Proteomic analysis of cervicovaginal fluid of patients with HPV-associated cervical lesions treated with activated glycyrrhizic acid
Objective: To investigate the proteome of cervicovaginal fluid (CVF) in patients with HPV-associated cervical lesions treated with activated glycyrrhizic acid (AGA).Frankevich V.E., Nazarova N.M., Dovlethanova E.R., Gusakov K.I., Kononikhin A.S., Bugrova A.E., Brzhozovskiy A.G., Prilepskaya V.N., Chagovets V.V., Starodubtseva N.L., Sukhikh G.T.
Materials and methods: The study included 80 HPV-positive females aged 18 to 49 with cytologically confirmed NILM and chronic cervicitis (group I, n=40) and LSIL (group II, n=40). All patients received a drug with the active AGA substance (Epigen Intim spray 0.1%) for 18 months according to the protocol. The proteomic composition of CVF was analyzed by HPLC-MS/MS at four time points (before therapy, after 6, 12, and 18 months).
Results: In a proteomic study, we compared two groups of CVF samples from 10 patients after AGA administration. Group 1 samples (n=5) showed positive changes (HPV elimination) and group 2 (n=5) had no changes (HPV persistence). Comparison of the proteomic composition of CVF samples in the dynamics revealed a change in 48 proteins. Levels of 27 proteins increased in the positive-change group, while the level of 21 proteins decreased compared to the no-change group. Annotation of proteins showing increase in their levels revealed their involvement in immune system processes (MPO, AZU1, LYZ, RNASE7, PPL, TXN, CD59, UBA52, CRISP3, ELANE), in particular, in the innate immune response (MPO, AZU1, LYZ, RNASE7, TXN, CD59, UBA52, CRISP3, ELANE) as well as in neutrophil degranulation (MPO, AZU1, LYZ, CD59, CRISP3, ELANE), and antimicrobial activity (AZU1, ELANE).
Conclusion: The study findings suggest that long-term topical application of AGA affects CVF protein levels in small cervical lesions, in particular reduces proinflammatory proteins during HPV elimination.
Keywords
Cervical cancer is a rapidly progressive malignancy that affects women at different stages of life, including reproductive age. The etiology of cervical cancer is strongly associated with highly oncogenic human papillomavirus (HPV) types (16, 18, 31, 33, 35, 39, 45, 51, 56, 52, 59, 68) [1]. Persistence of high oncogenic risk HPV types (HR-HPV) initially causes low-grade squamous intraepithelial lesions (LSIL), which are caused by virus replication and are usually eliminated by the immune system within one year. Persistence of a high carcinogenic risk virus for more than 24 months can lead to the development of cervical intraepithelial neoplasia (CIN). With continued persistence of HPV infection, the risk of progression to CIN III in 5 years is 5–7.4%, 8–10%, and 15–17% in patients with normal cytology (NILM), ASCUS, and LSIL, respectively [2].
Viral persistence with prolonged active expression of viral oncoproteins initiates a multistage process in which cervical epithelial cells undergo changes that contribute to tumor progression [3, 4]. Oncoproteins E6 and E7 play an essential role in the development of cervical cancer, as they are necessary to maintain the viral genome in the cell. Persistence is a consequence of decreased cellular immunity and local and general interferon deficiency. According to V. Colmenares et al. [5], HPV is able to inhibit the secretion of interferon gamma (IFN-γ) and the expression of some immune innate cell receptors. Immunoglobulin-like transcript 2 (ILT2) is a regulatory receptor that may be involved in the pathogenesis of viral infection. Induction of immunosuppressive cytokines (IL-10 or TGF-b) is an additional mechanism by which infected HPV cells can avoid immune-mediated elimination. A significant factor is that HPV inactivates the tumor suppressor p53, which plays an important role in the prevention of cancer development. Previous studies by Gusakov KI et al. demonstrated statistically significant differences in the expression of nine CVF proteins directly involved in the immune response (APOB, FABP5, GRN, HP, MUC5AC, OLFM4, PKP1, QSOX1, S100A8) in HPV-vaccinated women [6–10]. Understanding the role of HPV persistence is key to finding and developing effective immune agents that can completely eliminate viral infection.
Ammonium glycyrrhizinate (AMGZ, glycyram, ammonium salt of glycyrrhizin, ammonium salt of glycyrrhizic acid) is a substance extracted from licorice root. It can be obtained in the form of glycyrrhizin ammonium and glycyrrhizin monoammonium by extraction from the plant. Glycyrrhizic acid is a dry extract of licorice containing 26–28% glycyrrhizic acid obtained by extraction with acetone containing 0.1% sulfuric acid at room temperature. The obtained tricalic salt of the glycoside is converted to a monocalic salt by recrystallizing the latter from aqueous ethanol twice at a ratio respectively equal to (5: 1, V/V), and then by conversion to glycyrrhizinic acid by treating the one-pot salt with 1% sulfuric acid solution at 98-100 ° C and with chloroform at room temperature. Ammonium glycyrrhizinate has a wide range of pharmacological and biological activities. It is an HMGB1 protein inhibitor and has anti-inflammatory, antitumor, and antidiabetic effects. In particular, the administration of ammonium glycyrrhizinate and doxorubicin reduced the cardiotoxicity caused by the administration of doxorubicin by enhancing endogenous antioxidant activity. Ammonium glycyrrhizinate has antioxidant properties and can block the expression of genes involved in apoptosis (GDF15, ATF3, TNFRSF10A, NALP1) or include induced oxidative stress (HMOX1). The antiviral effect of activated glycyrrhizic acid has been demonstrated in hepatitis virus. Glycyrrhizin therapy was associated with increase in lymphocyte proliferation 4 days after the initiation of treatment.
Farooqui et al. studied the effect of glycyrrhizin on the HeLa cervical cancer cell line [11]. Cell viability analysis showed that exposure of HeLa cells to glycyrrhizin significantly reduced the viability of cervical cancer cells depending on time and dose. Glycyrrhizin exerts a cytotoxic effect on cervical cancer cells with no significant effect on normal cells. Furthermore, glycyrrhizin exhibited antiproliferative and apoptotic properties against cervical cancer cells, causing disruption of mitochondrial membrane potential, increased generation of AFK, activation of caspases in both external and internal cell death pathways, and induction of cell cycle arrest in the G0/G1 phase. Therefore, the examination of the proteomic composition of the cervicovaginal fluid (CVF) in HPV-positive women with small cervical lesions using activated glycyrrhizinic acid (AGA) is extremely relevant.
The present study aimed to investigate the proteome of cervicovaginal fluid in HPV-positive women treated with AGA to reduce the risk of developing CIN.
Materials and methods
The study enrolled 80 women aged 18 to 49 (mean age 30 years) treated at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Inclusion criteria were age 18 to 49 years, HR-HPV, low-grade squamous intraepithelial lesions (HPV-associated chronic cervicitis and LSIL), sexual activity with a condom during the entire study period, regular menstrual cycle, ability to meet protocol requirements, providing signed informed consent to take part in the study.
Exclusion criteria were patient desire to discontinue participation in the study, allergic reactions or individual intolerance to AGA during the study, noncompliance, and violation of the study protocol by the patient.
Criteria for noninclusion were sexually transmitted infections during the study (gonorrhea, chlamydia, trichomoniasis, syphilis, candidiasis, genital herpes), malignant neoplasms of the reproductive system, pregnancy and lactation, severe somatic diseases, cancer, diabetes, primary and secondary immunodeficiency states, systemic or local antibacterial therapy within one month before the study, and use of oral contraceptives use during the first month of the study.
The participants were divided into 2 groups classified by cytological findings. Group I (n=40) included patients with chronic cervicitis and HPV persistence; group II (n=40) comprised patients with LSIL and HPV. All patients received intravaginal 0.1% AGA intravaginal spray (Epigen Intim spray) three times a month for three months, and then a three-month break, followed by AGA according to the same schedule. Control time points were before the start of therapy and after 6, 12, and 18 months, according to the clinical guidelines of the Ministry of Health for the management of patients.
The comprehensive examination of the women included clinical and medical history, determination of gynecological status, cytological examination, HPV typing, lipidomic, and proteomic analysis of cervical epithelium scrapings. Samples for cytological examination of the cervical epithelium were collected by a Cervex-Brush with detachable brush heads using the standard technique. The cytological evaluation of cervical smears was performed according to the Bethesda system (2014).
Biological material (cervical epithelium scraping) for HPV typing was taken in test tubes with normal saline. Virus DNA was isolated using HS Sample kits (DNA-Technology, Russia). The method was based on the use of a strong chaotropic agent for cell lysis followed by sorption of nucleic acids on a solid carrier, subsequent washing of the sorbent, and elution of DNA from the sorbent. The volume of the sample after isolation was 100 µl. Amplification of type-specific fragments of HPV and human DNA (control of biomaterial adequacy) was performed using a set of reagents for detection and typing and quantitative determination of HPV type 21 by HPV PCR Kvant-21 (DNA-Technology, Russia). Amplification was performed in real time on a DT-964 device (DNA-Technology, Russia). Fluorescence levels were measured at each amplification cycle through FAM, HEX, ROX, and Cy5 channels. The results were processed automatically using the instrument software.
A Leisegang's colposcope (Germany) with ×7.5/15/30 magnification was used for colposcopic examination. The cervical mucosa was examined both without treatment and with subsequent application of a 3% acetic acid solution and then a 2% Lugol aqueous iodine solution (Schiller test). During colposcopy, we marked the sites for the targeted biopsy and histological examination of the biopsy material. The new IFCPC international colposcopic terminology/classification system adopted at the 14th World Congress in Rio de Janeiro was used to assess the colposcopic picture. Colposcopic images were documented graphically in all patients according to the clock position.
To obtain a CVF sample, the vagina and cervix were irrigated with normal saline before any manipulation to minimize the risk of sample contamination with blood. Then centrifugation was performed to remove epithelial cells and the supernatant was frozen and stored at -80°C. After rapid thawing, proteins were reduced with 100 mM dithiothreitol followed by alkylation with 50 mM iodacetamide, precipitation of the protein mixture with ice-cold acetone with 0.1% trifluoroacetic acid and trypsinolysis [9, 10]. The tryptic peptide mixture was analyzed by nanoflow high performance chromatography with tandem mass spectrometry (HPLC-MS/MS) on a Dionex Ultimate 3000 (Thermo Fisher Scientific, USA) coupled to a TIMS TOF Pro (Bruker Daltonics, USA) using a parallel accumulation and sequential fragmentation (PASEF) DDA (data dependent acquisition) data collection method.
The data obtained were analyzed using PEAKS Studio 8.5 software with the following parameters: the mass error of the parent ion was 20 ppm; the mass error of fragmentin was 0.03 Da. Methionine oxidation was set as possible variable modification. The search was performed using the SWISS-PROT Protein Sequence Data Bank. FDR thresholds for all steps were set at or below 0.01 (1%).
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. All participants provided signed informed consent to take part in the study.
Results and discussion
The study included HPV-positive women with cytologically confirmed NILM and chronic cervicitis (group I, n=40) and LSIL (group II, n=40).
We conducted a comparative evaluation of the incidence of high- and low-risk HPV in the study groups. At baseline, HR-HPV type 16 (38%) was predominant in group I patients. Eighteen months after AGA administration, HR-HPV and low-risk HPV were eliminated in 87.5% and 77% of women, respectively.
In group II, HR-HPV type 16 was also the most common at baseline (40%). After 18 months of topical and external application of 0.1% AGA spray, 88% of HR-HPV and 80% of HPV were eliminated.
At the dynamic follow-up of patients with small cervical lesions, the use of AGA was associated with an improved cytological picture in 62.5% of group II patients. No progression to CIN II+ was observed in either group.
All patients included in the study underwent colposcopy. The area of the junction of the multilayered squamous and cylindrical epithelium was fully visualized in 67 (58%) patients, and not fully visualized in 49 (42%). A normal colposcopic picture was observed in 22% of the patients. An abnormal colposcopic picture was revealed in 61 (78%) of the women. Mild changes in the cervical epithelium at colposcopy were observed in 64.4% of cases and included the presence of thin acetowhite epithelium with gentle mosaic and punctation (Fig. 1). Mild changes were the most common in patients in groups I and II. Severe changes occurred in 3.3% of cases (group II). It should be noted that a cervical biopsy with histological verification of LSIL (CIN I) diagnosis was performed in these cases. During the 18-month follow-up, no negative changes were observed in colposcopy in the study groups.
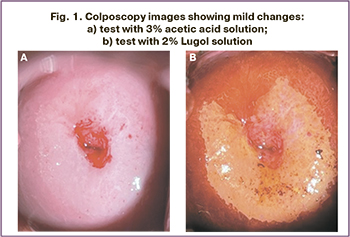
Cervical biopsy was performed after 18 months of follow-up for HPV persistence and cervical epithelial changes suspicious of CIN in 33 (42.3%) patients. LSIL (CIN I) was histologically diagnosed in 26 patients, benign cervix diseases in 6 [chronic cervicitis in four (5.1%), cervical leukoplakia in two (2.6%)].
During the follow-up, samples from 10 patients of the second group (LSIL) treated with AGA were examined to determine CVF proteomic composition. Five samples showed positive changes (HPV elimination) and five did not change (HPV persistence).
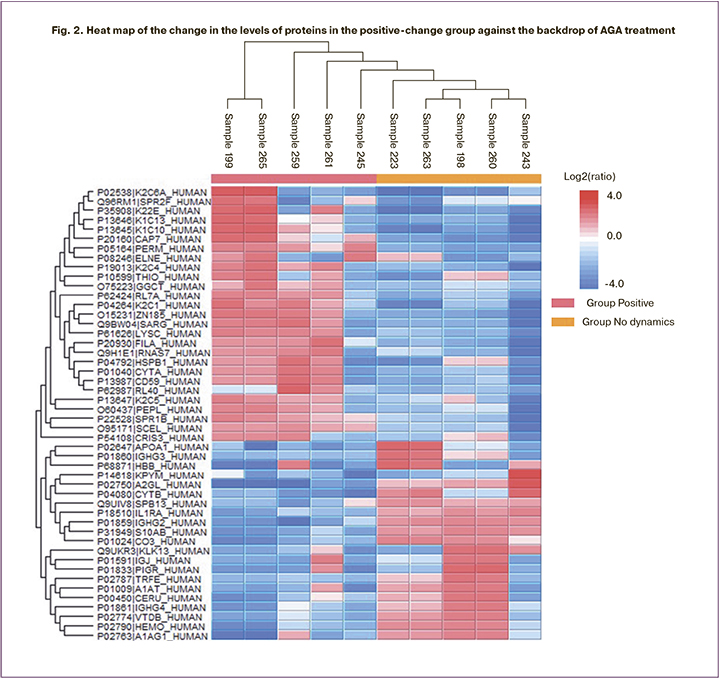
Comparison of the proteomic composition of CVF samples revealed a change in 48 proteins (FDR=0.01). The levels of 27 proteins increased in the positive-change group, while the level of 21 proteins decreased compared to the no-change group (Fig. 2).
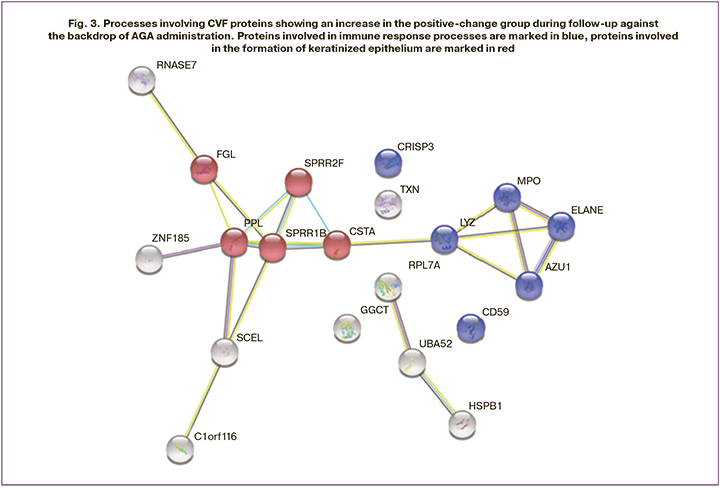
Annotation of proteins showing increase in their levels (Fig. 3), according to Gene Ontology database revealed their involvement in immune system processes (MPO, AZU1, LYZ, RNASE7, PPL, TXN, CD59, UBA52, CRISP3, ELANE), in particular, in the innate immune response (MPO, AZU1, LYZ, RNASE7, TXN, CD59, UBA52, CRISP3, ELANE), and in neutrophil degranulation (MPO, AZU1, LYZ, CD59, CRISP3, ELANE). AZU1, ELANE proteins have antimicrobial activity [12]. Thus, AZU1 is part of the innate defense - human neutrophils. Previous studies have reported that it plays a role in neutrophil-induced increases in vascular permeability [13]. In addition, an increase in the levels of proteins involved in the formation of keratinous epithelium (CSTA, SPRR1B, PPL, FLG, SPRR2F) and keratins (K1C10, K1C13, K22E, K2C1, K2C4, K2C5, K2C6A) should be noted. The increased level of keratin 13 in CVF in adenocarcinoma was confirmed by the the iTRAQ method in 2020 by Zhifang Ma group [14]. Altered levels of keratin 10 and 13 synthesis in epithelial cells have also been shown in cervical and vulvar neoplasia [15, 16].
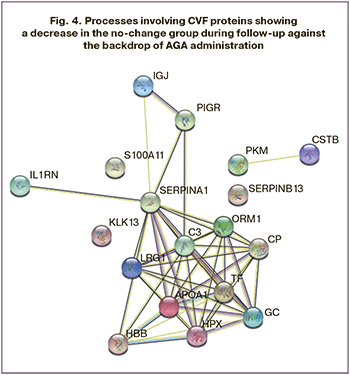
The annotation of proteins with decreasing levels (Fig. 4), according to the Gene Ontology database, also revealed their participation in the immune system processes (C3, IGJ, IL1RN, ORM1, S100A11, CSTB, LRG1, PKM, HBB, PIGR, SERPINA1), but their decrease was presumably due to virus elimination and decrease of pro-inflammatory activity of immune cells in the cervix epithelium.
Earlier studies that analyzed the changes in CVF protein composition in patients with HPV-associated small cervical lesions also revealed significant changes in the levels of proteins involved in the immune response. The level of 13 proteins changed more than 2-fold: A1BG, ACTR3, C4A, CAMP, CAP1, CSTB, GSTP1, HSPA8, LTA4H, LTF, MMP9, PPIA, and S100A11 [9, 10]. It is of particular note that the analysis of proteomic composition of the CVF in women vaccinated against HPV showed changes in the levels of proteins primarily involved in innate immunity processes (APOB, FABP5, GRN, HP, MUC5AC, OLFM4, PKP1, QSOX1 and S100A8). The decreased levels of proteins of the S100 group (S100A8 and S100A11) in this study may be associated with a decrease in the pro-inflammatory activity of the epithelial immune cells due to the elimination of HPV. Further studies should confirm this point of view.
There are more than 21 representatives of the calcium-binding protein family (S100) [17]. This family of proteins perform many intra- and extracellular functions, such as regulation of protein phosphorylation, Ca2+ homeostasis, enzyme activity, cell differentiation and inflammatory response [18]. Some of the S100 proteins are now used in clinical practice as markers of tumors such as melanoma, schwannoma, and neurofibroma [17] and are markers of inflammatory diseases [18].
The levels of HSPB1, IGHG2, and PIGR proteins changed significantly during follow-up. IGHG2 and PIGR protein levels decreased, while HSPB1 levels increased during AGA treatment. The IGHG2 protein is part of the immunoglobulin heavy chain and therefore participates in the recognition phase of the humoral immune response. Secreted immunoglobulins mediate the effector phase of humoral immunity, which leads to the elimination of bound antigens. PIGR is also an immunoglobulin receptor and mediates selective transcytosis of polymeric IgA and IgM through mucosal epithelial cells. The reduction of these proteins after the use of AGA may be associated with a reduction in inflammation during HPV elimination.
This study confirms the effect of long-term topical application of AGA on the CVF proteome in small cervical lesions. In particular, there is a decrease in proinflammatory proteins during HPV elimination.
Due to the high prevalence of papillomavirus infection, researchers face several challenges. Firstly, to identify severe cervical lesions. Second, to identify HPV-positive women with minor lesions who are at risk of pathological process progression to CIN III. Third, to determine CVF protein groups by mass spectrometry and identify their role in the immunopathogenesis of HPV infection, and to evaluate the effectiveness of local immunotherapy to prevent the progression of HPV infection. The present work also aimed at solving these problems. The preliminary findings suggest that long-term local use of 0.1% AGA spray is associated with a significant reduction in the expression of pro-inflammatory CVF proteins that may participate in the protective effect and prevent the development and progression of CIN. These proteins were determined in patients with HPV elimination.
Conclusion
The investigation of drug efficacy by studying the proteomic composition, at both the basic and applied levels, is important to contribute to future innovations for the widespread implementation of immunotherapy for HPV-associated diseases through long-term topical application of AGA.
References
- Garg M., Singhal T., Sharma H. Cardioprotective effect of ammonium glycyrrhizinate against doxorubicin-induced cardiomyopathy in experimental animals. Indian J. Pharmacol. 2014; 46(5): 527-30. https://dx.doi.org/10.4103/0253-7613.140585.
- Gage J.C., Hunt W.C., Schiffman M., Katki H.A., Cheung L.C., Cuzick J. et al.; New Mexico HPV Pap Registry Steering Committee. Risk stratification using human papillomavirus testing among women with equivocally abnormal cytology: results from a state-wide surveillance program. Cancer Epidemiol. Biomarkers Prev. 2016; 25(1): 36-42. https://dx.doi.org/10.1158/1055-9965.EPI-15-0669.
- Бебнева Т.Н., Прилепская В.Н. Папилломавирусная инфекция и патология шейки матки. Гинекология. 2013; 3(3): 77-81. [Bebneva T.N., Prilepskaya V.N. Papillomavirus infection and pathology of the cervix. Gynecology. 2013; 3(3): 77-81. (in Russian)].
- Kaufman R., Adam E., Vonka N. HPV-infection and cervical carcinoma. Clin. Obstet. Gynecol. 2013; 43(2): 363-80. https://dx.doi.org/10.1097/00003081-200006000-00016.
- Colmenares V., Noyola D.E., Monsiváis-Urenda A., Salgado-Bustamante M., Estrada-Capetillo L., González-Amaro R., Baranda L. Human papillomavirus immunization is associated with increased expression of different innate immune regulatory receptors. Clin. Vaccine Immunol. 2012; 19(7): 1005-11. https://dx.doi.org/10.1128/CVI.00043-12.
- Gusakov K., Nazarova N. , Frankevich V., Starodubtseva N., Prilepskaya V., Burmenskaya O., Kononikhin A., Bugrova A., Brzhozovskiy A., Dovletkhanova E., Abakarova P. Mass-spectrometry proteome analysis in QHPV vaccinated women. IPVC 2021 34th International Papillomavirus Conference Research and Education for HPV Elimination. Abstract E-book. 2021; 110.
- Гусаков К.И., Франкевич В.Е., Назарова Н.М., Прилепская В.Н., Стародубцева Н.В., Чаговец В.В., Кононихин А.С., Бржозовский А.Г. Определение ранних маркеров ВПЧ-ассоциированных заболеваний шейки матки в ЦВЖ вакцинированных женщин методом масс-спектрометрии Материалы XXVII Всероссийского конгресса с международным участием «Амбулаторно-поликлиническая помощь в эпицентре женского здоровья». Москва; 2021: 38-9. [Gusakov K.I., Frankevich V.E., Nazarova N.M., Prilepskaya V.N., Starodubtseva N.L., Chagovets V.V., Kononikhin A.S., Brzhozovskiy A.G. Early markers detection in women vaccinated against HPV by mass-spectroscopy. XXVII Congress Outpatient care in the center of women’s health. Moscow; 2021: 38-9. (in Russian)].
- Гусаков К.И., Назарова Н.М., Франкевич В.Е., Стародубцева Н.Л., Бурменская О.В., Прилепская В.Н., Сухих Г.Т. Результаты генотипирования вируса папилломы человека у женщин репродуктивного возраста, вакцинированных от вируса папилломы человека. Акушерство и гинекология. 2020; 9: 114-9. [Gusakov K.I., Nazarova N.M., Frankevich V.E., Starodubtseva N.L., Burmenskaya O.V., Prilepskaya V.N., Sukhikh G.T. Human papillomavirus genotyping results of human papillomavirus vaccinated women of reproductive age. Obstetrics and Gynecology. 2020; 9: 114-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.9.114-119.
- Starodubtseva N.L., Brzhozovskiy A.G., Bugrova A.E., Kononikhin A.S., Indeykina M.I., Gusakov K.I., Chagovets V.V., Nazarova N.M., Frankevich V.E., Sukhikh G.T., Nikolaev E.N. Label-free cervicovaginal fluid proteome profiling reflects the cervix neoplastic transformation. J. Mass Spectrom. 2019; 54(8): 693-703. https://dx.doi.org/10.1002/jms.4374.
- Стародубцева Н.Л., Бржозовский А.Г., Бугрова А.Е., Кононихин А.С., Гусаков К.И., Назарова Н.М. Характеристика динамических изменений протеомного состава цервиковагинальной жидкости при заболеваниях шейки матки, ассоциированных с ВПЧ-инфекцией. Акушерство и гинекология. 2020; 7: 111-6. [Starodubtseva N.L., Brzhozovskiy A.G., Bugrova A.E., Kononikhin A.S., Gusakov K.I., Nazarova N.M., Frankevich V.E. Characteristics of dynamic changes in proteomic composition of cervicovaginal fluid in cervical diseases associated with HPV infection. Obstetrics and Gynecology. 2020; 7: 111-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.7.111-116.
- Farooqui A., Khan F., Khan I., Ansari I.A. Glycyrrhizin induces reactive oxygen species-dependent apoptosis and cell cycle arrest at G0/G1 in HPV18+ human cervical cancer HeLa cell line. Biomed. Pharmacother. 2018; 97: 752-64. https://doi.org/10.1016/j.biopha.2017.10.147.
- Eggers C.T., Murray I.A., Delmar V.A., Day A.G., Craik C.S. The periplasmic serine protease inhibitor ecotin protects bacteria against neutrophil elastase. Biochem. J. 2004; 379(Pt 1): 107-18. https://dx.doi.org/10.1042/BJ20031790.
- McCabe D., Cukierman T., Gabay J.E. Basic residues in azurocidin/HBP contribute to both heparin binding and antimicrobial activity. J. Biol. Chem. 2002; 277(30): 27477-88. https://dx.doi.org/10.1074/jbc.M201586200.
- Ma Z., Chen J., Luan T., Chu C., Wu W., Zhu Y., Gu Y. Proteomic analysis of human cervical adenocarcinoma mucus to identify potential protein biomarkers. PeerJ. 2020; 8: e9527. https://dx.doi.org/10.7717/peerj.9527.
- Carrilho C., Alberto M., Buane L., David L. Keratins 8, 10, 13, and 17 are useful markers in the diagnosis of human cervix carcinomas. Hum. Pathol. 2004; 35(5): 546-51. https://dx.doi.org/10.1016/j.humpath.2004.01.021.
- Dasgupta S., Ewing-Graham P.C., van Kemenade F.J., van Doorn H.C., Hegt V.N., Koljenović S. Differentiated vulvar intraepithelial neoplasia (dVIN): the most helpful histological features and the utility of cytokeratins 13 and 17. Virchows Arch. 2018; 473(6): 739-47. https://dx.doi.org/10.1007/s00428-018-2436-8.
- Marenholz I., Heizmann C.W., Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem. Biophys. Res. Commun. 2004; 322(4): 1111-22. https://dx.doi.org/10.1016/j.bbrc.2004.07.096.
- Donato R. Intracellular and extracellular roles of S100 proteins. Microsc. Res.Tech. 2003; 60(6): 540-51. https://dx.doi.org/10.1002/jemt.10296.
Received 20.05.2022
Accepted 24.05.2022
About the Authors
Vladimir E. Frankevich, Ph.D., Head of the Department of System Biology in Reproduction, Academician V.I.Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(495)438-07-88 (ex. 2198), v_frankevich@oparina4.ru,117997, Russia, Moscow, Ac. Oparina str., 4.
Niso M. Nazarova, Dr. Med. Sci., Leading Researcher, Academician V.I.Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Healthcare of the Russian Federation, +7(495)438-14-03, grab2@yandex.ru, 117997, Russia, Moscow, Ac. Oparina str. 4.
Emira R. Dovletkhanova, PhD, Senior Researcher, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(495)438-14-03, e_dovletkhanova@oparina4.ru, https://orcid.org/0000-0002-8243-5272, 117997, Russia, Moscow, Ac. Oparina str., 4.
Kirill I. Gusakov, PhD, Researcher, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(495)438-14-03, kigusakov@gmail.com, https://orcid.org/0000-0003-3895-8225, 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksey S. Kononikhin, Ph.D., Researcher at the Laboratory of Proteomics of Human Reproduction, Academician V.I.Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation; Senior Researcher at the Laboratory of mass spectrometry, Skolkovo Institute of Science and Technology, 7(495)438-07-88 (ex. 2198), konoleha@yandex.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Anna E. Bugrova, Ph.D., Senior Researcher, at the Laboratory of Proteomics of Human Reproduction, Academician V.I.Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation; Senior Researcher at the Laboratory of neurochemistry, N.M. Emanuel Institute
of Biochemical Physics of RAS, +7(926)562-65-90, a_bugrova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alexander G. Brzhozovskiy, Researcher at the Laboratory of Proteomics of Human Reproduction, Academician V.I.Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation; Junior Researcher at the Laboratory of mass spectrometry, Skolkovo Institute of Science and Technology, +7(495)438-07-88 (ex. 2198), agb.imbp@gmail.com, 117997, Russia, Moscow, Ac. Oparina str., 4.
Vera N. Prilepskaya, Dr. Med. Sci., Professor, Honored Scientist of the Russian Federation, Head of the Scientific Polyclinic Department, Academician V.I. Kulakov
National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(495)438-14-03, vprilepskaya@mail.ru,
https://orcid.org/0000-0003-3993-7629, 117997, Russia, Moscow, Ac. Oparina str., 4.
Vitaliy V. Chagovets, PhD, Senior Researcher at the Laboratory of Proteomics and Metabolomics of Human Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(926)562-65-90, vvchagovets@gmail.com,
https://orcid.org/0000-0002-5120-376X, 117997, Russia, Moscow, Ac. Oparin str., 4.
Natalia L. Starodubtseva, Ph.D., Head of the Laboratory of Proteomics of Human Reproduction, Academician V.I.Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(916)463-98-67, n_starodubtseva@oparina4.ru,
117997, Russia, Moscow, Ac. Oparina str., 4.
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of the RAS, Director of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, g_sukhikh@oparina4.ru, https://orcid.org/0000-0002-7712-1260,
117997, Russia, Moscow, Ac. Oparina str., 4.
Authors' contributions: Sukhikh G.T., Gusakov K.I., Nazarova N.M., Dovlethanova E.R, Frankevich V.E., Starodubtseva N.L., Prilepskaya V.N. – conception and design of the study; Gusakov K.I., Nazarova N.M., Dovlethanova E.R., Bugrova A.E., Brzhozovskiy A.G., Kononikhin A.S. – collection and processing of material; Brzhozovskiy A.G., Starodubtseva N.L., Kononikhin A.S. – statistical analysis; Starodubtseva N.L., Nazarova N.M., Gusakov K.I., Kononikhin A.S., Bugrova A.E., Brzhozovskiy A.G. – manuscript drafting; Sukhikh G.T., Frankevich V.E., Bugrova A.E., Prilepskaya V.N. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Commission on Biomedical Research Ethics of the Academician V.I.Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Frankevich V.E., Nazarova N.M., Dovlethanova E.R.,
Gusakov K.I. , Kononikhin A.S., Bugrova A.E., Brzhozovskiy A.G., Prilepskaya V.N.,
Chagovets V.V., Starodubtseva N.L., Sukhikh G.T. Proteomic analysis of cervicovaginal fluid
of patients with HPV-associated cervical lesions treated with activated glycyrrhizic acid.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 5: 109-117 (in Russian)
https://dx.doi.org/10.18565/aig.2022.5.109-117



