Comparison of methods for detecting biofilm syndrome in bacterial vaginosis
Shalepo K.V., Spasibova E.V., Krysanova A.A., Khusnutdinova T.A., Budilovskaya O.V., Storozheva K.V., Tapilskaya N.I., Savicheva A.M., Kogan I.Yu.
In bacterial vaginosis, Gardnerella vaginalis and Fannyhessea vaginae form highly structured, dense biofilm consortia. Key cells serve as markers of biofilm organization within bacterial communities.
Objective: To compare microbiological methods for detecting biofilm syndrome in bacterial vaginosis.
Materials and methods: Twenty-eight women with complaints of vaginal discharge were examined. Vaginal discharge was used as the clinical material. Microscopy of preparations stained with Gram and methylene blue, as well as fluorescent in situ hybridization (FISH) or the RiGinaM method, were used. The Femoflor test was used for molecular analysis.
Results: The diagnosis of bacterial vaginosis in the Nugent study was established in 89.3% (25/28) of women. The microscopic evaluation of vaginal microbiocenosis using aniline dyes and the RiGinaM method yielded identical results (L:E ratio less than 4:1, with clue cells detected). Clue cells were not identified by any of the methods in only three cases. Among the 25 patients diagnosed with bacterial vaginosis by the Femoflor test, G. vaginalis was detected in all clinical samples, with an average logarithmic value of 7.55, whereas F. vaginae was detected in 22 cases. In three instances of physiological vaginal microbiocenosis, the total bacterial mass was predominantly represented by lactobacilli. G. vaginalis was found among these women, but their total bacterial mass was low (3.6, 4.1, and 6.8 lg), and F. vaginae was detected in only one case (2.1 lg).
Conclusion: Clue cells, as markers of biofilm bacterial vaginosis, can be detected using Gram staining, methylene blue staining, and the RiGinaM method. Microscopic methods using aniline dyes do not differentiate the types of bacteria present in the clue cells, whereas the RiGinaM method requires specific probes to identify microorganisms. The multiplex PCR method in real time can be utilized for the diagnosis of bacterial vaginosis in conjunction with microscopy methods.
Authors' contributions: Shalepo K.V. – methodology, design, and coordination of the study, drafting and editing of the manuscript; Spasibova E.V. – methodology, design, and coordination of the study; Storozheva K.V. – conducting the study, participation drafting and editing of the manuscript; Khusnutdinova T.A., Budilovskaya O.V., Krysanova A.A. – participation in drafting and editing of the manuscript; Tapilskaya N.I., Savicheva A.M. – conception and coordination of the study, editing of the manuscript, approval of the final version of the manuscript; Kogan I.Yu. – conception of the study. All authors contributed significantly to the research and analysis and drafting of the manuscript, read and approved the final version prior to submission.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the Federal Research Institute's scientific topic, "Universal and Specific Mechanisms of Implementation and Disorders of Reproductive Function in the Family", reg. No. 1021062812133-0-3.2.2.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of D.O. Ott Research Institute for OG&R.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' data sharing statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Shalepo K.V., Spasibova E.V., Krysanova A.A., Khusnutdinova T.A., Budilovskaya O.V.,
Storozheva K.V., Tapilskaya N.I., Savicheva A.M., Kogan I.Yu. Comparison of
methods for detecting biofilm syndrome in bacterial vaginosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (7): 121-129 (in Russian)
https://dx.doi.org/10.18565/aig.2025.117
Keywords
Bacterial vaginosis (BV) is a non-inflammatory, biofilm-forming polymicrobial syndrome characterized by complete or partial replacement of the vaginal microflora, predominantly represented by lactobacilli, with high concentrations of other mainly anaerobic microorganisms. BV is prevalent in various regions of the world, occurring in 23–29% of women [1].
Asymptomatic BV is observed in 5–25% of women. On average, BV recurs in approximately 30% of women within the first three months and in 80% of patients within nine months after treatment [1]. Additionally, BV increases susceptibility to sexually transmitted infections such as human immunodeficiency virus, Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, human papillomavirus, and herpes simplex virus [2]. During pregnancy, BV increases the risk of miscarriage, premature rupture of membranes, preterm birth, chorioamnionitis, and postpartum infections. Consequently, screening and treatment for BV are recommended for pregnant women at increased risk of preterm birth between 12 and 16 weeks, even if asymptomatic [3].
Laboratory diagnostics play a crucial role in the early detection of diseases and monitoring the effectiveness of therapy.
Since the publication of the CDC guidelines for the treatment of sexually transmitted infections in 2015 [4], several methods have been used to diagnose BV: the Amsel criteria with microscopy of native preparations; Hallen, Ison–Hay, and Nugent criteria for evaluating Gram-stained preparations [5]; a test for vaginal sialidase activity; a test for metabolic byproducts of G. vaginalis; and a test using oligonucleotide probes that detect high concentrations of G. vaginalis (more than 5×105 CFU/ml of vaginal fluid). The sensitivity and specificity of these tests have recently been summarized by Coleman et al. [6]. Currently, commercial tests based on nucleic acid amplification are employed for the diagnosis, featuring high sensitivity and specificity. These tests detect specific bacterial nucleic acids, including G. vaginalis, A. vaginae, BVAB2, Megasphaera spp., and certain types of lactobacilli, such as L. crispatus, L. jensenii, and L. gasseri [2].
The primary requirement for laboratory diagnostics is a rapid, accurate, and easily reproducible method with a high sensitivity and specificity. Microscopy methods are quick and cost-effective, allowing for direct visualization and approximate characterization of bacteria based on their cell wall structure, while also enabling the assessment of the cellular composition of the vaginal biotope and leukocyte response. Advances in clinical microbiology have led to the development of molecular methods such as real-time polymerase chain reaction (PCR) in a multiplex format. The combination of microscopic and molecular biological methods facilitates accurate diagnosis of vaginal infections.
Researchers are currently increasingly interested in bacterial biofilms formed by various bacteria colonizing the vagina, both in healthy conditions and in diseases, particularly BV, and its recurrent forms.
In physiological microbiocenosis, commensal Lactobacillus species exist as weakly attached conglomerates that are classified as aggregate-type biofilms. They can be found in vaginal samples from women without clinical symptoms [7]. In BV, dense biofilms are formed, predominantly consisting of Gardnerella vaginalis and Fannyhessea vaginae, and these biofilms are firmly adhered to the vaginal epithelial cells [7, 8].
The diversity of vaginal microflora in BV was first described by Schröder in 1921 [9]. In 1955, Gardner H.L. and Dukes C.D. described squamous epithelial cells covered with a layer of vaginal microorganisms, coining the term "clue cells" due to their central role in diagnosing BV. These cells are absent in healthy women [10]. Clue cells are now considered part of the vaginal bacterial biofilm. The emergence of new species of Gardnerella [11] and their detection in healthy women prompted researchers to seek new diagnostic tests to ascertain the significance of these species in disease development, particularly in recurrent BV. Gushchin A.E. developed molecular tests to identify different genotypes of Gardnerella, which were subsequently classified as separate species. Krysanova et al. (2021) demonstrated for the first time that the simultaneous detection of multiple genotypes of G. vaginalis indicates recurrent BV, likely in its biofilm form [12].
Study by Swidsinski A. (2010) revealed that G. vaginalis can exist in two forms: bound (conglomerates) and dispersed (single bacteria). In the bound form, a significant number of Gardnerella attached to epithelial cells in all patients with confirmed BV and their sexual partners. Single Gardnerella were found among a mass of other microorganisms and were identified not only in healthy women but also in men, suggesting a potential sexual route of transmission for BV [13].
Swidsinski A. was among the first to demonstrate the utility of the FISH method (fluorescent in situ hybridization), later termed RiGinaM (ribosomal in situ hybridization), for detecting bacterial biofilms using specific molecular probes. This method can also utilize probes developed by Gushchin A.E. to identify different species of Gardnerella [14].
Thus, the emergence of new methods for visualizing clue cells as a variant of biofilm syndrome has encouraged us to conduct comparative studies using microscopy, RiGinaM, and multiplex real-time PCR methods.
This study aimed to compare microbiological methods for detecting the biofilm syndrome in bacterial vaginosis.
Materials and methods
The study included clinical materials from 28 reproductive age women, aged 22 to 35 years, who presented with complaints of discomfort and discharge from the genital tract.
Vaginal discharge samples were collected using three cotton swabs. One swab was placed in an Eppendorf tube for real-time PCR analysis; the second swab was used to apply the material to two slides for microscopic examination and assessment of vaginal microbiocenosis; the third swab was utilized for RiGinaM analysis, where the material was placed on a slide and immediately fixed by immersion in Carnoy's solution [15, 16].
For microscopic examination, the preparations were stained using the Gram staining method and methylene blue. The results of the Gram staining were evaluated according to Nugent's criteria. When staining with methylene blue, the ratio of polymorphonuclear leukocytes to vaginal epithelial cells, presence or absence of clue cells, and morphotypes of lactobacilli and other microorganisms were assessed. The real-time PCR method, specifically the Femoflor 16 test (DNA-technology, Moscow, Russia), was employed for molecular research.
The same clinical materials were examined using FISH or the RiGinaM method. RiGinaM technology is based on the FISH method, which employs fluorescent microscopy and a panel of various fluorescently labeled 16S rRNA probes.
Multiplex hybridization was conducted on a SuperFrost Plus slide (SuperFrost, Langenbrinck, Emmendingen, Germany) in a 5 mm² quadrant. A hybridization buffer was prepared according to the protocol provided at http://www.swidsinski.de/zusatzdateien/fishmethode/fishmethode.htm.
Microscopic examination was performed using a fluorescence microscope (BX51 WI; Olympus, Japan). Individual bacterial cells were classified as scattered bacteria, whereas aggregates of bacterial cells attached to vaginal epithelial cells were defined as adhesive bacteria that formed a biofilm. A species-specific signal was considered positive only if it corresponded to a positive analog in DAPI staining and demonstrated a positive signal simultaneously with a broad-spectrum probe.
The diagnosis of BV was established based on the Nugent method, which is recognized as the gold standard for diagnosis according to published data [9].
Results
The table presents data from a comparative evaluation of laboratory diagnostic methods for BV.
As can be seen from the data presented in the table, clinical materials from 28 women of reproductive age were evaluated using the Nugent scale. A microscopic picture of BV was detected in 89.3 % (25/28) of cases. Microscopic examination of vaginal microbiocenosis revealed that all women in the vaginal biotope showed no inflammatory reaction (L:E ratio was less than 4:1); clue cells were detected both when stained with methylene blue and by the RiGinaM method. In only three cases, clue cells were not detected simultaneously using these two methods, and the Nugent criteria indicated the absence of BV.
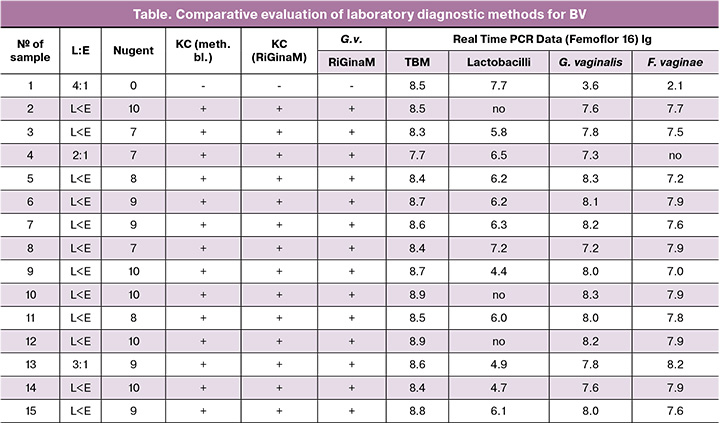
When using the Femoflor test, it was found that the total bacterial mass in all clinical materials was high – from 7.7 to 8.9 lg, with an average logarithm of 8.5. Lactobacillus spp. was detected in 22 (78.5%) samples, with an average logarithm of 5.7. In three cases of physiological vaginal microbiocenosis (samples 1, 16, and 17), the total bacterial mass was mainly represented by lactobacilli. G. vaginalis was detected in these women, but their total bacterial mass was low (3.6; 4.1 and 6.8 lg), F. vaginae was detected in only one case (2.1 lg). Lactobacilli were not detected in 6/25 (21.4%) patients diagnosed with BV. In these women, G. vaginalis was detected in all clinical samples with an average logarithm of 7.55, and F. vaginae was detected in 22 cases.
For example, we present the data obtained from patient D., aged 28 years, with complaints of vaginal discharge. The results are presented in the table under number 1. The results of microscopic examination of the vaginal samples are presented in Figure 1.
Figure 1 shows a microscopic picture of physiological vaginal microbiocenosis. Cells of the stratified squamous epithelium are visible, with the absence of a leukocytic reaction and the presence of lactobacilli according to light microscopy (gram-positive rods). The same picture can be seen in the micropreparations on the right. When staining with specific probes, lactobacilli were detected, and the species Lactobacillus crispatus was identified.
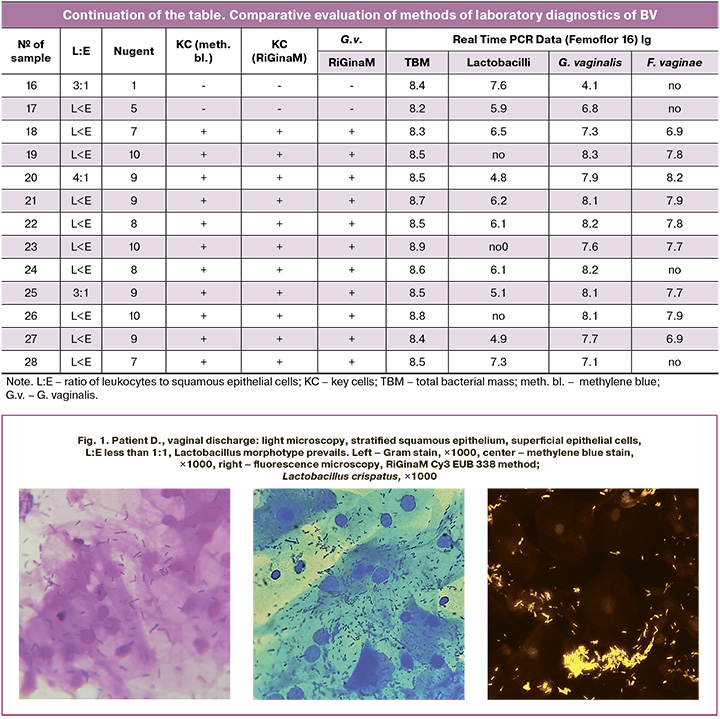
Figure 2 presents the data obtained from patient K., 32 years of age, with complaints of vaginal discharge. They are presented in the table under number 24. The microphotographs show clue cells, which were visualized using all the microscopic methods. When specific probes were used, Gardnerella vaginalis was detected.
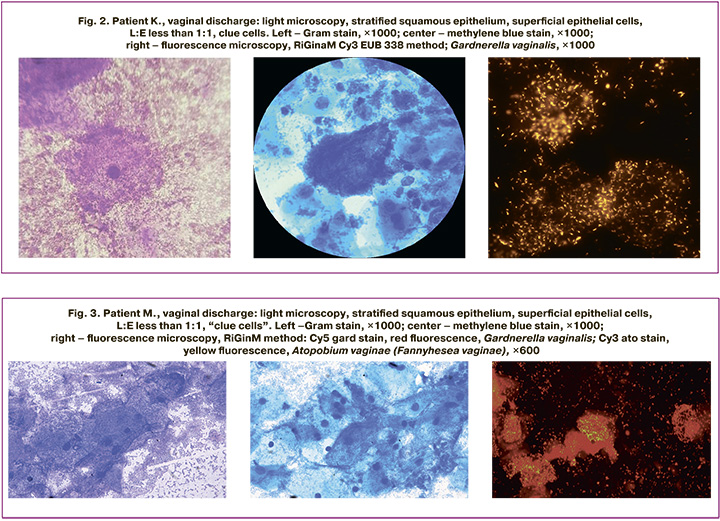
Figure 3 presents the data obtained from patient M., 34 years of age, with complaints of vaginal discharge. They are presented in the table under number 26. Clue cells were visualized using all the staining methods. When specific Cy5 gard probes were used, Gardnerella vaginalis was detected, and Cy3 ato probes detected Atopobium vaginae (Fannyhesеa vaginae).
Thus, when comparing microscopic methods for visualizing clue cells as a marker of biofilm BV, it was noted that bacterial films were detected by any method when staining preparations with Gram, methylene blue, or the RiGinaM method. Owing to the subjectivity of microscopic methods, the experience of the personnel conducting microscopic examinations is important. Staining with aniline dyes does not differentiate the species of bacteria that make up the clue cells. The RiGinaM method requires specific probes to determine the species of the microorganisms. Of the 28 women we examined with complaints of vaginal discharge, only three patients were not diagnosed with BV; clue cells were not detected by any method, and real-time PCR revealed the dominance of lactobacilli with a low concentration of Gardnerella vaginalis and Fannyhesеa vaginae.
Discussion
Microscopy has historically served as the first diagnostic method. Currently, it remains at the initial stage of diagnosis when examining microbiocenosis of the reproductive tract. The routine use of microscopy minimizes its impact on clinical material, allowing for a comprehensive assessment of vaginal microbiocenosis. Staining clinical materials with aniline dyes enables the determination of leukocyte counts, assessment of epithelial cells, evaluation of mucus presence, and analysis of the ratios between leukocytes and epithelial cells and bacterial morphotypes [17]. Light microscopy evaluates the quality of the collected clinical material [18], identifying basal and parabasal cells resulting from epithelial desquamation, elements of yeast-like fungi, the presence of Trichomonas vaginalis protozoa, and absolute pathogens such as Neisseria gonorrhoeae. A key aspect is the ability to assess the ratio of polymorphonuclear leukocytes to squamous epithelial cells, which provides insight into the presence or absence of local inflammation—something other methods do not offer. The determination of leukocytes in the vagina is not standardized; however, leukocyte counting can be performed in a microscopic field using stained vaginal smears or wet vaginal preparations. Standardizing the thickness and concentration of these preparations is a challenging task. Randjelovic I. et al. (2018) reported that a leukocyte counts greater than 4 per epithelial cell in phase-contrast microscopy of a vaginal wet preparation indicates local inflammation [19]. Our study incorporated a microscopic criterion, specifically the ratio of leukocytes to epithelial cells [19]. We evaluated microbial morphotypes rather than their species composition, with routine microscopy enabling assessment of the diversity of the present bacterial morphotypes. However, a limitation exists in the resolving power of the microscope for detecting microorganisms larger than 4 µm.
A significant aspect of our study was our consideration of not only the presence of lactobacilli, but also their ratio with other bacteria, whether lactobacilli are in the minority or predominating. In microscopic examinations of vaginal discharge samples, lactobacilli were identified as large gram-positive rods with smooth blunt ends.
In 1995, Kira E.F. developed a classification for assessing vaginal microbiocenosis, distinguishing four types. This classification integrates microscopic interpretation with clinical characteristics corresponding to specific nosological forms [20].
Amsel's criteria, first published in 1983, are most commonly used for the clinical diagnosis of bacterial vaginosis (BV) [21]. However, these methods have not yet been widely applied in clinical practice. In many countries, two traditional systems are employed to evaluate microscopy data for BV diagnosis. The first was described by Spiegel et al. in 1983 and was later modified by Nugent et al. in 1991 [22]. These methods are thoroughly detailed and used according to a scoring system for diagnosing BV.
In our study, we applied the Nugent's criteria to diagnose BV. Among the 28 women who reported vaginal discharge, 25 (89,3%) were diagnosed with BV. Notably, all parameters from our microscopic and molecular biological studies correlated with each other.
Thus, clue cells, as the main criterion for biofilm BV, were found in a microscopic examination of the clinical materials of all 25 women who were diagnosed with BV according to Nugent's criteria (score from 7 to 10). When viewing preparations stained with Gram, methylene blue, and FISH, we found clue cells. It should be emphasized that when using light microscopy of preparations stained with aniline dyes, it is impossible to determine the species of microorganisms that make up the biofilms and, accordingly, adhere to the clue cells [18]. This can be achieved by RiGinaM when using fluorescent probes for specific species and even genotypes of microorganisms. In three cases, BV was not diagnosed in women according to Nugent’s criteria. In all these women, when using the microscopy method, clue cells were not found; at the same time, lactobacilli were detected, and RiGinaM made it possible to detect a specific species of lactobacilli when using the corresponding DNA probes.
A more modern method for assessing vaginal microbiocenosis and diagnosing BV is the multiplex real-time PCR method, which allows for qualitative and quantitative evaluation of microorganisms. In our study, we utilized the Femoflor test. This method enabled us to determine the total bacterial mass, which was notably high and ranging from 7.7 to 8.9 lg. In three cases of physiological vaginal microbiocenosis, the total bacterial mass was predominantly composed of lactobacilli, with Gardnerella vaginalis present at low concentrations, and Fannyhesae vaginae was detected in one case at a level of 2.1 lg. This information is crucial to evaluate normal vaginal microbiocenosis. In cases of diagnosed BV, the total bacterial mass was primarily represented by Gardnerella species, with both G. vaginalis and F. vaginae constituting the main components of the vaginal microbiocenosis and were detected at sufficiently high concentrations. A limitation of multiplex real-time PCR is the inability to visualize the preparation, which hinders the detection of cellular composition and assessment of the inflammatory response.
Regarding FISH or RiGinaM methods, it is important to note that this technique enables the determination and identification of microorganisms in a sample. FISH is based on hybridization of a fluorescently labeled nucleic acid probe with a complementary sequence present within the microbial cell, typically in the form of ribosomal RNA (rRNA). The hybridized cell was identified because of the abundance of fluorescent particles inside the cell corresponding to the available targets [15]. A panel of various fluorescently labeled probes is employed to diagnose BV, including pathogens/groups such as Gardnerella spp., F. vaginae, Bifidobacteriaceae, and lactobacilli, or bacteria in general (Eubacteria). FISH allows taxonomic identification and assessment of the spatial distribution of microorganisms in clinical samples. 16S rRNA, present in large quantities of bacterial ribosomes (10³–10⁵ copies), contains both species-specific and group-specific or universal bacterial sites [8].
In our study, probes labeled with different fluorochromes were used for simultaneous analysis using a luminescence microscope (multicolor FISH) to identify specific species within a group of microorganisms. The advantage of the in situ fluorescent hybridization method is the integration of microscopic capabilities with nucleic acid amplification techniques.
Thus, the presence of a highly structured polymicrobial biofilm on the vaginal epithelium contributes to the development of disease relapse, and the detection of clue cells in preparations serves as a primary diagnostic marker for BV. It is essential to differentiate between true and false clue cells. True clue cells form when Gardnerella and other bacteria constituting the biofilm adhere to the surface of the epithelial cells. In contrast, when bacteria are separately located in the vaginal mucus, a uniform coating of the bacterial suspension can be observed on the squamous epithelial cells and intercellular spaces. Consequently, only the characteristic arrangement of adhered bacterial conglomerates on the surface of stratified squamous epithelial cells represents a biofilm. Many researchers concur that the detection of true clue cells indicates biofilm-associated BV [9].
Our study also demonstrated that clue cells can be visualized using light microscopy with aniline dye staining, which aligns with the FISH results.
Conclusions
- Microbiological methods for detecting biofilm syndrome in BV were compared, including light microscopy with Gram and methylene blue staining, luminescence microscopy using RiGinaM technology, and multiplex real-time PCR (Femoflor).
- It was observed that clue cells, as a marker of biofilm-associated BV, could be detected by all methods, including Gram staining, methylene blue, or RiGinaM. The subjectivity of microscopic methods highlights the importance of personnel experience in conducting examinations. Aniline dye staining does not differentiate the bacterial species comprising the clue cells, whereas RiGinaM requires specific probes to identify the microorganism species.
- Multiplex real-time PCR represents a modern approach for assessing vaginal microbiocenosis and can be effectively combined with microscopic methods for diagnosing BV.
References
- Peebles K., Velloza J., Balkus J.E., McClelland R.S., Barnabas R.V. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex. Transm. Dis. 2019; 46(5): 304-11. https://dx.doi.org/10.1097/OLQ.0000000000000972
- Muzny C.A., Balkus J., Mitchell C., Sobel J.D., Workowski K., Marrazzo J. et al. Diagnosis and management of bacterial vaginosis: summary of evidence reviewed for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines. Clin. Infect. Dis. 2022;74(Suppl. 2): S144-51. https://dx.doi.org/10.1093/cid/ciac021
- Yudin M.H., Money D.M. No. 211 – Screening and management of bacterial vaginosis in pregnancy. J. Obstet. Gynaecol. Can. 2017; 39(8): e184-91. https://dx.doi.org/10.1016/j.jogc.2017.04.018
- Workowski K.A., Bolan G.A.; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015; 64(RR-03): 1-137.
- Ison C.A., Hay P.E. Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex. Transm. Infect. 2002; 78(6): 413-5. https://dx.doi.org/10.1136/sti.78.6.413
- Coleman J.S., Gaydos C.A. Molecular diagnosis of bacterial vaginosis: an update. J. Clin. Microbiol. 2018; 56(9): e00342-18. https://dx.doi.org/10.1128/JCM.00342-18
- Swidsinski A., Mendling W., Loening-Baucke V., Ladhoff A., Swidsinski S., Hale L.P. et al. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 2005; 106(5 Pt 1): 1013-23. https://dx.doi.org/10.1097/01.aog.0000183594.45524.d2
- Hardy L., Jespers V., Dahchour N., Mwambarangwe L., Musengamana V., Vaneechoutte M. et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PLOS One. 2015; 10(8): e0136658. https://dx.doi.org/10.1371/journal.pone.0136658
- Abou Chacra L., Fenollar F., Diop K. Bacterial vaginosis: what do we currently know? Front. Cell. Infect. Microbiol. 2022; 11: 672429. https://dx.doi.org/10.3389/fcimb.2021.672429
- Gardner H.L., Dukes C.D. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified non-specific vaginitis. Am. J. Obstet. Gynecol. 1955; 69(5): 962-76. https://dx.doi.org/10.1016/0002-9378(55)90095-8
- Vaneechoutte M., Guschin A., Van Simaey L., Gansemans Y., Van Nieuwerburgh F., Cools P. Emended description Gardnerella vaginalis and description Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int. J. Syst. Evol. Microbiol. 2019; 69(3): 679-87. https://dx.doi.org/10.1099/ijsem.0.003200
- Крысанова А.А., Гущин А.Е., Савичева А.М. Значение определения генотипов Gardnerella vaginalis в диагностике рецидивирующего бактериального вагиноза. Медицинский алфавит. 2021; 30: 48-52. [Krysanova A.A., Gushchin A.E., Savicheva A.M. The importance of determining Gardnerella vaginalis genotypes in the diagnosis of recurrent bacterial vaginosis. Medical Alphabet. 2021; 30: 48-52 (in Russian)]. https://dx.doi.org/10.33667/2078-5631-2021-30-48-52
- Swidsinski A., Doerffel Y., Loening-Baucke V., Swidsinski S., Verstraelen H., Vaneechoutte M. et al. Gardnerella biofilm involves females and males and is transmitted sexually. Gynecol. Obstet. Invest. 2010; 70(4): 256-63. https://dx.doi.org/10.1159/000314015
- Swidsinski A., Loening-Baucke V., Swidsinski S., Sobel J.D., Dörffel Y., Guschin A. Clue cells and pseudo clue cells in different morphotypes of bacterial vaginosis. Front. Cell. Infect. Microbiol. 2022; 12: 905739. https://dx.doi.org/10.3389/fcimb.2022.905739
- Swidsinski A., Amann R., Guschin A., Swidsinski S., Loening-Baucke V., Mendling W. et al. Polymicrobial consortia in the pathogenesis of biofilm vaginosis visualized by FISH. Historic review outlining the basic principles of the polymicrobial infection theory. Microbes Infect. 2024; 26(8): 105403. https://dx.doi.org/10.1016/j.micinf.2024.105403
- Oliveira R., Almeida C., Azevedo N.F. Detection of microorganisms by fluorescence in situ hybridization using peptide nucleic acid. Methods Mol. Biol. 2020; 2105: 217-30. https://dx.doi.org/10.1007/978-1-0716-0243-0_13
- Савичева А.М. Современные представления о лабораторной диагностике репродуктивно значимых инфекций у женщин репродуктивного возраста. Мнение эксперта. Вопросы практической кольпоскопии. Генитальные инфекции. 2022; (3): 34-9. [Savicheva A.M. Modern ideas about the laboratory diagnosis of reproductively significant infections in women of reproductive age. Expert opinion. Issues of Practical Colposcopy & Genital Infections. 2022; 3: 34-9 (in Russian)]. https://dx.doi.org/10.46393/27826392_2022_3_34
- Савичева А.М., Крысанова А.А., Шалепо К.В., Спасибова Е.В., Будиловская О.В., Хуснутдинова Т.А., Тапильская Н.И., Коган И.Ю., Свидзинский А.В., Свидзинская С. Применение метода флуоресцентной гибридизации in situ в диагностике бактериального вагиноза. Акушерство и гинекология. 2023; 12: 68-77. [Savicheva A.M., Krysanova A.A., Shalepo K.V., Spasibova E.V., Budilovskaya O.V., Khusnutdinova T.A., Tapilskaya N.I., Kogan I.Yu., Swidsinski A.V., Swidsinski S. Application of fluorescent in situ hybridization in the diagnosis of bacterial vaginosis. Obstetrics and Gynecology. 2023; (12): 68-77 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.129
- Randjelovic I., Moghaddam A., Freiesleben de Blasio B., Moi H. The role of polymorphonuclear leukocyte counts from urethra, cervix, and vaginal wet mount in diagnosis of nongonococcal lower genital tract infection. Infect. Dis. Obstet. Gynecol. 2018; 2018: 8236575. https://dx.doi.org/10.1155/2018/8236575
- Кира Е.Ф. Бактериальный вагиноз. М.: МИА; 2012. 470 c. [Kira E.F. Bacterial vaginosis. Moscow: MIA; 2012. 470 p. (in Russian)].
- Amsel R., Totten P.A., Spiegel C.A., Chen K.C., Eschenbach D., Holmes K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983; 74(1): 14-22. https://dx.doi.org/10.1016/0002-9343(83)91112-9
- Nugent R.P., Krohn M.A., Hillier S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991; 29(2): 297-301. https://dx.doi.org/10.1128/jcm.29.2.297-301.1991
Received 30.04.2025
Accepted 18.06.2025
About the Authors
Kira V. Shalepo, PhD, Senior Researcher at the Experimental Microbiology Group, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,3 Mendeleyevskaya Line, 199034, St. Petersburg, Russia, +7(911)2474151, 2474151@mail.ru, https://orcid.org/ 0000-0002-3002-3874
Elena V. Spasibova, Bacteriologist at the Laboratory of Clinical Microbiology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 3 Mendeleyevskaya Line, 199034, St. Petersburg, Russia, elena.graciosae@gmail.com, https://orcid.org/0009-0002-6070-4651
Anna A. Krysanova, PhD, Senior Researcher at the Experimental Microbiology Group, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
3 Mendeleyevskaya Line, 199034, St. Petersburg, Russia, krusanova.anna@mail.ru, https://orcid.org/ 0000-0003-4798-1881
Olga V. Budilovskaya, PhD, Senior Researcher at the Experimental Microbiology Group, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
3 Mendeleyevskaya Line, 199034, St. Petersburg, Russia, o.budilovskaya@gmail.com, https://orcid.org/0000-0001-7673-6274
Tatiana A. Khusnutdinova, PhD, Senior Researcher at the Experimental Microbiology Group, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
3 Mendeleyevskaya Line, 199034, St. Petersburg, Russia, husnutdinovat@yandex.ru, https://orcid.org/0000-0002-2742-2655
Kseniia V. Storozheva, PhD Student, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 3 Mendeleyevskaya Line,
199034, St. Petersburg, Russia, kvstorozheva@mail.ru, https://orcid.org/0009-0005-8954-0234
Natalya I. Tapilskaya, Dr. Med. Sci., Professor, Leading Researcher at the Reproduction Department, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 3 Mendeleyevskaya Line, 199034, St. Petersburg, Russia, tapnatalia@mail.ru, https://orcid.org/ 0000-0001-5309-0087
Alevtina M. Savicheva, Dr. Med. Sci., Professor, Head of the Department of Medical Microbiology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 3 Mendeleyevskaya Line, 199034, St. Petersburg, Russia, savitcheva@mail.ru, https://orcid.org/ 0000-0003-3870-5930
Igor Yu. Kogan, Corresponding Member of the RAS, Dr. Med. Sci., Professor, Director, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
3 Mendeleyevskaya Line, 199034, St. Petersburg, Russia, ovr@ott.ru, https://orcid.org/0000-0002-7351-6900
Corresponding author: Kira V. Shalepo, 2474151@mail.ru



