Bisphenol A in infertile patients: impact on assisted reproductive technologies outcomes
Aim. To investigate the relationship between bisphenol A levels in infertile patients and the outcomes of assisted reproductive technologies (ART).Syrkasheva A.G., Kindysheva S.V., Starodubtseva N.L., Frankevich V.E., Dolgushina N.V.
Materials and methods. Three hundred one married couples underwent assisted reproductive technology treatment. Blood and follicular fluid (FF) levels of bisphenol A were determined by chromatography-mass spectrometry. The study analyzed the impact of bisphenol A level on early embryogenesis parameters and ART outcomes.
Results. Bisphenol A was detected in 92.9% (277/298) of blood and 16.8% (49/292) of FF samples. No statistical relationship was found between the blood and FF bisphenol levels in the same patients. There were no differences in main early embryogenesis parameters and pregnancy rates in the quartile subgroups of blood bisphenol A. When dividing patients into groups based on the presence or absence of a detectable (i.e., 0.1 ng/ml and higher) bisphenol A in FF, no statistically significant differences in embryological parameters were revealed. Among 48 patients with available bisphenol A data both in the blood and FF, 24 had higher FF bisphenol A levels, and in the other 24 patients, it was higher in the blood. Patients with bisphenol A levels in FF higher than that in the blood had decreased number and quality of oocytes and embryos.
Conclusion. Increased level of bisphenol A in FF compared with its level in the blood is associated with a decrease in the number and quality of oocytes in ART.
Keywords
Bisphenol is a chemical mainly used as a monomer in polymers' manufacturing, particularly polycarbonate plastics and epoxy-polyester. Bisphenol A exposure is conveyed via food from plastic utensils and bottles. Bisphenol A is also used in some dental materials, including dental sealants. It is also found in the lining of aluminum and tin cans, inkless cash register receipts, etc. Given the widespread exposure to polycarbonate plastics in everyday life, bisphenol A is detected in 95-100% of adults in the population [1, 2].
Bisphenol A is an endocrine disruptor. According to the American Society of Endocrinologists, endocrine-disrupting chemicals comprise a wide variety of exogenous chemicals, including synthetic compounds able to affect hormones synthesis, metabolism, and function
Biochemical studies have shown that bisphenol A possesses a binding affinity for estrogens receptors. Bisphenol A mimics the effect of estradiol by binding to both ER-α and ER-β receptors; its affinity for ER-β is significantly higher [3]. Also, bisphenol A has antiandrogenic, mutagenic, and epigenetic effects, which may have a transgenerational effect predisposing the subsequent generations to the risk of developing BPA-related diseases [4]. Bisphenol A penetrates the histohematogenous barriers, and its accumulation was found in follicular fluid (FF) in patients undergoing assisted reproductive technologies (ART). However, the relationship between bisphenol A level, granulosa cell steroidogenesis, and the oocyte quality remains unclear [5, 6].
The ubiquity of bisphenol A containing plastics with estrogenic properties has stimulated interest in investigating their adverse effects of endocrine disruptors on the reproductive system.
This study aimed to investigate the relationship between bisphenol A levels in infertile patients and the outcomes of assisted reproductive technologies.
Materials and methods
Study design
This prospective study included 301 married couples seeking ART treatment from 2017 to 2018, with no contraindications to ART. The Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia approved this study (17.10.2016). All participants provided signed informed consent to take part in the study.
The inclusion criteria were normal karyotype of both spouses, the absence of pronounced pathospermia (100% teratozoospermia, absolute asthenozoospermia, all types of azoospermia), the age of women from 18 to 39 years, the body mass index (BMI) of women from 19 to 25 kg/m2. The exclusion criteria were the use of donor gametes or surrogacy, as well as the receipt of 3 or fewer oocytes on the day of transvaginal ovarian puncture (TVP).
All married couples included in the study were examined following the order of the Ministry of Health of Russia No. 107n of 30.08.2012 “On the procedure for using assisted reproductive technologies, contraindications, and restrictions on their use” [3].
All recruited women underwent ovarian stimulations using gonadotropin-releasing hormone (GnRH) antagonist protocols; the dose of gonadotropins was selected on a case-by-case basis. The ovulation trigger was administered when follicle dimensions on transvaginal ultrasound reached 17 mm or more. GnRH- agonist (0.2mg) or human chorionic gonadotropin (hCG, 8000–10,000 IU) were used as an ovulation trigger. The luteal phase and post-transfer support were applied according to the standard protocol.
Oocytes were fertilized by in vitro oocyte insemination ("classical" IVF, from now on IVF – fertilization method) or intracytoplasmic sperm injection into the oocyte (ICSI). During fertilization, morphological assessment of oocytes was carried out using light microscopy, and the presence of cytoplasmic dysmorphisms was determined. Cultivation and embryo transfer were carried out according to the methods accepted in clinical practice.
On day 14 days after embryo transfer, serum β-hCG concentration was determined. Clinical pregnancy was confirmed by fetal cardiac activity on transvaginal ultrasonography five weeks after the embryo transfer. At week 40, after the embryo transfer, the patients were interviewed by telephone to analyze the pregnancy outcomes. The pregnancy rate was defined as the ratio of the number of clinical pregnancies to the total number of native cycles with embryo transfer. The live birth rate was defined as the number of live births to the total number of native cycles with embryo transfer. The cumulative live birth rate was defined as the ratio of the number of live births in a given cycle and live births after the transfer of thawed embryos obtained in a given IVF or ICSI cycle to the total number of cycles.
Sample collection and pre-processing
Venous blood samples were collected on the day of TVP and cryopreserved at t=-80°C. FF samples were taken immediately after oocyte retrieval. FF samples contaminated with blood were excluded from the study.
Bisphenol A level was determined by mass spectrometry; the laboratory had no access to the patients' clinical characteristics.
Quantitative analysis of serum bisphenol A levels by gas chromatography-mass spectrometry (GC-MS)
Benzophenone (Ph2CO, 182.22 g / mol, Sigma-aldrich, 99% purity) was used as an internal standard. To increase the sensitivity, preliminary derivatization with dansyl chloride (99%, HPLC-grade, Sigma-aldrich) was carried out. All stock solutions were prepared by dissolving the required amount of bisphenol A (4,4'-isopropyl-idenediphenol, 228.29 g/mol, Sigma-aldrich) or benzophenone in 100% MeOH (99.9%, HPLC Basic, Scharlau). Before use, the solutions were stored in a refrigerator at 40°C for no more than one week in glass vials. All glass cups were washed with hexane to eliminate possible contamination during sample preparation.
The 70 μl IS (10 μg/ml benzophenone in 100% MeOH) was added to 700 μl of blood serum, followed by vortexing and centrifugation. After adding 700 μl MTBE (≥ 99.5%, HPLC grade, Fisher Chemical), vortexing for 1 minute, centrifuging at 13,000 rpm at room temperature for 10 minutes, the upper phase was taken. Extraction was carried out two more times; then, the organic phase was dried in a stream of N2 at 40°C until the solution was dehydrated. To derivatize bisphenol A, 100 μl of a freshly prepared mixture of dansyl chloride (1 mg/ml in acetonitrile) and Na2CO3 (1 mg/ml in water) (1: 1) was added, incubated at 70°C for 10 minutes, centrifuged at 13000 rpm at room temperature for 10 minutes. The 90 μL of supernatant was transferred into a glass vial with an insert for subsequent gas chromatography-mass spectrometric analysis.
Samples were prepared in 2 ml Eppendorf tubes free of bisphenol A. This was verified by shortened sample preparation, during which 1.8 ml MTBE was kept for 3 hours in empty Eppendorf tubes, then 70 μl IS was added and dried in a stream of N2, at a temperature of 40°C until the complete drying, after which standard sample preparation was continued. No BPA was detected in the obtained washings from Eppendorf tubes.
The calibration curve was constructed using MS-grade water as a matrix, which allowed obtaining the sensitivity threshold of 0.1ng/ml. To 700 μL of H2O, 70 μL of IS and 70 μL of a stock solution of the required concentration were added. A calibration curve was drawn from 9 points (correlation coefficient above 0.99) with BPA concentrations in the resulting sample ranging from 0.1 ng to 100 ng. Four QCs were taken as quality control: QC1=0.2 ng, QC2=0.6 ng, QC3=50 ng, and QC4=75 ng of bisphenol A. The LOQ sensitivity limit was 0.2 ng.
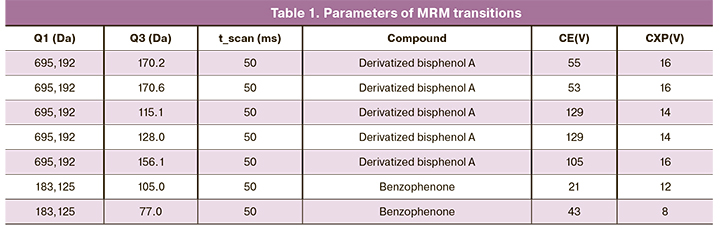
Chromatographic separation was performed using an Agilent Technologies 1260 infinity mass spectrometric detection (QTRAP 5500 ABSciex) in electrospray mode (ESI-MS/MS) positive mode in MRM mode. Analysis was conducted using a Zorbax Eclipse Plus C18 column sized 3×50 mm, particle size 1.8 μm. Mobile phase A consisted of distilled H2O with 0.1% formic acid, mobile phase B consisted of acetonitrile with 0.1% formic acid. The measurements were carried out in an isocratic mode for 5 minutes with a constant phase ratio A/B = 4/96 at a 450 μL/min flow rate, a column temperature of 35°C, and a 5 μL sample volume. GC-MS analysis was performed in three technical repetitions. The system dead time under these conditions did not exceed 0.5 min, the IS exit time was 0.76 min, and the BPA exit time was 1.48 min. Table 1 shows the parameters of the used MRM transitions.
Statistical analysis
Statistical analysis was conducted using Statistica 10 (USA) software. Data showing normal distribution are presented as the mean (standard deviation) or median (interquartile range). Categorical variables were compared by the χ2 test, and the Kruskal–Wallis test was used for comparing medians. Correlation analysis was conducted by calculating Spearman's rank correlation coefficients. Differences between the groups were considered statistically significant at p<0.05.
Results
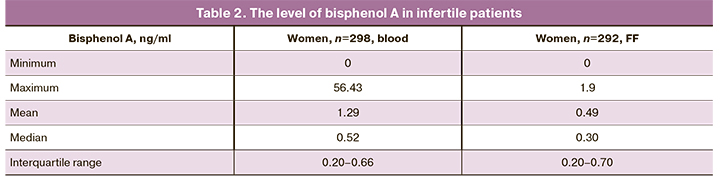
The threshold for determining the level of bisphenol A (both in blood and FF) was 0.1 ng/ml. Bisphenol A was detected in 92.9% (277/298) and 16.8% (49/292) of blood and FF samples, respectively.
Correlation between levels of bisphenol A in the blood and the FF of the same patients was statistically non-significant (r=-0.157, p=0.288).
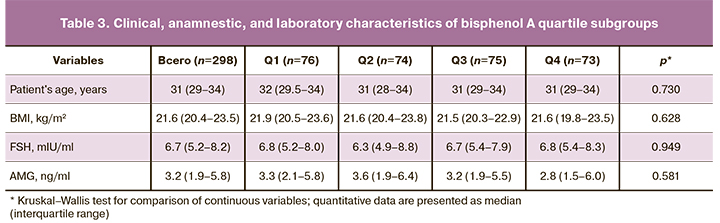
Next, we analyzed the clinical and anamnestic characteristics according to quartiles of bisphenol A (Table 3). There were no statistically significant differences between subgroups in age and BMI. Median levels of major hormones were also comparable across quartile subgroups. No differences were found in the assessment of the main parameters of folliculogenesis and oogenesis.
Further, we analyzed characteristics of the embryological stage in bisphenol A quartile subgroups (Table 4).
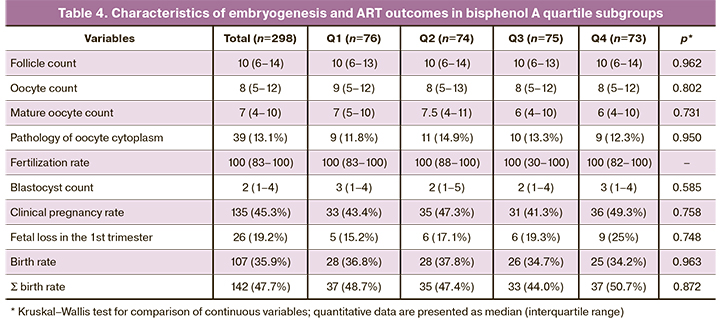
The median number of follicles, oocytes, mature oocytes, oocyte morphology, oocyte fertilization rate, and the number of blastocysts did not differ between the groups. The rates of clinical pregnancy, childbirth, and cumulative birth rate (Ʃ birth rate) did not differ significantly between the groups. There was a tendency for an increase in the rate of pregnancy losses in the first trimester with an increase in bisphenol A level; however, the differences did not reach statistical significance.
There was no statistically significant relationship between bisphenol A level in FF and the number of oocytes (p=0.323), the number of mature oocytes (p=0.220), and the number of blastocysts (p=0.503). There were no statistically significant differences in embryological parameters when patients were divided into groups based on the presence or absence of a detectable (i.e., 0.1 ng/ml and higher) level of bisphenol A in FF. The pregnancy rate was slightly higher in the subgroup of patients with an undetectable level of bisphenol A in FF, but the differences were not statistically significant (Table 5).
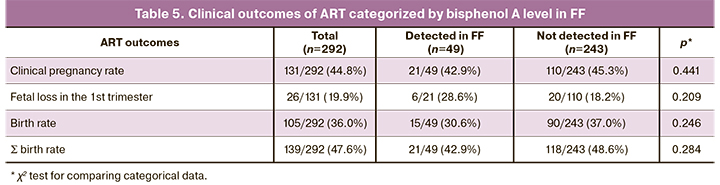
Then, we conducted a subgroup analysis of patients who had FF bisphenol A level above the detection threshold (i.e., ≥ 0.1 ng / ml). It was found that among 48 patients with available bisphenol A data both in the blood and FF, 24 had higher FF bisphenol A levels, and in the remaining 24 patients, it was higher in the blood.
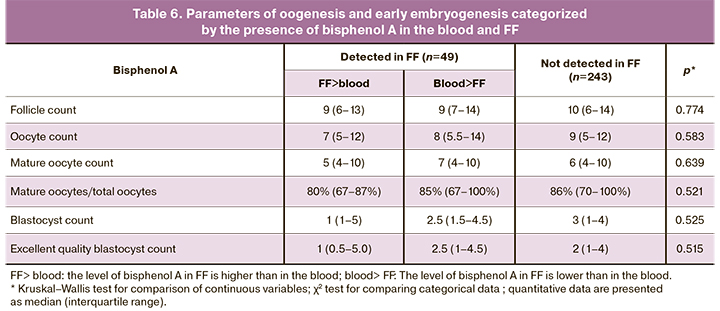
Table 6 shows the main oogenesis and early embryogenesis parameters categorized by bisphenol A in FF and the relationship between the level of bisphenol A in the blood and FF.
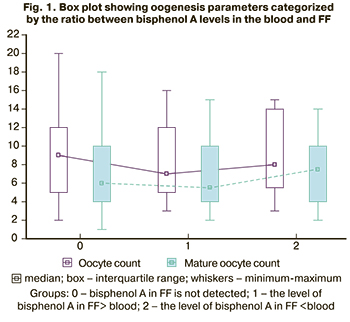 The median oocyte yields and mature oocyte counts were lower among patients in whom the level of bisphenol A in FF was higher than that in the blood (Fig. 1).
The median oocyte yields and mature oocyte counts were lower among patients in whom the level of bisphenol A in FF was higher than that in the blood (Fig. 1).
Also, patients with bisphenol A levels in FF higher than that in the blood had a decreased ratio between mature oocyte counts and oocyte yields. None of the patients in this subgroup received 100% of mature oocytes in the total cohort.
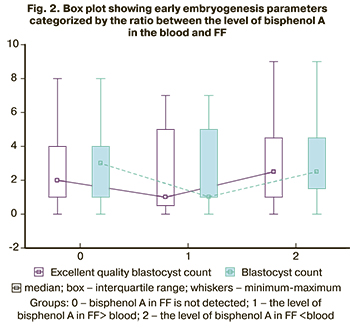
As a result, these patients had decreased blastocyst yields and low counts of blastocysts of excellent quality (Fig. 2).
Therefore, although the differences did not reach statistical significance, there was a tendency towards a decrease in the number and quality of oocytes and embryos in the subgroup of patients in whom the bisphenol level in FF exceeded its level in the blood.
 Considering the measurement error of bisphenol A in biological fluids, it is appropriate to look for the value of the target parameter using the formula (Cfolfluid + 0.1 ng/ml)/(Сblood + 0.1 ng/ml), where 0.1 ng/ml is the measurement error. This additional indicator, which ranged between 0.03 and 15, was included in statistical analysis (Bisphenol A FF/Blood Coefficient).
Considering the measurement error of bisphenol A in biological fluids, it is appropriate to look for the value of the target parameter using the formula (Cfolfluid + 0.1 ng/ml)/(Сblood + 0.1 ng/ml), where 0.1 ng/ml is the measurement error. This additional indicator, which ranged between 0.03 and 15, was included in statistical analysis (Bisphenol A FF/Blood Coefficient).
Correlation analysis of bisphenol A in blood and FF, weight and BMI of the patient, AMH level, and the number of oocytes and embryos was carried out. A statistically significant weak positive correlation was found between this indicator and the patient's BMI (r=0.14, р=0.0488).
Discussion
According to our study, bisphenol A in detectable concentrations was found in most patients, consistent with the data reported in other countries and various categories of patients [1, 3, 7].
Bisphenol A was detected in 16.7% of FF samples from infertile patients, and no association was found between bisphenol A levels in blood and FF. Current literature lacks studies evaluating the level of endocrine disruptors in the body fluids of patients. The results of such studies are contradictory since all studies use different methods for determining endocrine disruptors A, the objects of study are different categories of patients, and various substances are being investigated [8]. We hypothesize that the lack of a relationship between the level of bisphenol A in the blood and FF may be associated with a different metabolic rate of bisphenol A in FF (possibly due to the genetic characteristics of the patients) or different duration of exposure to endocrine disruptors, which is a prerequisite for further research.
When assessing the embryological stage of ART programs, we found no significant effect of the blood bisphenol A on oocyte count, fertilization rate, number, and quality of blastocysts. Clinical pregnancy rats also did not differ in quartile groups. However, there was a tendency to increase in early reproductive losses with an increase in bisphenol A level.
The pathogenesis of early spontaneous pregnancy loss due to the adverse effect of bisphenol A has not been determined. The most common cause of early reproductive loss is embryo genetic abnormalities. At the same time, experimental animal models have shown the effect of endocrine disruptors on meiosis [9, 10].
The main object of our interest was to assess the effect of bisphenol A level in FF on the parameters of oogenesis and early embryogenesis, since, probably, the concentrations of various substances in FF correlate more accurately with the quality of oocytes, compared with blood concentrations of the same substances. However, in our study, no association was found between bisphenol A level in FF and the number of oocytes and embryos. In a similar study, Poormoosavi et al. reported that the levels of endocrine disruptors in FF adversely affect oocyte count and the incidence of morphological oocyte abnormalities [11]. Also, no differences were found when comparing the parameters of oogenesis and early embryogenesis in patients with detectable and undetectable concentrations of bisphenol A in FF.
It should be noted that the average and maximum levels of bisphenol A in FF are significantly lower compared to the blood bisphenol A level; similar results are described in the literature [11]. However, a more detailed analysis of the group of patients with a detectable FF bisphenol A level showed that in half of the cases (n=24), the level of bisphenol A in the FF was higher than in the blood. In this subgroup of patients, a tendency towards a decrease in the number of oocytes and embryos was observed. The adverse effect of endocrine disruptors on the transcriptional profile of cumulus cell genes has been shown in various studies [12, 13], which may explain the decrease in blastulation rate in these patients. However, the question remains open why such a trend is characteristic only for patients with FF bisphenol A levels higher than that in the blood. It should be borne in mind that bisphenol A can enter the ovary and, accordingly, into the FF only through the systemic circulation. The relationship between endocrine disruptors’ levels in various biological tissues is probably determined by the individual characteristics of the histohematogenous barriers and the detoxification system.
Further analysis revealed a relationship between the FF/blood ratio of bisphenol A and the patient's BMI. These findings suggest the need for further research.
Conclusion
According to our findings, bisphenol A is detected in most infertile patients undergoing ART. Bisphenol A can cross histohematogenous barriers and enter the FF, which is associated with a decrease in the quality of oocytes and embryos.
Increased level of bisphenol A in FF compared with its level in the blood is associated with a decrease in the number and quality of oocytes in ART.
References
- Morgan M.K., Nash M., Barr D.B., Starr J.M., Scott Clifton M., Sobus J.R. Distribution, variability, and predictors of urinary bisphenol A levels in 50 North Carolina adults over a six-week monitoring period. Environ. Int. 2018; 112: 85-99. https://dx.doi.org/10.1016/j.envint.2017.12.014.
- Arya S., Dwivedi A.K., Alvarado L., Kupesic-Plavsic S. Exposure of U.S. population to endocrine disruptive chemicals (Parabens, Benzophenone-3, Bisphenol-A and Triclosan) and their associations with female infertility. Environ. Pollut. 2020; 265(Pt A): 114763. https://dx.doi.org/10.1016/j.envpol.2020.114763.
- Ma Y., Liu H., Wu J., Yuan L., Wang Y., Du X. et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019; 176: 108575. https://dx.doi.org/10.1016/j.envres.2019.108575.
- Onuzulu C.D., Rotimi O.A., Rotimi S.O. Epigenetic modifications associated with in utero exposure to endocrine disrupting chemicals BPA, DDT and Pb. Rev. Envirn. Health. 2019; 34(4): 309-25. https://dx.doi.org/10.1515/reveh-2018-0059.
- Paoli D., Pallotti F., Dima A.P., Albani E., Alviggi C., Causio F. et al. Phthalates and bisphenol A: presence in blood serum and follicular fluid of Italian women undergoing assisted reproduction techniques. Toxics. 2020; 8(4): 91. https://dx.doi.org/10.3390/toxics8040091.
- Kim H.K., Ko D.H., Lee W., Kim K.R., Chun S., Song J. et al. Body fluid concentrations of bisphenol A and their association with in vitro fertilization outcomes. Hum. Fertil. (Camb). 2019; May 17; 1-9. https://dx.doi.org/10.1080/14647273.2019.1612104.
- Rancière F., Botton J., Slama R., Lacroix M.Z., Debrauwer L., Charles M.A. et al. Exposure to bisphenol A and bisphenol S and incident type 2 diabetes: A Case-Cohort Study in the French Cohort D.E.S.I.R. Environ. Health Perspect. 2019; 127(10): 107013. https://dx.doi.org/10.1289/EHP5159.
- Ren X., Zhang T., Chen X., Wei X., Tian Y., Li G. et al. Early-life exposure to bisphenol A and reproductive-related outcomes in rodent models: a systematic review and meta-analysis. Aging (Albany NY). 2020; 12(18): 18099-126. https://dx.doi.org/10.18632/aging.103620.
- Mirihagalle S., You T., Suh L., Patel C., Gao L., Rattan S. et al. Prenatal exposure to di-(2-ethylhexyl) phthalate and high-fat diet synergistically disrupts mouse fetal oogenesis and affects folliculogenesis†. Biol. Reprod. 2019; 100(6): 1561-70. https://dx.doi.org/10.1093/biolre/ioz051.
- Tu Z., Mu X., Chen X., Geng Y., Zhang Y., Li Q. et al. Dibutyl phthalate exposure disrupts the progression of meiotic prophase I by interfering with homologous recombination in fetal mouse oocytes. Environ. Pollut. 2019; 252(Pt A): 388-98. https://dx.doi.org/10.1016/j.envpol.2019.05.107.
- Poormoosavi S.M., Behmanesh M.A. Level of bisphenol A in follicular fluid and serum and oocyte morphology in patients undergoing IVF treatment. J. Family Reprod. Health. 2019; 13(3): 154-9.
- Rodosthenous R.S., Baccarelli A.A., Mansour A., Adir M., Israel A., Racowsky C. et al. Supraphysiological concentrations of bisphenol A alter the expression of extracellular vesicle-enriched miRNAs from human primary granulosa cells. Toxicol. Sci. 2019; 169(1): 5-13. https://dx.doi.org/10.1093/toxsci/kfz020.
- Mansur A., Adir M., Racowsky C., Combelles C.M., Landa N., Machtinger R. Susceptibility of human cumulus cells to bisphenol A in vitro. Reprod. Toxicol. 2017; 74: 189-94. https://dx.doi.org/10.1016/j.reprotox.2017.09.008.
Поступила 11.02.2021
Принята в печать 19.04.2021
About the Authors
Anastasia G. Syrkasheva, Ph.D., Senior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(926)363-17-20. E-mail: a_syrkasheva@oparina4.ru.117997, Russia, Moscow, st. Academician Oparin, 4.
Svetlana V. Kindysheva, Ph.D., Senior Researcher at the Laboratory of Proteomics and Metabolomics of Human Reproduction, Department of Systems Biology
in Reproduction, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-07-88. E-mail: s_kindysheva@oparina4.ru.
117997, Russia, Moscow, st. Academician Oparin, 4.
Natalia L. Starodubtseva, Ph.D. (biol.sci.), Head of the Laboratory of Proteomics and Metabolomics of Human Reproduction, Department of Systems Biology
in Reproduction, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-07-88. E-mail: n_starodubtseva@oparina4.ru.
117997, Russia, Moscow, st. Academician Oparin, 4.
Vladimir E. Frankevich, Ph.D. (Physical and Mathematical Sciences), Head of the Department of Systems Biology in Reproduction, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. Tel.: +7(495)438-07-88. E-mail: v_frankevich@oparina4.ru. 117997, Russia, Moscow, st. Academician Oparin, 4.
Nataliya V. Dolgushina, Dr. Med. Sci., Deputy Director – Head of the Department of Research Administration, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. Tel.: +7(495)438-49-77. E-mail: n_dolgushina@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
For citation: Syrkasheva A.G., Kindysheva S.V., Starodubtseva N.L., Frankevich V.E., Dolgushina N.V. Bisphenol A in infertile patients: impact on assisted reproductive technologies outcomes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 5: 113-120 (in Russian)
https://dx.doi.org/10.18565/aig.2021.5.113-120



