Effect of vaginal microbiota composition in early pregnancy on pregnancy course and outcomes
Shadrova P.A., Bondarenko K.R., Guschin A.E., Zatevalov A.M., Metalnikova V.O., Dobrokhotova Yu.E.
Objective: This study aimed to investigate the outcomes of pregnancy in relation to the quantitative and qualitative composition of cervicovaginal microbiota in early pregnancy using real-time polymerase chain reaction (RT-PCR).
Materials and methods: The study included 153 pregnant women at 7–12 weeks of gestation, with 73 experiencing a threatened miscarriage and 80 having a healthy pregnancy. The entire cohort was divided into three groups based on the outcomes. Group I (n=122) consisted of women who delivered at term, group II (n=7) included patients with preterm delivery, and group III (n=24) comprised women who experienced spontaneous miscarriage. The analysis involved laboratory investigation that quantitatively determined the DNA of opportunistic flora associated with the development of bacterial vaginosis (BV), aerobic vaginitis (AV), and candidal vulvovaginitis (CVV). Additionally, DNA from pathogens of four sexually transmitted infections (Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis) was determined. All study participants underwent diagnostic procedures in accordance with regulatory documents and received treatment in Moscow hospitals or outpatient settings, under the supervision of an obstetrician-gynecologist.
Results: Patients whose pregnancies were terminated in the first half of gestation had a higher incidence of BV (70.8%) than those who delivered at term (22.2%, p<0.05). The frequency of AV in women with spontaneous abortion was twice as high (8.3%) as that in patients who delivered at term (3.9%, p<0.05). The prevalence of VVC in the three groups was not statistically different. The presence of more than five microorganism markers involved in the development of BV, AV, and VVC, as well as opportunistic genital mycoplasmas detected in the vagina of a pregnant woman in the first trimester, was associated with the development of spontaneous miscarriage in the first trimester and preterm birth (p<0.01).
Conclusion: Laboratory evaluation of the reproductive tract using RT-PCR revealed higher detection rates
of certain nosologies and syndromes in pregnant women with adverse pregnancy outcomes. The early detection of reproductive tract infections is associated with an increased risk of early pregnancy loss and preterm delivery.
Authors' contributions: Shadrova P.A. – work with literature sources, drafting of the manuscript; Bondarenko K.R. – drafting
of the manuscript; Guschin A.E. – conception of the study; Zatevalov A.M. – statistical analysis; Metalnikova V.O. – work with literature sources; Dobrokhotova Yu.E. – development of the topic of the article.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Pirogov RNRMU of Minzdrav of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Shadrova P.A., Bondarenko K.R., Guschin A.E., Zatevalov A.M., Metalnikova V.O., Dobrokhotova Yu.E.
Effect of vaginal microbiota composition in early pregnancy on pregnancy course and outcomes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (8): 70-78 (in Russian)
https://dx.doi.org/10.18565/aig.2024.49
Keywords
The prevention of early and late pregnancy losses is considered one of the most important tasks in modern obstetrics and gynecology, especially under the current conditions of negative natural population growth in the Russian Federation (Rosstat, 2021). There are 23 million cases of spontaneous miscarriages registered annually worldwide, with a total risk of 15.3% of all diagnosed pregnancies [1]. Early reproductive losses can be classified as preventable or unpreventable, depending on the etiological factors. Approximately 50% of sporadic miscarriages are caused by "non-modifiable" chromosomal defects, which cannot be corrected at this stage of scientific and practical medical development, even when parents have a normal karyotype [2]. Therefore, clinicians should focus on addressing the so-called "modifiable" factors of early pregnancy losses, including behavioral, anatomical, endocrine, toxic, immunological, and infectious factors [3–6].
Before the COVID-19 pandemic, it was established that infectious causes, including a wide range of viral, bacterial, and protozoal human diseases, accounted for up to 15% of pregnancy losses before 20 weeks [7]. However, the specific impact of abnormal composition of the cervicovaginal microbiome in the early stages of pregnancy remains unclear [8]. Furthermore, research on the consequences of disturbances in the microbial balance of the cervicovaginal locus before the 12th week of pregnancy on the incidence of preterm birth is limited. Available studies demonstrate that acute infections and the exacerbation of chronic infections in the early stages of gestation can disrupt delicate immune-dependent mechanisms associated with ovum implantation, cytotrophoblastic invasion, remodeling of spiral arteries, and placentation. These disruptions ultimately lead to spontaneous miscarriage or the development of "great obstetric syndromes" [9, 10].
According to the World Health Organization (WHO), the incidence of premature births worldwide ranges from 5% to 18% of the total number of births depending on the population. This has resulted in an annual birth of 15 million premature babies [11]. On average, 40–50% of spontaneous preterm births, most commonly occurring between 22 and 32 weeks of pregnancy, are associated with direct exposure to various infectious agents [12].
Currently, scientific progress in the fields of molecular biology and genetics has significantly expanded the possibilities of studying the vaginal microbiome as an active participant in the gestational process. This may provide physicians with an effective tool for predicting and preventing obstetric complications through early assessment of cervicovaginal microbial community parameters. Therefore, the aim of our study was to investigate the course and outcomes of pregnancy in relation to the quantitative and qualitative composition of the cervicovaginal microbiota in early pregnancy.
Materials and methods
The study included 153 women of reproductive age who underwent laboratory examination and signed voluntary informed consent to participate in the study in accordance with the order of the Ministry of Health of the Russian Federation dated October 20, 2020, N 1130n "On approval of the Procedure for the provision of medical care in the profile of "obstetrics and gynecology"". Of these, 73 patients experiencing threatened miscarriage during the first trimester were hospitalized in the gynecology department of N.I. Pirogov City Clinical Hospital No. 1 and F.I. Inozemtsev City Clinical Hospital No. 36 in Moscow.
Recruitment of patients to the study group was carried out according to the following inclusion criteria: age of patients in the range of 18–35 years; singleton intrauterine pregnancy, gestational age of 7–12 weeks; diagnosis of threatened miscarriage, verified on the basis of bloody discharge from the genital tract. The exclusion criteria were pregnancy achieved with the use of assisted reproductive technologies, severe non-obstetric diseases, HIV infection, syphilis, infectious hepatitis (B and C), use of antimicrobial drugs during the last 4 weeks before inclusion in the protocol, presence of other factors leading to threatened pregnancy loss, recurrent miscarriage, use of narcotic and psychoactive substances, and alcohol abuse. The control group consisted of 80 women with healthy pregnancies at the time of enrollment and contact with an obstetrician-gynecologist. Biological material was collected on an outpatient basis.
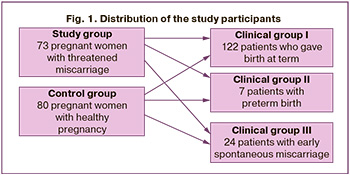
The study groups were comparable; the median age of patients in the study and control groups was 30 and 29 years, respectively (p>0.05). The groups did not differ in parity; the percentages of repeated pregnancies in the study and control groups were 42.7% and 41%, respectively (p>0.05). No statistically significant differences were found in gynecological history: the mean age at menarche was 13 years (p>0.05), regular menstrual rhythm was observed in 85.3% of women in the study group, and in 75.6% of the control group (p>0.05). All patients were diagnosed using standard methods in accordance with current regulatory documents, including general clinical laboratory and functional investigations as well as special methods. To assess the impact of the cervicovaginal microbiome in the first trimester on the outcome of gestation, the women included in the study were retrospectively divided into three groups: group I (n=122) consisted of women who delivered at term, group II (n=7) included patients with preterm delivery, and group III (n = 24) comprised women who experienced spontaneous miscarriage (Fig. 1).
Detection of the main pathogens of sexually transmitted infections (Chlamydia, Gonococcus, Trichomoniasis, M. genitalium infections); diagnosis of infectious diseases associated with an imbalance of the vaginal microbiome – bacterial vaginosis (BV), aerobic vaginitis (AV), candidal vulvovaginitis (CVV); detection of opportunistic genital mycoplasma (OGM) was performed using AmpliPrime-NCMT and FLOROSKRIN (AmpliPrime-FLOROSKRIN-Bacterial vaginosis (AmpliPrime-FLOROSKRIN-Aerobes, AmpliPrime-FLOROSKRIN-Candida, AmliPrime-FLOROSKRIN-Mycoplasmas) tests manufactured by NextBio LLC (Moscow, Russia)) based on multiplex real time polymerase chain reaction (RT-PCR) with quantitative assessment of the DNA concentration of microorganisms-markers of the corresponding infections in samples of biological material obtained by collecting cervicovaginal discharge with disposable swab probes into transport medium (TM). The FRT-manager software (Interlabservice LLC) was used to automatically calculate the concentrations (in genomic equivalents per 1 ml, GE/ml) and the ratio of DNA of microorganism markers and to issue a laboratory report, on the basis of which a diagnosis was made, taking into account the clinical data of the patients. The diagnoses of "chlamydial infection,” "gonococcal infection,” "trichomoniasis,” "infection caused by M. genitalium (MG infection)" were established based on the detection of DNA of microorganisms including Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Mycoplasma genitalium, respectively, using the AmpliPrime-NCMT reagent kit. BV was diagnosed by assessing the difference in the logarithms of lactobacillary flora DNA concentrations, total amount of bacterial DNA, and Gardnerella vaginalis (s.l.) and Atopobium vaginae (new Fannyhessea vaginae) DNA. The diagnosis of BV was based on a laboratory report stating that "the ratio of microorganism DNA concentrations is consistent with bacterial vaginosis”. The clinical significance of OGM, including Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum, was taken into account when the concentration of any of them was more than 105GE/ml and the patient had BV. The diagnosis of AV was verified based on the presence of a clinical picture of vaginitis with the detection of DNA of facultative anaerobic microorganisms, including Enterobacteriaceae, Streptococcus spp., and Staphylococcus spp., in concentrations exceeding lactobacilli DNA (laboratory report of the FLOROSKRIN test stating that “aerobic bacteria predominate”).
The diagnosis of CVV was made based on the presence of a characteristic clinical picture of vaginitis and the detection of DNA of Candida species (C. albicans, C. glabrata, C. krusei, C. parapsilosis/tropicalis) in a concentration higher than 102 GE/ml.
The diagnosis of “mixed infections” was based on the detection of a simultaneous combination of two or more infectious nosologies/syndromes.
Statistical analysis
Statistical analysis was performed using Statistica 8.0 and MS Office Excel 2010 software. The normality of the distribution of continuous variables was tested using the Shapiro–Wilk and Kolmogorov–Smirnov tests. Continuous variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range [Q1–Q3] was reported. The Kruskal–Wallis test was used to compare numerical data between the groups. The multiplicity of the risk of pregnancy outcomes depending on the clinical diagnosis was determined using the relative risk formula as the ratio of the proportion of the influencing factor in the study group to the proportion of the influencing factor in the control group. The percentages of categorical variables were compared using the χ2 test or Fisher’s exact test when appropriate. Differences were considered statistically significant at p<0,05.
Results
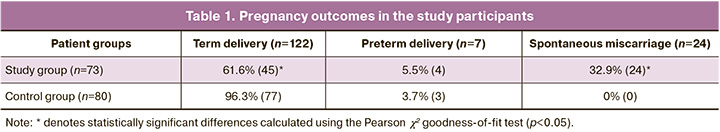
In 24/73 patients in the study group (32.9%), pregnancy ended in early spontaneous miscarriage, in 4/73 (5.5%) in preterm delivery (before 37 weeks), and in 45/73 (61.6%) in term delivery. In the control group, pregnancy outcomes were as follows: 77/80 (96.3%) women delivered at term; 3/80 (3.7%) pregnancies ended in preterm delivery; and there were no cases of early pregnancy loss (Table 1).
To assess the effect of the cervicovaginal microbiome in the first trimester on gestational outcomes, all patients included in the study were retrospectively divided into three groups. Group I (n=122) consisted of women who delivered at term, group II (n=7) included patients with preterm delivery, and group III (n=24) comprised women who experienced spontaneous miscarriage.
Among the patients in the entire cohort, two episodes of sexually transmitted infections (STIs) were detected as a part of mixed infections. In one case of chlamydial infection in combination with BV and AV, pregnancy was terminated at 12 weeks of gestation. In the case of M. genitalium infection in combination with BV and CVV, the pregnancy ended with miscarriage at 14 weeks. Data analysis (Fig. 2) revealed a significant increase in the incidence of BV (up to 70.8%) in women whose pregnancy was terminated in the first half of pregnancy compared to the term (22.2%, p<0.05) and preterm (28.6%, p<0.05) groups, indicating the importance of this syndrome as a risk factor for pregnancy complications. The risk of infection-induced spontaneous miscarriage increased 6-fold in the presence of BV in pregnant women in the first trimester (OR=5.7; p<0.01). BV was detected in 8.3% of cases of spontaneous miscarriage, which was twice the frequency of its detection in patients in group I (3.9%, p<0.05). AV was not detected in the preterm birth group (p<0.01). The prevalence of CVV in the three groups was not statistically different, allowing us to conclude that there was no isolated effect of CVV occurring in the first trimester on implantation, placentation, and pregnancy progression.
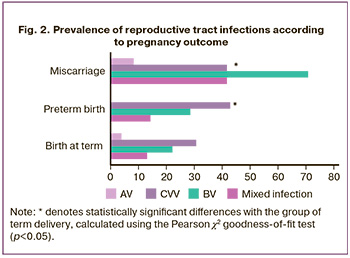
The combination of different infections (mixed infections) in women whose pregnancy ended in early spontaneous miscarriage was observed in 41.7% of cases, which was significantly higher than that in the term (13.1%, p<0.01) and preterm (14.3%, p<0.01) groups. Mixed infection, BV, and CVV were found in 10.5% (n=16) of adverse pregnancy outcomes, BV and AV in 1.3% (n=2), and BV, AV, and chlamydial infection in 0.65% (n=1). Moreover, the most common combination of infections was BV with CVV, indicating the significance of this combination as a risk factor for pregnancy disorders. The risk of infection-induced spontaneous miscarriage increased 4-fold when a mixed infection (BV in combination with CVV) was detected in pregnant women in the first trimester (OR=4.2; p<0.01).
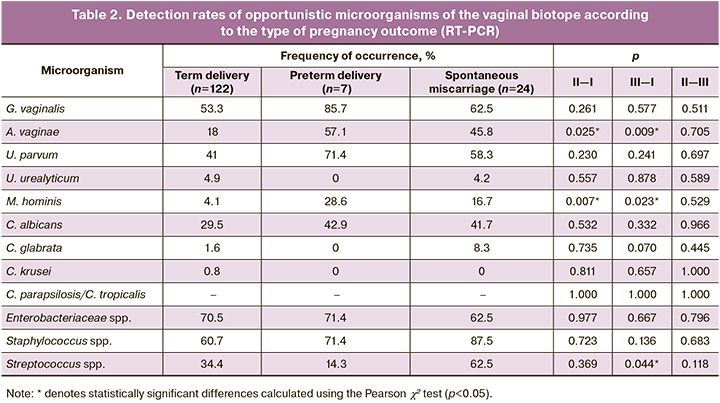
To establish the relationships between individual representatives of the opportunistic microbiome of the vaginal biotope in the first trimester and various pregnancy outcomes, a comparative analysis of their prevalence and concentration in the three study groups was performed (Table 2). The detection rate of BV-associated microorganisms in the vaginal microflora during early pregnancy was significantly higher among women with adverse outcomes. The detection rates of A. vaginae in Group II (57.1%) and in the spontaneous miscarriage group (45.8%) were significantly higher than those in the term delivery group (18%, p=0.025 and p=0.009, respectively). The presence of M. hominis in the vaginal microbial community in the first trimester was confirmed in only 4.1% of women whose pregnancy ended at term delivery. Compared with the participants in group I, M. hominis was registered four times more often among patients in group III (16.7%, p=0.023) and 6.9 times more often in the preterm birth group (28.6%, p=0.007), which may confirm the participation of BV-associated pathogens in the development of adverse pregnancy outcomes. Among the three potential families of AV pathogens detected by the AmpliPrime-FLOROSKRIN test, statistically significant differences in the detection rate from the vagina of women in the compared samples were observed for Streptococcus spp., which was detected in the first trimester in 62.5% of women with spontaneous miscarriage, which was almost two times higher than that in group I (34.4%, p=0.044). Lactobacilli were detected in 100% of samples.
The number of opportunistic bacteria simultaneously isolated from the vagina of one patient in the first trimester reached 4.5 species per participant in the early spontaneous miscarriage group, which was statistically significantly higher than that in the term delivery group (3.1 species, p<0.01) and was comparable with that in group II (4.4 bacterial species per woman).
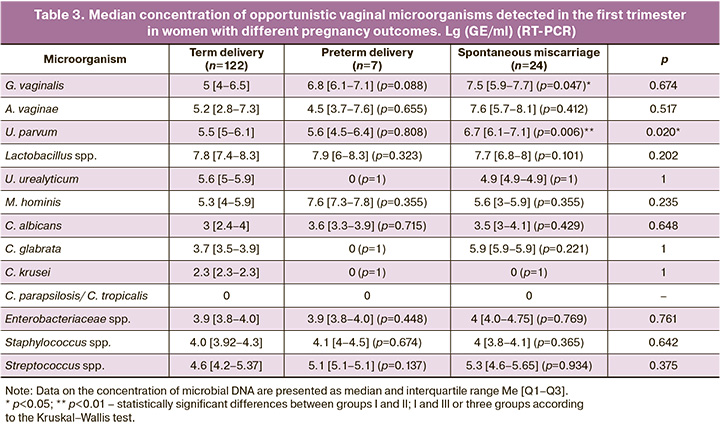
The intensity of contamination of the vaginal biotope with various types of opportunistic microorganisms among women in the study groups was distributed as follows (Table 3): the median concentration of G. vaginalis in the term delivery group was significantly lower (5 [4–6.5] GE/ml) than in the early spontaneous miscarriage group (7.5 [5.9–7.7] GE/ml, p=0.047). Another representative BV-associated microorganism, U. parvum, was detected at higher concentrations in women who had miscarriage in the first half of pregnancy (6.7 [6.1–7.1] GE/ml) than in the term delivery group (5.5 [5–6.1] GE/ml, p=0.006). The absence of U. urealyticum and C. glabrata in the preterm delivery group may be due to the small sample size. The intensity of Lactobacillus spp. contamination did not differ significantly between the study groups and was 7.8 [7.4–8.3] GE/ml– in the term delivery group, 7.9 [6–8.3] GE/ml (p=0.323) in the preterm delivery group, and 7.7 [6.8–8] GE/ml (p=0.101) in women with early spontaneous miscarriage.
Factorial correspondence analysis based on the results of the AmpliPrime-NCMT test showed that the simultaneous detection of four or fewer opportunistic representatives of vaginal bacteria in early pregnancy is a prognostically favorable factor, indicating a high probability of completing pregnancy at term (p<0.01). In contrast, the presence of more than five microorganism markers involved in the development of BV, AV, CVV, and OGM, detected in the vagina of one pregnant woman in the first trimester using the AmpliPrime FLOROSKRIN tests based on RT-PCR, was associated with the development of spontaneous miscarriage in the first trimester and preterm birth (p<0.01).
Discussion
The study findings confirmed a link between the cervicovaginal microbiome during early pregnancy and pregnancy outcomes. Among women with adverse pregnancy outcomes such as miscarriage and preterm birth, conditions such as BV, AV, and STIs were more common, which is consistent with existing literature [8, 13–17]. However, it is important to note that the presence of candida vulvovaginitis in the first half of pregnancy does not increase the risk of miscarriage or preterm birth, as shown in previous studies [18].
To assess the impact of quantitative and qualitative indicators of the cervicovaginal microbiome in early pregnancy on pregnancy outcomes, study participants were divided into three groups retrospectively. The results showed a statistically significant increase in the prevalence of BV among women with spontaneous miscarriages and preterm births, with G. vaginalis and A. vaginae as the main contributors. These microorganisms are known to form biofilms, which are the main pathogenic substrates of BV [19]. Previous studies have shown that biofilms created by opportunistic G. vaginalis can migrate from the vagina to the endometrium and fallopian tubes, partially explaining the mechanisms underlying early reproductive losses in BV. This finding suggests that BV is a significant risk factor for pregnancy-related complications. The frequency and concentration of BV-associated microorganisms in the cervicovaginal microbiome were significantly higher in women with adverse outcomes. It is possible that infection-induced abnormal placentation, one of the causes of preterm birth, is facilitated by the ability of pathogens to activate macrophages, ultimately leading to caspase-dependent apoptosis in trophoblasts [21].
Opportunistic mycoplasmas (U. parvum, U. urealyticum, and M. hominis) are often considered independent infectious risk factors for subfertility and pregnancy termination at any stage. Our findings align with those of previous research [13, 22], indicating a higher frequency of U. parvum and M. hominis among pregnant women with preterm birth and no negative effect of U. urealyticum on pregnancy outcomes. Furthermore, we found that the concentration of U. parvum was higher in patients who delivered before 37 weeks of gestation or had spontaneous termination during the first half of pregnancy. M. hominis was more frequently detected in the vagina during the early stages of pregnancy that ended in spontaneous miscarriage, but its concentrations were comparable to those in the term delivery group and higher than those in women with preterm termination of pregnancy. However, it is not currently recommended to consider U. parvum and M. hominis as the only therapeutic targets, as the use of various antibacterial therapy regimens does not improve pregnancy outcomes when opportunistic mycoplasmas are detected [13]. It is advisable to exclusively consider these microorganisms in the context of BV [22, 23].
Compared to subjects who delivered at term, AV was more frequently diagnosed in women whose pregnancy ended in early miscarriage. The diagnosis of AV was not established during the first trimester in patients with preterm birth. However, our data analysis showed that the development of miscarriage is significantly more often accompanied by an increase in the frequency of detection of opportunistic bacteria, such as Staphylococcus spp., Streptococcus spp., and the Enterobacteriaceae spp., which are involved in the development of AV and are consistent with the literature [10].
According to our data, CVV did not affect the pregnancy outcomes.
Mixed infections occur in 30% of vaginitis episodes, and the pathogens worsen the course of the disease [24]. Previously, we demonstrated [14] that the presence of a diagnosis of mixed lower reproductive tract infection in the third trimester, combined with two or more independent syndromes/diseases, was associated with the risk of preterm birth. However, there is no available literature on the negative impact of mixed infections occurring in early pregnancy stages on pregnancy outcomes. In this study, we found that a combination of various vaginal infections in the first trimester was recorded four times more often in women whose pregnancy ended in spontaneous termination in the first trimester and preterm birth than in women who gave birth at term. The results of the comparison of the average number of opportunistic microorganisms colonizing the cervicovaginal biotope in the first trimester (out of 13 identified key markers of cervicovaginal infections) were consistent with the literature on the lower microbial diversity of the vaginal microbiome during the normal course of pregnancy [25, 26]. The highest species diversity in this location was observed in women with spontaneous miscarriages and preterm births compared with those who gave birth at term.
Conclusion
Infection of the lower female reproductive tract during the first trimester of pregnancy is associated with an increased risk of early pregnancy loss and preterm births. The question of whether vaginal infections or specific pathogens are the cause of spontaneous miscarriage and preterm birth or whether the abnormal microbiome is merely involved in the implementation of the pathophysiological process induced by other factors remains unanswered. Further research is necessary to successfully manage the infectious factors and reduce reproductive losses.
References
- Quenby S., Gallos I.D., Dhillon-Smith R.K., Podesek M., Stephenson M.D., Fisher J. et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021; 397(10285): 1658-67. https://dx.doi.org/10.1016/S0140-6736(21)00682-6.
- Smits M.A.J., van Maarle M., Hamer G., Mastenbroek S., Goddijn M., van Wely M. Cytogenetic testing of pregnancy loss tissue: a meta-analysis. Reprod. Biomed. Online. 2020; 40(6): 867-79. https://dx.doi.org/10.1016/j.rbmo.2020.02.001.
- Larsen E.C., Christiansen O.B., Kolte A.M., Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013; 11: 154. https://dx.doi.org/10.1186/1741-7015-11-154.
- Bottomley C., Bourne T. Diagnosing miscarriage. Best. Pract. Res. Clin. Obstet. Gynaecol. 2009; 23(4): 463-77. https://dx.doi.org/10.1016/j.bpobgyn.2009.02.004.
- Calleja-Agius J., Jauniaux E., Pizzey A.R., Muttukrishna S. Investigation of systemic inflammatory response in first trimester pregnancy failure. Hum. Reprod. 2012; 27(2): 349-57. https://dx.doi.org/10.1093/humrep/der402.
- Cocksedge K.A., Saravelos S.H., Metwally M., Li T.C. How common is polycystic ovary syndrome in recurrent miscarriage? Reprod. Biomed. Online. 2009; 19(4): 572-6. https://dx.doi.org/10.1016/j.rbmo.2009.06.003.
- Giakoumelou S., Wheelhouse N., Cuschieri K., Entrican G., Howie S.E.M., Horne A.W. The role of infection in miscarriage. Hum. Reprod. Update. 2016; 22(1): 116-33. https://dx.doi.org/10.1093/humupd/dmv041.
- Al-Memar M., Bobdiwala S., Fourie H., Mannino R., Lee Y.S., Smith A. et al. The association between vaginal bacterial composition and miscarriage: a nested case-control study. BJOG. 2020; 127(2): 264-74. https://dx.doi.org/10.1111/1471-0528.15972.
- Brosens I., Pijnenborg R., Vercruysse L., Romero R. The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011; 204(3): 193-201. https://dx.doi.org/10.1016/j.ajog.2010.08.009.
- Donders G.G., Van Calsteren K., Bellen G., Reybrouck R., Van den Bosch T., Riphagen I. et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG. 2009; 116(10): 1315-24. https://dx.doi.org/10.1111/j.1471-0528.2009.02237.x.
- Всемирная организация здравоохранения. Информационный бюллетень «Преждевременные роды», февраль 2018. Доступно по: https://www.who.int/ru/news-room/fact-sheets/detail/preterm-birth [WHO. Fact sheets “Preterm birth”, February 2018].
- Romero R., Dey S.K., Fisher S.J. Preterm labor: one syndrome, many causes. Science. 2014; 345(6198): 760-5. https://dx.doi.org/10.1126/science.1251816.
- Donders G.G.G., Ruban K., Bellen G., Petricevic L. Mycoplasma/Ureaplasma infection in pregnancy: to screen or not to screen. J. Perinat. Med. 2017; 45(5): 505-15. https://dx.doi.org/10.1515/jpm-2016-0111.
- Доброхотова Ю.Э., Бондаренко К.Р., Гущин А.Е., Румянцева Т.А., Долгова Т.В., Кузнецов П.А., Джохадзе Л.С. Результаты исследования цервико-вагинальной микробиоты методом ПЦР в реальном времени у беременных с угрожающими преждевременными родами. Акушерство и гинекология. 2018; 11: 50-9. [Dobrokhotova Yu.E., Bondarenko K.R., Gushchin A.E., Rumyantseva T.A., Dolgova T.V., Kuznetsov P.A., Dzhokhadze L.S. The results of the examination of cervical-vaginal microbiota in pregnant women with threatened preterm birth using a real-time polymerase chain reaction. Obstetrics and Gynecology. 2018; (11): 50-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.11.50-59.
- van Oostrum N., De Sutter P., Meys J., Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum. Reprod. 2013; 28(7):1809-15. https://dx.doi.org/10.1093/humrep/det096.
- Donders G.G., Van Bulck B., Caudron J., Londers L., Vereecken A., Spitz B. Relationship of bacterial vaginosis and mycoplasmas to the risk of spontaneous abortion. Am. J. Obstet. Gynecol. 2000; 183(2): 431-7. https://dx.doi.org/10.1067/mob.2000.105738.
- Ravel J., Moreno I., Simón C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am. J. Obstet. Gynecol. 2021; 224(3): 251-7. https://dx.doi.org/10.1016/j.ajog.2020.10.019.
- Aguin T.J., Sobel J.D. Vulvovaginal candidiasis in pregnancy. Curr. Infect. Dis. Rep. 2015; 17(6): 462. https://dx.doi.org/10.1007/s11908-015-0462-0.
- Verstraelen H., Swidsinski A. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment Curr. Opin. Infect. Dis. 2013; 26(1): 86-9. https://dx.doi.org/10.1097/QCO.0b013e32835c20cd.
- Swidsinski A., Verstraelen H., Loening-Baucke V., Swidsinski S., Mendling W., Halwani Z. Presence of a polymicrobial endometrial biofilm in patients with bacterial vaginosis. PLoS One. 2013; 8(1): e53997. https://dx.doi.org/10.1371/journal.pone.0053997.
- Wu Z.M., Yang H., Li M., Yeh C.C., Schatz F., Lockwood C.J. et al. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta. 2012; 33(3): 188-94. https://dx.doi.org/10.1016/j.placenta.2011.12.007.
- Horner P., Donders G., Cusini M., Gomberg M., Jensen J.S., Unemo M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women? - a position statement from the European STI Guidelines Editorial Board. J. Eur. Acad. Dermatol. Venereol. 2018; 32(11): 1845-51. https://dx.doi.org/10.1111/jdv.15146.
- Taylor-Robinson D., Lamont R.F. Mycoplasmas in pregnancy. BJOG. 2011; 118(2): 164-74. https://dx.doi.org/10.1111/j.1471-0528.2010.02766.x.
- Sobel J.D., Subramanian C., Foxman B., Fairfax M., Gygax S.E. Mixed vaginitis - more than coinfection and with therapeutic implications. Curr. Infect. Dis. Rep. 2013; 15(2): 104-8. https://dx.doi.org/10.1007/s11908-013-0325-5.
- Romero R., Hassan S.S., Gajer P., Tarca A.L., Fadrosh D.W., Bieda J. et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014; 27(2): 18. https://dx.doi.org/10.1186/2049-2618-2-18.
- Romero R., Hassan S.S., Gajer P., Tarca A.L., Fadrosh D.W., Nikita L. et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014; 2(1): 4. https://dx.doi.org/10.1186/2049-2618-2-4.
Received 04.06.2024
Accepted 26.07.2024
About the Authors
Polina A. Shadrova, PhD, Teaching Assistant at the Department of Obstetrics and Gynecology of Medical Faculty, Pirogov RNRMU, Ministry of Health of Russia,117997, Russia, Moscow, Ostrovityanov str., 1/9; Student of Master’s Program HSE Health Care Administration and Economy, 101000, Russia, Moscow, Myasnitskaya str., 20,
+7(925)033-70-99, shadrovapolina@hotmail.com, SPIN-code: 9935-8003, https://orcid.org/0000-0002-3721-1421
Karina R. Bondarenko, Dr. Med. Sci., Clinic of Modern Ozone Therapy, 117628, Russia, Moscow, Starokachalovskaya str., 6, karinabond@mail.ru,
https://orcid.org/0000-0003-4147-1151
Alexander E. Guschin, PhD (Bio), Leading Researcher at the Moscow Research and Practical Center for Dermatovenereology and Cosmetology,
119071, Russia, Moscow, Leninsky Ave., 17, mcdik@zdrav.mos.ru, https://orcid.org/0000-0002-0399-1167
Alexander M. Zatevalov, PhD (Bio), Chief Researcher at the Laboratory of Diagnosis and Prevention of Infectious Diseases, G.N. Gabrichevsky Research Institute
for Epidemiology and Microbiology, 125212, Russia, Moscow, Admiral Makarov str., 10, 89057149114@mail.ru, https://orcid.org/0000-0002-1460-4362
Valeria O. Metalnikova, Resident at the Department of Obstetrics and Gynecology of Medical Faculty, Pirogov RNRMU, Ministry of Health of Russia,
117997, Russia, Moscow, Ostrovityanov str., 1/9, metalnikova-valeri@mail.ru, https://orcid.org/0000-0002-3432-7023
Yulia E. Dobrokhotova, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology of Medical Faculty, Pirogov RNRMU, Ministry of Health of Russia,
117997, Russia, Moscow, Ostrovityanov str., 1/9, +7(495)722-63-99, pr.dobrohotova@mail.ru, https://orcid.org/0000-0002-7830-2290
Corresponding author: Polina A. Shadrova, shadrovapolina@hotmail.com



