fatty acids, omega-3, olive oil, and vitamin D in 55 couples starting six weeks before ovarian stimulation. Serum samples were collected from married couples before and after treatment, and FF was obtained from both ovaries on the day of transvaginal puncture. In the control group, post-treatment serum lipids were strongly correlated with FF profiles and the quality of the obtained oocytes. Furthermore, a diet enriched in polyunsaturated fatty acids was associated with a lower arachidonic acid content in FF [27].
Considering the literature on the possible therapeutic effects on the lipid composition of blood plasma and FF, the study of lipid profiles allows for differentiated personalized preparation of patients before ovarian stimulation to improve oocyte quality. The development of models for predicting the effectiveness of infertility therapy using the ART method, including machine learning methods, based on the blood lipidomic profile and FF using gas chromatography-mass spectrometry, is another relevant and important task [28, 29].
Conclusion
Currently, many studies are focused on the integrated analysis of omics technologies, including lipidomic data, genomics, proteomics, and metabolomics. Our results suggest that the FF lipid profile may be associated with the serum lipid profile. The determination of blood lipid profile is a minimally invasive and convenient marker not only for the assessment of oocyte quality but can also be used as a marker of treatment efficacy.
The study results suggest the potential for personalized infertility treatment and preparation for ART programs as well as a deeper understanding of the mechanisms underlying impaired oocyte maturation and the causes of low fertilization rates.
References
- Министерство здравоохранения Российской Федерации. Женское бесплодие (современные подходы к диагностике и лечению). Клинические рекомендации (протокол лечения). 2019: 33. [Ministry of Health of the Russian Federation. Female infertility (modern approaches to diagnosis and treatment). Clinical guidelines (treatment protocol). 2019: 33. (in Russian)].
- Назаренко Т.А. Вспомогательная репродукция в клинической практике. Разбор клинических случаев с использованием международных и отечественных рекомендаций. М.: МедКом-Про; 2020. 121с. [Nazarenko T.A. Assisted reproduction in clinical practice. Analysis of clinical cases using international and domestic recommendations. M.: MedCom-Pro; 2020. 121p. (in Russian)].
- Макарова Н.П., Лобанова Н.Н., Кулакова Е.В., Непша О.С., Екимов А.Н., Калинина Е.А. Влияние преимплантационного генетического тестирования на результаты программ вспомогательных репродуктивных технологий у супружеских пар с мужским фактором бесплодия. Акушерство и гинекология. 2021; 11: 154-64. [Makarova N.P., Lobanova N.N., Kulakova E.V., Nepsha O.S., Ekimov A.N., Kalinina E.A. Impact of preimplantation genetic testing on assisted reproductive technology outcomes in couples with male factor infertility. Obstetrics and Gynecology. 2021; (11): 154-64 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.154-164.
- Чараева А.В., Макарова Н.П., Драпкина Ю.С., Калинина Е.А. Новые достижения в понимании молекулярных механизмов имплантации эмбриона человека в программах экcтракорпорального оплодотворения. Акушерство и гинекология. 2023; 3: 21-8. [Charaeva A.V., Makarova N.P., Drapkina Yu.S., Kalinina E.A. New advances in understanding the molecular mechanisms of human embryo implantation in in vitro fertilization programs. Obstetrics and Gynecology. 2023; (3): 21-8 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.281.
- Cordeiro F.B., Montani D.A., Pilau E.J., Gozzo F.C., Fraietta R., Turco E.G.L. Ovarian environment aging: follicular fluid lipidomic and related metabolic pathways. J. Assist. Reprod. Genet. 2018; 35(8): 1385-93. https://dx.doi.org/10.1007/s10815-018-1259-5.
- Shehadeh A., Bruck-Haimson R., Saidemberg D., Zacharia A., Herzberg S., Ben-Meir A. et al. A shift in follicular fluid from triacylglycerols to membrane lipids is associated with positive pregnancy outcome. FASEB J. 2019; 33(9): 10291-9. https://dx.doi.org/10.1096/fj.201900318RR.
- Nagy R.A., van Montfoort A.P.A., Groen H., Homminga I., Andrei D., Mistry R.H. et al. Anti-oxidative function of follicular fluid HDL and outcomes of modified natural cycle-IVF. Sci. Rep. 2019; 9(1): 12817. https://dx.doi.org/10.1038/s41598-019-49091-3.
- Николенко И.Г., Смольникова В.Ю., Чаговец В.В. Возможности прогнозирования исходов программ вспомогательных репродуктивных технологий у пациенток с эндометриоидными кистами яичников на основании метаболомного профиля фолликулярной жидкости. Акушерство и гинекология. 2020; 11: 44-8. [ Nikolenko I.G., Smolnikova V.Yu., Chagovets V.V. Possibilities of predicting the outcomes of assisted reproductive technology programs in patients with ovarian endometrioid cysts on the basis of the metabolic profile of follicular fluid. Obstetrics and Gynecology. 2020; (11): 44-8 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.44-48.
- Wen X., Kuang Y., Zhou L., Yu B., Chen Q., Fu Y. et al. Lipidomic components alterations of human follicular fluid reveal the relevance of improving clinical outcomes in women using progestin-primed ovarian stimulation compared to short-term protocol. Med. Sci. Monit. 2018; 24: 3357-65. https://dx.doi.org/10.12659/MSM.906602.
- Matorras R., Martinez-Arranz I., Arretxe E., Iruarrizaga-Lejarreta M., Corral B., Ibañez-Perez J. et al. The lipidome of endometrial fluid differs between implantative and non-implantative IVF cycles. J. Assist. Reprod. Genet. 2020; 37(2): 385-94. https://dx.doi.org/10.1007/s10815-019-01670-z.
- Bouet P.E., Chao de la Barca J.M., El Hachem H., Descamps P., Legendre G., Reynier P. et al. Metabolomics shows no impairment of the microenvironment of the cumulus-oocyte complex in women with isolated endometriosis. Reprod. Biomed. Online. 2019; 39(6): 885-92. https://dx.doi.org/10.1016/j.rbmo.2019.08.001.
- Ban Y., Ran H., Chen Y., Ma L. Lipidomics analysis of human follicular fluid form normal-weight patients with polycystic ovary syndrome: a pilot study. J. Ovarian Res. 2021; 14(1): 135. https://dx.doi.org/10.1186/s13048-021-00885-y.
- Luan C.X., Xie W.D., Liu D., Li W., Yuan Z.W. Candidate circulating biomarkers of spontaneous miscarriage after IVF-ET identified via coupling machine learning and serum lipidomics profiling. Reprod. Sci. 2022; 29(3): 750-60. https://dx.doi.org/10.1007/s43032-021-00830-w.
- Núñez Calonge R., Guijarro J.A., Andrés C., Cortés S., Saladino M., Caballero P., Kireev R. Relationships between lipids levels in blood plasma, follicular fluid and seminal plasma with ovarian response and sperm concentration regardless of age and body mass index. Rev. Int. Androl. 2022; 20(3): 178-88. https://dx.doi.org/10.1016/j.androl.2021.02.004.
- Khan R., Jiang X., Hameed U., Shi Q. Role of lipid metabolism and signaling in mammalian oocyte maturation, quality, and acquisition of competence. Front. Cell. Dev. Biol. 2021; (9): 639704. https://dx.doi.org/10.3389/fcell.2021.639704.
- Фортыгина Ю.А., Макарова Н.П., Непша О.С., Лобанова Н.Н., Калинина Е.А. Роль липидомных исследований в репродукции человека и исходах программ лечения бесплодия методами вспомогательных репродуктивных технологий. Акушерство и гинекология. 2022; 10: 14-20. [Fortygina Yu.A., Makarova N.P., Nepsha O.S., Lobanova N.N., Kalinina E.A. The role of lipidomic studies in human reproduction and in the outcomes of infertility treatment programs using assisted reproductive technologies. Obstetrics and Gynecology. 2022; (10): 14-20 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.10.14-20.
- Szczuko M., Kikut J., Komorniak N., Bilicki J., Celewicz Z., Ziętek M. The role of arachidonic and linoleic acid derivatives in pathological pregnancies and the human reproduction process. Int. J. Mol. Sci. 2020; 21(24): 9628. https://dx.doi.org/10.3390/ijms21249628.
- Wang J., Zheng W., Zhang S., Yan K., Jin M., Hu H. et al. An increase of phosphatidylcholines in follicular fluid implies attenuation of embryo quality on day 3 post-fertilization. BMC Biol. 2021; 19: 200. https://dx.doi.org/10.1186/s12915-021-01118-w.
- Liu L., Yin T.L., Chen Y., Li Y., Yin L., Ding J. et al. Follicular dynamics of glycerophospholipid and sphingolipid metabolisms in polycystic ovary syndrome patients. J. Steroid Biochem. Mol. Biol. 2019; 185: 142-9. https://dx.doi.org/10.1016/j.jsbmb.2018.08.008.
- Zhang Y., Zhang X., Lu M., Zou X. Ceramide-1-phosphate and its transfer proteins in eukaryotes. Chem. Phys. Lipids. 2021; 240: 105135. https://dx.doi.org/10.1016/j.chemphyslip.2021.105135.
- Timur B., Aldemir O., İnan N., Kaplanoğlu İ., Dilbaz S. Clinical significance of serum and follicular fluid ceramide levels in women with low ovarian reserve. Turk. J. Obstet. Gynecol. 2022; 19(3): 207-14. https://dx.doi.org/10.4274/tjod.galenos.2022.05760.
- Eliyahu E., Shtraizent N., Martinuzzi K., Barritt J., He X., Wei H. et al. Acid ceramidase improves the quality of oocytes and embryos and the outcome of in vitro fertilization. FASEB J. 2010; 24(4): 1229-38. https://dx.doi.org/10.1096/fj.09-145508.
- Meng X., Zhang J., Wan Q., Huang J., Han T., Qu T. et al. Influence of vitamin D supplementation on reproductive outcomes of infertile patients: a systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023; 21(1): 17. https://dx.doi.org/10.1186/s12958-023-01068-8.
- Fernando M., Ellery S.J., Marquina C., Lim S., Naderpoor N., Mousa A. Vitamin D-Binding protein in pregnancy and reproductive health. Nutrients. 2020; 12(5): 1489. https://dx.doi.org/10.3390/nu12051489.
- Ciepiela P., Dulęba A.J., Kowaleczko E., Chełstowski K., Kurzawa R. Vitamin D as a follicular marker of human oocyte quality and a serum marker of in vitro fertilization outcome. J. Assist. Reprod. Genet. 2018; 35(7): 1265-76. https://dx.doi.org/10.1007/s10815-018-1179-4.
- Antunes R.A., Mancebo A.C.A., Reginatto M.W., Deriquehem V.A.S., Areas P., Bloise E. et al. Lower follicular fluid vitamin D concentration is related to a higher number of large ovarian follicles. Reprod. Biomed. Online. 2018; 36(3): 277-84. https://dx.doi.org/10.1016/j.rbmo.2017.12.010.
- Kermack A.J., Wellstead S.J., Fisk H.L., Cheong Y., Houghton F.D., Macklon N.S. et al. The fatty acid composition of human follicular fluid is altered by a 6-week dietary intervention that includes marine omega-3 fatty acids. Lipids. 2021; 56(2): 201-9. https://dx.doi.org/10.1002/lipd.12288.
- Драпкина Ю.С., Калинина Е.А., Макарова Н.П., Мильчаков К.С., Франкевич В.Е. Искусственный интеллект в репродуктивной медицине: этические и клинические аспекты. Акушерство и гинекология. 2022; 11: 37-44. [Drapkina Yu.S., Kalinina E.A., Makarova N.P., Milchakov K.S., Frankevich V.E. Artificial intelligence in reproductive medicine: ethical and clinical aspects. Obstetrics and Gynecology. 2022; (11): 37-44 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.11.37-44.
- Драпкина Ю.С., Макарова Н.П., Татаурова П.Д., Калинина Е.A. Поддержка врачебных решений с помощью глубокого машинного обучения при лечении бесплодия методами вспомогательных репродуктивных технологий. Медицинский Совет. 2023;(15):27-37. [Drapkina J.S., Makarova N.Р., Tataurova P.D., Kalinina E.A. Deep machine learning applied to support clinical decision-making in the treatment of infertility using assisted reproductive technologies. Medical Council. 2023; (15): 27-37. (in Russian)]. https://doi.org/10.21518/ms2023-368.
Received 20.03.2024
Accepted 02.04.2024
About the Authors
Yulia A. Fortygina, graduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, yu_fortygina@oparina4.ru, https://orcid.org/0000-0002-1251-0505Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher, Department of IVF named after Professor B.V. Leonov, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, np_makarova@oparina4.ru,
https://orcid.org/0000-0003-8922-2878
Yulia S. Drapkina, PhD, Researcher, Department of IVF named after Professor B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, yu_drapkina@oparina4.ru, https://orcid.org/0000-0002-0545-1607
Anastasia V. Novoselova, Researcher, Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, a_novoselova@oparina4.ru
Alina M. Gamisonia, Researcher, Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, a_gamisoniya@oparina4.ru, https://orcid.org/0000-0001-8532-4714
Vitaly V. Chagovets, PhD, Head of the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, vvchagovets@gmail.com, https://orcid.org/0000-0002-5120-376X
Vladimir E. Frankevich, Dr. Sci. in physics and mathematics, Head of the Department of Systems Biology in Human Reproduction, Deputy Director of the Institute of Translational Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Siberian State Medical University, Ministry of Health of Russia, v_frankevich@oparina4.ru, https://orcid.org/0000-0002-9780-4579
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the IVF Department named after Professor B.V. Leonov, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, e_kalinina@oparina4.ru,
https://orcid.org/0000-0002-8922-2878



