Диагноз рецидивирующего вульвовагинального кандидоза (РВВК) ставится при наличии 3 и более эпизодов инфекции, регистрируемых в течение одного года [1–3]. В большинстве случаев рецидив заболевания обусловлен реактивацией грибковой инфекции из персистирующих резервуаров во влагалище или кишечнике.
Candida albicans является причиной 80–90% случаев РВВК [2; 4], однако перед началом терапии целесообразно провести культуральное исследование отделяемого из влагалища с видовой идентификацией возбудителя (С. albicans, C. non-albicans, грибы не рода Candida) для уточнения его чувствительности к лекарственным препаратам. Целью терапии является облегчение симптомов и предотвращение рецидива заболевания [1, 5].
Для индукционной терапии используются системные и местные препараты, эффективность которых равнозначна [6–8]. Выбор определяется доступностью и предпочтением пациента по способу введению лекарства. Из системных препаратов чаще используется флуконазол в связи с его хорошей переносимостью и низкой стоимостью. Он назначается по 150 мг каждые 72 ч (всего 3 дозы) с последующим переходом на поддерживающую терапию – по 150 мг перорально 1 раз в неделю в течение 6–12 месяцев. Рецидив кандидозного вульвовагинита после окончания терапии происходит примерно у 55% пациентов [9].
Не менее эффективным является назначение топических азолов. Индукционное лечение включает клотримазол (вагинальная таблетка 100 мг 1 раз в сутки 7 дней), миконазол (суппозитории 100 мг 7 дней), бутоконазол (2% крем 5 г однократно) или итраконазол (вагинальная таблетка 200 мг 10 дней). При наличии выраженных объективных симптомов кандидозного вульвовагинита интравагинальную терапию продлевают до 10–14 дней. После достижения положительного клинико-микробиологического результата поддерживающая терапия проводится в течение 6 месяцев препаратами натамицина (суппозитории 100 мг 1 раз в неделю) или клотримазола (вагинальная таблетка 500 мг один раз в неделю) [8, 10]. Такое лечение приводит к улучшению качества жизни у 80% пациенток; в течение последующих 6–12 месяцев рецидивы развиваются у 50% женщин [11].
В большинстве случаев РВВК протекает на фоне условного нормоценоза влагалища, что снижает эффективность монотерапии пероральными азолами и делает предпочтительным использование местных средств с более широким спектром активности, таких как фентиконазол. Фентиконазола нитрат является производным имидазола с широким спектром антимикотической активности в отношении дерматофитов, дрожжевых грибов, с антибактериальным и противопаразитарным действием [3]. Высокая эффективность препарата обусловлена двойным механизмом противогрибкового действия – фунгистатическим и фунгицидным. Фентиконазол нарушает структуру клеточной мембраны гриба и провоцирует его саморазрушение за счет ингибирования синтеза ферментов, отвечающих за прикрепление к поверхности эпителиальных клеток. Фунгицидный механизм действия связан с ингибированием биосинтеза эргостерола, изменяющего проницаемость клеточной стенки грибов, приводя к ее разрушению. Фентиконазол обладает выраженным антибактериальным действием на грамположительную флору (Streptococcus spp., Staphylococcus aureus), на Trichomonas vaginalis; активен в отношении Malassezia furfur, Trichophyton spp., Microsporum spp., Epidermophyton floccosum, Bacillus sublilis и Gardnerella vaginalis [12].
В исследовании Sanguinetti M. et al. (2019) была продемонстрирована высокая эффективность фентиконазола в отношении Staphylococcus aureus, E. coli, S. agalactiae, C. albicans, С. non-albicans и Gardnerella vaginalis [13]. Показатель излечения составлял 85% при вагините бактериального происхождения и 97,5% – при вагините, вызванном Candida albicans.
Рекомендуемые схемы лечения пероральными и местными средствами эффективны не для всех женщин [14, 15]. В связи с этим нами было проведено проспективное обсервационное исследование, включившее 206 пациенток с целью изучения эффективности продленного курса местной терапии фентиконазолом для профилактики рецидива кандидозного вульвовагинита у пациенток с его хроническим рецидивирующим течением.
Материалы и методы
Набор пациенток проводился на базах кафедры акушерства и гинекологии лечебного факультета РНИМУ им. Н.И. Пирогова г. Москвы в течение 2022–2023 гг. Работа соответствовала международным стандартам качественной клинической практики и принципам Хельсинкской декларации.
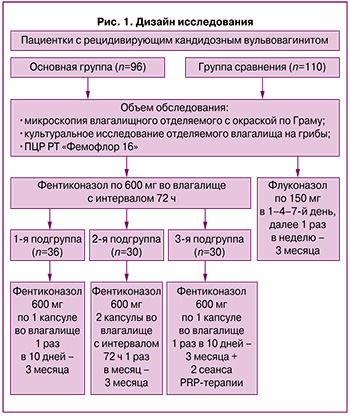
Было сформировано 2 группы: основная (n=96) и группа сравнения (n=110). Основная группа была разделена на 3 подгруппы. Стратификация осуществлялась с учетом пожеланий пациенток по варианту поддерживающей терапии и способу введения препарата. В 1-й подгруппе (n=36) женщины получали стартовую терапию фентиконазолом вагинально по 600 мг дважды с интервалом в 72 ч, далее в качестве поддерживающего лечения по 600 мг во влагалище 1 раз в 10 дней в течение 3 месяцев. Во 2-й подгруппе (n=30) после стартовой терапии фентиконазолом вагинально по 600 мг 2 раза с интервалом 72 ч в последующем препарат вводился вагинально по 600 мг 2 раза с интервалом 72 ч ежемесячно в течение 3 месяцев. Время для повторного введения фентиконазола определялось календарно (ровно через месяц после предыдущего введения) или при появлении дискомфорта и зуда во влагалище. Для уточнения причин дискомфорта пациентки использовали тест Frautest Candida, положительный результат которого свидетельствовал об обострении инфекции.
В 3-й подгруппе (n=30) терапия соответствовала 1-й подгруппе, но в дополнение проводился курс PRP-терапии (Platelet Rich Plasma – плазма, обогащенная тромбоцитами) влагалища. Лечение PRP выполнялось 2 раза с интервалом в 1,5 месяца, вне периода введения фентиконазола. Использовались синие пробирки «Реген Лаб СА» (Швейцария). Перед процедурой проводился забор крови из кубитальной вены в пробирки с антикоагулянтом и сепарирующим гелем с последующим центрифугированием. Полученная плазма методом линейно-ретроградной веерной техники с использованием иглы G30 вводилась в подслизистый слой стенок влагалища.
Группа сравнения включила 110 женщин с рецидивирующим течением кандидозного вульвовагинита, получавших терапию флуконазолом (индукционный курс 150 мг перорально в 1–4–7-й дни, далее по 150 мг 1 раз в неделю 3 месяца). После окончания лечения наблюдение продолжалось в течение 3 месяцев с оценкой частоты рецидивов заболевания и качества жизни женщин (опросник SF-36).
Критерии включения были едины для обеих групп.
1. Добровольно подписанное пациенткой информированное согласие на участие в исследовании.
2. Возраст от 20 до 45 лет включительно.
3. Рецидивирующее течение кандидозного вульвовагинита (не менее 3 эпизодов в год).
4. Подтвержденная культуральным исследованием Candida albicans с сохраненной чувствительностью к препаратам группы азолов.
5. Согласие на использование контрацепции в ходе всего исследования.
Критериями исключения явились следующие.
1. Специфический вагинит.
2. Выявленные по данным культурального исследования Candida non-albicans (Glabrata, Crusei) или Candida albicans, резистентная к азолам.
3. Беременность, лактация или планируемая беременность в период исследования.
4. Наличие других заболеваний вульвы или влагалища, которые могут затруднить интерпретацию результатов исследования.
5. Гиперчувствительность к компонентам исследуемого препарата.
6. Сахарный диабет без достижения целевых значений гликемии в течение 3 мес. до начала терапии.
7. Необходимость в приеме антибиотиков во время участия в исследовании.
8. Прием иммунодепрессантов, системных и топических глюкокортикостероидов в процессе исследования.
9. Органические заболевания центральной нервной системы и системы кроветворения (лейкоз, нарушение гемопоэза).
10. Первичные и вторичные иммунодефициты, в т.ч. ВИЧ-инфекция.
11. Тяжелые заболевания печени (цирроз печени, хронический гепатит) и органов желудочно-кишечного тракта (болезнь Крона, язвенный колит).
12. Известная или подозреваемая наркотическая/алкогольная зависимость.
13. Предполагаемая низкая приверженность пациентки лечению или неспособность выполнять процедуры и соблюдать ограничения согласно протоколу исследования.
Пациентки подписали информированное согласие на участие в исследовании, использование биологического материала и обработку персональных данных.
Дизайн исследования представлен на рисунке 1.
Забор материала для микроскопического исследования производили из заднего свода влагалища стерильным ватным тампоном и наносили на сухое предметное стекло с последующим стандартным высушиванием и окрашиванием по Граму. Культуральное исследование с видовой идентификацией грибов проводилось с использованием угольной транспортной среды с последующим посевом на питательную среду Сабуро. Микроскопическое и культуральное исследование выполнялись однократно на этапе включения в исследование.
Изучение влагалищной микробиоты проводили с использованием молекулярно-биологического метода полимеразной цепной реакции (ПЦР) с детекцией результатов в режиме реального времени (ПЦР-РВ) при помощи тест-системы «Фемофлор-16» (ООО «НПО ДНК-Технология»). Полученный клинический образец помещался в пробирку типа «Эппендорф», содержащую транспортную среду, и в течение 2–4 ч с соблюдением температурного режима (+4°С) доставлялся в лабораторию «Гемотест». Количественная оценка биоценоза проводилась в абсолютных и относительных показателях на приборах для ПЦР-РВ (ДТ96, ДТ322) с учетом номера «порогового» цикла. Количество ДНК искомого микроорганизма в образце выражалось в геном-эквивалентах (ГЭ), пропорциональных количеству микроорганизма. Оценка биоценоза выполнялась дважды: на этапе включения в исследование и через 3 месяца от начала поддерживающей терапии.
Оценка эффективности терапии проводилась на основании субъективных ощущений (заполнение опросника SF-36), лабораторных данных (ПЦР-РВ «Фемофлор-16») и частоты рецидивов на фоне и после окончания лечения. Длительность терапии составила 3 месяца, длительность последующего наблюдения – 3 месяца.
Статистический анализ
Статистический анализ результатов исследования выполнен с использованием программы Statistica 10 (StatSoft, Russia). Проверка количественных данных на нормальность распределения проводилась с использованием методов графического (график Q–Q) и численного (критерий Шапиро–Уилка) методов. При распределении, отличном от нормального, переменные описывались с помощью медианы (Ме) и интерквартильного размаха (Q1–Q3). Для анализа использовались методы непараметрической статистики. При межгрупповом сравнении использован критерий Краскела–Уоллиса с определением одного общего значения р для всех групп. Затем для сравнений с положительным результатом (р меньше 0,05) проводились попарные сравнения групп для уточнения того, между какими именно группами имеются различия с помощью критерия Манна–Уитни с поправкой Бонферрони. Сравнение количественных данных в двух несвязанных группах выполнено с применением U-критерия Манна–Уитни. Для показателей, характеризующих качественные признаки, указывали абсолютное значение и относительную величину в процентах, проверку статистических гипотез о совпадении наблюдаемых и ожидаемых частот осуществляли с использованием точного критерия Фишера. Различия считали статистически значимыми при уровне критерия менее 0,05.
Результаты
Проспективное обсервационное исследование включило 206 пациенток. Общим элементом лечения в основной группе было назначение местного лечения фентиконазолом. В группе сравнения проводилось системное лечение флуконазолом.
Возраст пациенток сформированных групп составил в среднем 35 лет в 1-й подгруппе, 32,5 и 36 лет – во 2-й и 3-й подгруппах и 37 лет – в группе сравнения. Межгрупповых различий по возрасту и индексу массы тела (ИМТ) выявлено не было (табл. 1).
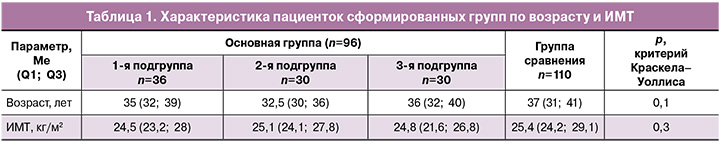
В структуре экстрагенитальных заболеваний в сравниваемых группах значимых различий выявлено не было. Наиболее распространенной патологией был хронический гастрит (11,1% (4/36), 10% (3/30), 13,3% (4/30) и 9,09% (10/110) соответственно) и гипотиреоз (8,3% (3/36), 6,7% (2/30), 6,7% (2/30) и 10% (11/110) соответственно); в структуре гинекологических заболеваний в анамнезе инфекции, передающиеся половым путем (ИППП), выявлялись у 8,8% (3/36), 6,7% (2/30), 10% (3/30) и 12,7% (14/110) пациенток соответственно. Значимых различий по частоте гинекологической патологии выявлено не было (табл. 2).
Основным критерием включения в исследование было наличие 3 и более рецидивов подтвержденной кандидозной инфекции в течение 1 года. Перед началом терапии всем пациенткам было проведено культуральное исследование, позволившее идентифицировать возбудитель как Candida albicans с сохраненной чувствительностью к азол-содержащим препаратам.
Исследование вагинального микробиоценоза выявило, что кандидозная инфекция часто сочеталась с дисбиозом влагалища различной степени выраженности (табл. 3). Условный нормоценоз, при котором сохранялось доминирование лактобацилл (более 80%) и присутствовали грибы Candida spp. в количестве более 104 ГЭ/мл, выявлялся у 19/36 (52,78%), 15/30 (50%) и 16/30 (53,33%) пациенток основной группы и у 53/110 (48,18%) женщин группы сравнения (р≥0,05). Таким образом, почти в половине случаев рецидивирующее течение кандидозного вульвовагинита сопровождалось нарушением микробиоценоза влагалища. У 47,22% (17/36) пациенток 1-й подгруппы, 30% (15/30) – 2-й, 46,67% (14/30) – 3-й подгруппы и у 51,82% (57/110) женщин группы сравнения были выявлены изменения во влагалищной флоре. При межгрупповом сравнении значимо чаще они диагностировались у пациенток группы сравнения по сравнению с пациентками 2-й подгруппы основной группы (р=0,04).
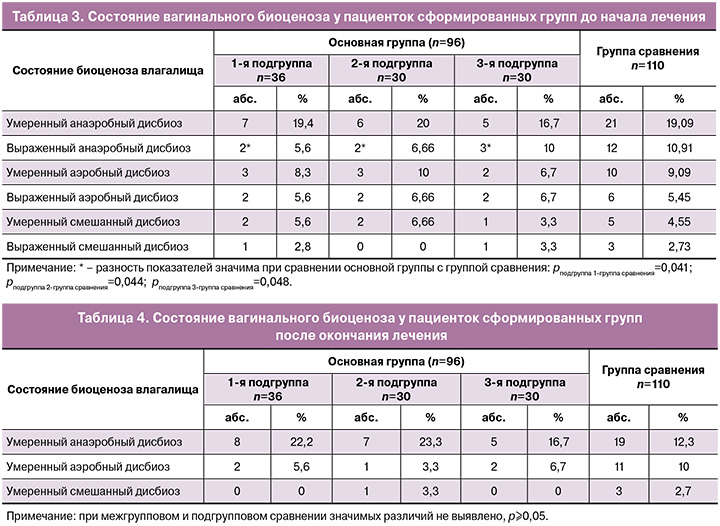
По частоте встречаемости типы нарушений влагалищного биоценоза не имели значимых различий между сформированными группами. Наиболее часто выявлялся анаэробный дисбиоз. Это состояние характеризовалось преобладанием облигатно-анаэробных бактерий и снижением доли лактобацилл от общей бактериальной массы (ОБМ) до 20–80% (при умеренном дисбиозе) и менее 20% (при выраженном дисбиозе). Среди пациенток сформированных групп преобладал умеренный анаэробный дисбиоз, встречающийся у 19,4% (7/36), 20% (6/30), 16,7% (5/30) и 19,1% (21/110) пациенток соответственно (р=0,69).
Выраженный анаэробный дисбиоз значимо чаще выявлялся у пациенток группы сравнения, чем в подгруппах основной группы (точный критерий Фишера р=0,04). Его диагностика потребовала проведения дополнительного лечения в группе сравнения с применением метронидазола (0,75% гель 5 г во влагалище 5 дней). В основной группе дополнительные препараты не назначались, так как спектр активности фентиконазола перекрывал чувствительность выявленных условно-патогенных микроорганизмов.
Умеренный аэробный дисбиоз, характеризующийся доминированием факультативно-анаэробных бактерий при снижении доли лактобацилл, был диагностирован у 8,3% (3/36), 10% (3/30), 6,7 (2/30) и 9,1% (10/110) пациенток соответственно. Выраженный аэробный дисбиоз (доля лактобацилл в составе биоценоза менее 20%) суммарно выявлялся у 5–6% пациенток в каждой группе и по частоте не имел значимых различий. При диагностике аэробного вагинита пациенткам группы сравнения дополнительно назначался клиндамицин (вагинальные суппозитории 100 мг 5 дней). В основной группе дополнительная терапия не проводилась в связи с эффективностью местного применения фентиконазола в отношении выявленных условно-патогенных микроорганизмов.
Смешанный дисбиоз или аэробно-анаэробный дисбиоз – это вариант микробиоценоза влагалища, характеризующийся доминированием разнообразных условно-патогенных бактерий из числа облигатно-анаэробных и факультативно-анаэробных бактерий, при соответствующем снижении доли лактобацилл. Выраженный дисбиоз характеризовался снижением доли лактобацилл менее 20% от ОБМ, умеренный – до 20–80% от ОБМ. У пациенток сформированных групп смешанный дисбиоз встречался редко и суммарно (умеренный и выраженный) не имел значимых различий. При смешанном дисбиозе в группе сравнения дополнительно назначался клиндамицин (100 мг суппозитории 5 дней). В основной группе дополнительная терапия не требовалась, так как фентиконазол был эффективен в отношении выявленных микроорганизмов.
После окончания трехмесячного курса поддерживающей терапии исследование вагинального микробиоценоза выявило, что абсолютный нормоценоз, характеризующийся доминированием лактобацилл (более 80% ОБМ) и минимальным (менее 104 ГЭ/мл) количеством или полным отсутствием ассоциантов микробиоценоза (Ureaplasma spp., Mycoplasma hominis, Candida spp.), присутствовал у 26/36 (72,2%), 21/30 (70%) и 23/30 (76,7%) пациенток основной группы и у 77/110 (70%) женщин группы сравнения (при межгрупповом сравнении р≥0,05).
У 27,8% (10/36) пациенток 1-й подгруппы, 30% (9/30) – 2-й, 23,3% (7/30) – 3-й подгруппы и у 30% (33/110) женщин группы сравнения сохранялись изменения во влагалищной флоре (табл. 4). Все изменения носили умеренный характер, выраженного анаэробного, аэробного и смешанного дисбиоза не было обнаружено ни в одном случае.
Межгрупповых различий в частоте развития дисбиотических состояний после окончания терапии выявлено не было (p=0,59).
На фоне 3 месяцев проводимой терапии частота рецидивов кандидозного вульвовагинита не имела значимых различий между группами и составила 9/96 (9,4%) при топическом и 6/110 (5,5%) – при пероральном лечении (табл. 5).

Межгрупповой анализ не выявил значимых различий в частоте рецидивов кандидозного вагинита на фоне проводимой терапии и наличие таковых в течение последующих 3 месяцев наблюдения (табл. 6).
При анализе частоты рецидивов в основной группе не было отмечено различий между подгруппами; то есть эффективность продленного курса применения фентиконазола, вне зависимости от кратности введения препарата, оказалась одинаковой. Также дополнительная терапия PRP не улучшила показатели частоты рецидивов как на фоне лечения, так и в течение последующих 3 месяцев наблюдения. На фоне поддерживающей терапии рецидив в группе топического лечения развивался в 11,1% (4/36), 10% (3/30) и 6,7% (2/30) соответственно подгруппам и суммарно составил 9,4% (9/96). В группе перорального применения флуконазола частота рецидивов на фоне лечения составила 5,5% (6/110) и не имела значимых отличий по сравнению с основной группой (р≥0,05).
В течение 3 месяцев без поддерживающего лечения в основной группе рецидив кандидозного вульвовагинита зафиксирован суммарно у 12,5% (12/96) пациенток. В группе сравнения частота рецидива была значимо выше и составила 23,6% (26/110) (р=0,043).
После окончания индукционного курса, на фоне поддерживающей терапии пациентки заполняли опросник самооценки качества жизни SF-36. Сравнительный анализ полученных результатов представлен в таблице 7.
Показатели интенсивности боли и ее влияние на способность заниматься повседневной деятельностью, включая работу по дому и вне дома, свидетельствовали о том, что боль не ограничивала активность пациенток исследуемых групп. В основной группе и группе сравнения показатель интенсивности боли равнялся 100 баллам (р=0,48). При оценке показателя общего состояния здоровья также не было выявлено значимых различий (р=0,27). Жизненная активность у пациенток в основной группе была несколько ниже и составила 71 балл, в группе сравнения – 74 балла (р=0,97).
Показатели социального функционирования, определяемого степенью, в которой физическое или эмоциональное состояние ограничивало бы социальную активность, и ролевого функционирования, обусловленного эмоциональным состоянием, в которой эмоциональное состояние мешало бы выполнению работы или другой повседневной деятельности, в исследуемых группах составили 100 баллов.
Полученные результаты свидетельствуют об отсутствии ограничения социальных контактов, уровня общения, выполнения повседневной работы, связанного с физическим и эмоциональным состоянием. Показатель психического здоровья, характеризующий настроение, отсутствие депрессивных, тревожных переживаний и психического неблагополучия в обеих группах, составил 74 и 76 баллов (р=0,93). Физический компонент здоровья, включающий шкалы физического функционирования, ролевого функционирования, обусловленного физическим состоянием, интенсивность боли, общее состояние здоровья в основной группе составил 92 балла, в группе сравнения – 93 балла (р=0,67). Психологический компонент здоровья исследуемых групп, включающий психическое здоровье, ролевое функционирование, обусловленное эмоциональным состоянием, социальное функционирование и жизненную активность, составил 83 и 82 баллов соответственно (р=0,54). Усредненная оценка качества жизни в группах не имела отличий и составила 88 и 87 баллов (р=0,72).
Основные жалобы пациенток сформированных групп, выявленные в течение 3 месяцев наблюдения без поддерживающей терапии, представлены в таблице 8.

Жалобы на зуд, жжение и творожистые выделения из половых путей были отмечены у пациенток с рецидивом вульвовагинальной кандидозной инфекции после завершения поддерживающей терапии. Частота данных симптомов значимо чаще выявлялась в группе сравнения после окончания системной терапии флуконазолом (р=0,043). При рецидиве пациенткам повторно назначали курс индукционной терапии с последующим поддерживающим лечением еще на 3 месяца. Выбор варианта терапии основывался на предпочтениях пациенток. Обращают на себя внимание сохраняющиеся жалобы на сухость слизистых влагалища, которые присутствовали значимо чаще у пациенток группы сравнения, даже без учета рецидивов кандидозной инфекции (р=0,031).
Внутригрупповой анализ показал, что сухость слизистых влагалища выявлялась значимо реже в 3-й подгруппе, в которой проводилась PRP-терапия (р=0,03). По подгруппам сухость слизистых значимо чаще диагностирована во 2-й подгруппе (12/36, 40%), чем в 1-й (5/30, 13,9%) и 3-й (2/30, 6,7%). Полученные результаты могут быть обусловлены положительным эффектом от применения PRP.
Обсуждение
До 5% женщин страдают РВВК, который определяют как 3 и более эпизода инфекции в течение 1 года [5]. В глобальном масштабе РВВК ежегодно поражает до 138 млн женщин (от 103 млн до 172 млн), а его распространенность составляет 3871 случай на 100 000 женщин [16].
Традиционная терапия РВВК не всегда бывает успешной [15]. Примерно у 50% женщин наблюдается рецидив симптомов в течение нескольких месяцев после окончания лечения [17].
Систематические обзоры показали, что пероральное и интравагинальное лечение одинаково эффективно у женщин с неосложненным острым и рецидивирующим кандидозным вагинитом [15, 18].
Рекомендуемые в настоящее время схемы терапии РВВК направлены на подавление симптомов и предупреждение развития рецидива инфекции [15, 17].
Для перорального лечения чаще всего используется флуконазол, поскольку он хорошо переносится, прост в использовании и имеет низкую стоимость. При стартовом лечении препарат назначается по 150 мг каждые 72 ч (всего 3 дозы) с последующим переходом на поддерживающую терапию – по 150 мг перорально 1 раз в неделю. Длительность лечения может достигать 6–12 месяцев. Рецидивы кандидозного вульвовагинита в течение последующих 6 месяцев развиваются у 55% пациентов [9].
Не менее эффективным является назначение азолов для местного применения. В метаанализе трех исследований с участием 106 пациентов с РВВК частота клинических рецидивов у пациенток с системным и местным лечением была статистически одинаковой в течение 6 и 12 месяцев наблюдения. К концу 6 месяца терапии пероральными или местными азолами рецидивы развивались у 21 и 12% (ОР 1,66; 95% ДИ 0,83–3,31), а через 12 месяцев – у 47 и 49% (ОР 0,95; 95% ДИ 0,71–1,27) соответственно [6].
В нашем исследовании в группе пациенток с системным применением флуконазола эффективность поддерживающей терапии составила 94,55%, а после окончания терапии эффект сохранялся в течение последующих 3 месяцев у 76,36%. Суммарная частота рецидивов во время проведения работы составила 29,09%; при этом на фоне применения флуконазола она была 5,45%, а после прекращения лечения – 23,64%.
Местное лечение фентиконазолом было эффективным у 92,7% пациенток, и эффект сохранялся в течение последующих трех месяцев у 87,5%.
В исследовании Sobel J.D. после местной продленной терапии клотримазолом частота рецидивов достигала 38% (18/48) [17]. В нашем исследовании суммарная частота рецидивов в группе топического применения фентиконазола составила 19,79%. На фоне терапии препаратом рецидивы кандидозного вагинита развивались у 7,29%, а в течение последующих 3 месяцев без лечения – у 12,5%.
Таким образом, в нашем исследовании были получены сопоставимые результаты, свидетельствующие о высокой эффективности локального применения фентиконазола при РВВК как на этапе индукционной, так и поддерживающей терапии.
На фоне длительного поддерживающего курса лечения флуконазолом могут развиваться такие побочные эффекты, как головная боль, боли в животе, тошнота и диспареуния. В нашем исследовании не было отмечено подобных эффектов, и все пациентки смогли провести полный назначенный курс терапии.
В последнее время увеличилась частота выявления сочетанных инфекций, что в ряде случаев требует применения многокомпонентной терапии, снижает общие показатели эффективности лечения и повышает его стоимость.
В проведенном нами исследовании было выявлено, что практически у 50% женщин с РВВК присутствуют различные нарушения вагинального биоценоза. В основной группе мы не назначали дополнительных препаратов в связи с широким спектром антибактериальной активности фентиконазола. В то же время в группе пероральной терапии флуконазолом потребовалось дополнительное назначение метронидазола или клиндамицина.
Влияние РВВК на интимные отношения и повседневную жизнь женщин может быть значительным. Показано, что женщины с РВВК чаще сообщают о признаках выгорания, эмоционального и физического стресса, а также дисбаланса между работой и личной жизнью [19]. Своевременное и эффективное лечение позволяет улучшить качество жизни женщины. В нашем исследовании не было выявлено значимых различий в оценке качества жизни пациенток на фоне поддерживающей системной и местной терапии рецидивирующей кандидозной инфекции.
По данным Pirotta М. et al., до 40% женщин с РВВК используют такое дополнительное нетрадиционное лечение, как спринцевание маслом чайного дерева, введение пробиотиков, вагинальных подкисляющих агентов, терапию СО2-лазером и PRP [20]. В проведенном исследовании дополнительное назначение 2 курсов терапии PRP не привело к снижению частоты рецидивов, но позволило улучшить субъективные ощущения увлажненности слизистой влагалища.
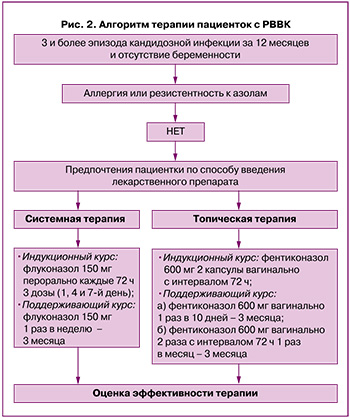
Заключение
По результатам исследования было показано, что топическая терапия фентиконазолом эффективна в купировании рецидивов вульвовагинального кандидоза и в профилактике его рецидивов. Назначение индукционного и продленного курса фентиконазолом может быть приемлемой альтернативой системной терапии (рис. 2).



