Грибы рода Candida за последние два десятилетия превратились в серьезную проблему здравоохранения. Вульвовагинальный кандидоз (ВВК) негативно влияет на качество жизни женщин во всем мире, в том числе качество сексуальной жизни [1, 2]. Зачастую эпизоды обострения рецидивирующего ВВК сопровождаются повышением тревожности вплоть до проявлений клинической депрессии [1, 3, 4]. Согласно данным оценки распространенности ВВК с помощью опроса женщин из 7 стран (n=7345), вероятность возникновения единичного эпизода ВВК к 50 годам варьируется от 23% до 49%, а вероятность перехода в рецидивирующий ВВК – от 14% до 28% [2]. В течение одного года до 9% женщин в различных популяциях переживают более 3–4 эпизодов ВВК, что уже считается рецидивирующим ВВК (РВВК) [5]. Глобальные данные о распространенности и эпидемиологические данные неточны, потому что они чаще основаны на самоотчетах и диагнозах местного врача общей практики. В связи с этим Denning et al., оценив эпидемиологические исследования с 1985 по 2016 гг. и 6000 онлайн-опросов из 5 западноевропейских стран и США, проведенных Foxman et al., задокументировали ежегодную распространенность 3871 случая РВВК на 100 тыс. женщин с самой высокой частотой (9%) у пациентов в возрасте от 25 до 34 лет [5, 6].
В 90% случаев заболевание вызывается грибами вида Candida albicans, в 10% – другими видами Candida (non-albicans) [7]. С развитием методов молекулярной идентификации виды Candida, не относящиеся к C. albicans, но вызывающие проявлений кандидоза, стали выявляться значительно чаще. В эту группу входят C. glabrata, C. tropicalis, C. parapsilosis и C. krusei [8]. Candida – диморфный гриб из группы Ascomycota, который в течение жизни населяет дыхательные пути, желудочно-кишечный и мочеполовой тракты более 30% здоровых людей [9, 10]. Изменения в симбиотическом взаимодействии между грибом и экосистемой слизистой связаны с грибковым дисбиозом легкой или умеренной степени в зависимости от инфицированной области, а также от состояния здоровья пациента. После анаэробного бактериального вагиноза ВВК считается второй по распространенности вагинальной инфекцией, поражающей хотя бы раз в жизни 75–80% женщин [11, 12].
Изменения в иммунной системе хозяина, стресс, резидентная микробиота и другие факторы могут привести к чрезмерному росту C. albicans, вызывая широкий спектр инфекционных заболеваний: от легкого поражения поверхностных слизистых оболочек до гематогенно-диссеминированного кандидоза [13]. Помимо поражения женских половых органов, C. albicans является причиной обострения ряда хронических воспалительных заболеваний кишечника, а иногда приобретает системный характер у соматически отягощенных пациентов, приводя к летальному исходу [14].
Биопленки как более агрессивная форма существования грибов рода Candida
Патогенность грибов Candida обусловлена определенными факторами вирулентности, такими как способность преодолевать защитные механизмы хозяина, адгезия и образование биопленок (на тканях хозяина и/или на медицинских устройствах), а также выработка повреждающих ткани гидролитических ферментов, таких как протеазы, фосфолипазы и гемолизины [13]. Биопленки – это биологические сообщества с необычайной степенью организации, в которых клетки Candida образуют структурированные, скоординированные и функциональные сообщества, встроенные в самосекретируемый внеклеточный матрикс [15]. Формирование грибковой биопленки происходит в ходе последовательного процесса, включающего адгезию планктонных клеток к соответствующему субстрату, колонизацию, продукцию экстрацеллюлярного матрикса, созревание биопленки и дисперсию. Научно доказано, что биопленка является более устойчивой к противогрибковым средствам формой существования грибов рода Candida. Механизмами устойчивости служат колонизация тканей хозяина, проявление у условного патогена вирулентных свойств, метаболическое взаимодействие внутри биопленки, эффективный захват питательных веществ хозяина, межклеточная коммуникация и обмен генетическим материалом [16]. К ним же можно отнести наличие клеток-персистеров, влияние высокой плотности клеток, снижение скорости роста, внеклеточной продукции матрикса, изменения экспрессии генов, поддержание кворума (оптимального количества клеток), активность оттока продуктов жизнедеятельности, стрессовые реакции, сверхэкспрессию мишеней для лекарств и увеличение содержания стеринов на мембране клетки Candida [13, 15].
Все типы грибковых биопленок имеют отличные от планктонных дрожжевых клеток свойства и увеличивают устойчивость к противогрибковым препаратам до 1000 раз [17, 18]. Биопленка представляет собой клетки-персистеры, которые напрямую связаны с накоплением высоких концентраций антимикробных агентов. По сути, персистер – это спящая клетка, и в ней практически отсутствует синтез клеточной стенки: лекарства связываются со своими молекулами-мишенями, но не могут способствовать гибели клеток инфекционного агента. В отличие от бактерий эти клетки были обнаружены только в биопленках дрожжей, но не в планктонных популяциях. Благодаря экстрацеллюлярному матриксу персистеры, присутствующие в биопленке, могут выдерживать как противогрибковое лечение, так и действие иммунной системы хозяина. Персистеры могут быть ответственными за повторное инфицирование, когда концентрация противомикробных препаратов снижается, и они могут повторно заселить биопленку.
Разные виды Candida образуют разные типы биопленок с собственными характеристиками и типичными локализациями. Например, биопленки, образованные C. albicans, представляют собой базальный слой бластоспор с плотным вышележащим матриксом, состоящим из экзополисахаридов и гиф. Такие биопленки могут вызывать эндокардит, кариес, риносинусит, выстилать питательные трубки для энтерального питания, сопутствовать некоторым заболеваниям органов дыхания (муковисцидоз, аллергические бронхолегочные заболевания), присоединяться к «диабетической стопе» [13]. Таким образом, способность образовывать биопленку связана не только с видом Candida, но и с локализацией поражения. Кроме того, на формирование биопленок влияют такие факторы, как pH, температура, осмолярность, окружающая среда, иммунные факторы хозяина и наличие антимикробных агентов [19]. Способность Candida образовывать устойчивые к противомикотикам формы – биопленки требует от науки создания новых подходов к лечению ВВК.
Клинические проявления ВВК
ВВК – инфекционное заболевание, сопровождающееся поражением кожи вульвы и слизистой влагалища, вызванное грибами рода Candida. Ведущим симптомом данного заболевания являются характерные творожистые серо-белые выделения из половых путей. Также могут быть следующие клинические проявления [20]: зуд, жжение, диспареуния, боль и неприятные ощущения при мочеиспускании, гиперемия и отек вульвы, при тяжелом течении: трещины кожных покровов и слизистых; при рецидивирующем течении: сухость, атрофия слизистой оболочки.
Чаще заболевают ВВК женщины в репродуктивном возрасте: после полового созревания и до наступления менопаузы, так как под влиянием эстрогенов эпителий влагалища производит гликоген, которым питаются дрожжевые грибы (например, C. albicans). Именно поэтому кандидоз редко предшествует половой зрелости, редко встречается у женщин в период лактации (в связи с низким уровнем эстрогенов) и в постменопаузе, если женщина не использует менопаузальную гормональную терапию (МГТ) или не страдает сахарным диабетом (СД). У большинства пациенток с рецидивирующим ВВК не было выявлено фактора, провоцирующего очередное обострение [1, 2].
Среди факторов риска развития ВВК первое место занимает бесконтрольное применение антибиотиков, далее идут половой акт, в т.ч. и защищенный, и нерациональное использование средств личной гигиены [21]. Кроме того, на рост Candida влияет состав вагинальной микробиоты: данные литературы весьма противоречивы, в частности, J.H. van de Wijgert et al. отметили, что колонизация влагалища Candida spp. даже чаще встречается у женщин с преобладанием лактобацилл, чем у женщин с дисбактериозом, оспаривая общепринятую аксиому «микробиом с преобладанием Lactobacilli является оптимальным» [22]. Таким образом, рост грибов не подавляется лактофлорой, и закисление среды влагалища не решит проблему РВВК.
Влагалищный микробиом обычно населен бактериальными сообществами, в основном представленными родом Lactobacillus, такими как L. iners и L. crispatus, и грибами. Грибы Candida – наиболее многочисленные грибковые организмы микробиома влагалища, следовательно, они могут быть возбудителями вагинальных инфекций при определенных условиях. Несколько факторов могут изменить микробиоту влагалища у пациентов с РВВК. Во-первых, изменения в сообществе Lactobacillus, продуцирующих H2O2 (например, L. acidophilus, L. gasseri и L. vaginalis). А во-вторых, высокий уровень эстрогена (например, МГТ эстрогенами, лютеиновая фаза или беременность) и разнообразие источников углерода, короткоцепочечных жирных кислот или состава эйкозаноидов. Было доказано, что эти факторы нарушают баланс между устойчивостью и инвазией, что приводит к «прилипанию» Candida к эпителию слизистой, аномальному росту дрожжей и повышению риска заражения Candida-инфекциями [19].
Кроме того, предполагается, что СД 2 типа, иммуносупрессия, терапия антибиотиками, а также поведенческие факторы (использование противозачаточных средств и внутриматочных спиралей) могут способствовать развитию ВВК. Но поскольку около 20–30% пациентов с ВВК – здоровые женщины без предрасполагающих факторов, было также высказано предположение, что индивидуальные различия, такие как генетические особенности и этническая принадлежность, а также вид грибов Candida, колонизирующий влагалище, могут играть ключевую роль в идиопатическом патогенезе РВВК [19]. Кросс-секционные исследования в Бразилии подтвердили, что уровень колонизации Candida выше у женщин с СД (18,8%), чем у пациенток без СД (11,8%), при этом в группе с СД чаще наблюдается симптоматическое течение (ВВК + РВВК = 66,6%), чем у здоровых пациенток (33,3%). Несмотря на то что неконтролируемый СД вызывает метаболические изменения, которые могут обуславливать предрасположенность к симптоматическому вагиниту, только небольшая часть женщин страдают от РВВК, что обусловлено индивидуальными различиями или дополнительными факторами, которые могут влиять на восприимчивость к инфекциям, вызванным грибами рода Candida [23].
Диагностика ВВК
Лабораторная диагностика кандидозов в настоящий момент базируется на следующих методах:
- световая микроскопия нативных препаратов или микроскопия мазков, окрашенных по Граму (обнаружение дрожжевых почкующихся клеток, псевдомицелия). Диагностическая ценность прямой микроскопии низка, что связано с низкой чувствительностью метода (для визуализации дрожжевых клеток или псевдомицелия гриба обсемененность биологического материала должна составлять не менее 104 КОЕ/мл). При низком титре возбудителя в биологическом материале получение корректного результата микроскопического исследования затруднительно или невозможно;
- методы амплификации нуклеиновых кислот (МАНК). В практике акушера-гинеколога широкое распространение получил анализ влагалищного соскоба методом ПЦР, с помощью тест-системы Фемофлор-16, которая позволяет из одной биопробы, помимо количественной оценки общей бактериальной массы, влагалищной нормофлоры (лактобактерий) и комплекса аэробных, анаэробных микроорганизмов и микоплазм, участвующих в развитии дисбиотических процессов в нижних отделах полового тракта, провести также скрининг на наличие дрожжевых грибов.
Иногда клиническая диагностика кандидоза может быть затруднена из-за отсутствия специфических симптомов и клинических признаков. Чаще всего стертое течение кандидоза вызывают non-albicans виды Candida [19]. Широкое использование противогрибковых средств изменило эпидемиологический ландшафт грибковых инфекций, где виды грибов, демонстрирующие устойчивость к одному и/или нескольким классам противогрибковых средств, все чаще выявляются в клинических условиях и связаны с терапевтической неудачей. Наиболее яркими примерами являются появление во всем мире устойчивых к триазолам Aspergillus fumigatus, C. tropicalis, C. parapsilosis и C. auris с множественной лекарственной устойчивостью, а также растущая распространенность множественной лекарственной устойчивости у C. glabrata, особенно в США. Следует отметить, что устойчивость к противогрибковым препаратам может быть либо приобретенной, когда грибы становятся устойчивыми во время противогрибковой терапии, либо врожденной, как у C. krusei, которая по своей природе устойчива к азолам. Когда гриб подвергается воздействию противогрибковых средств, пути устойчивости позволяют клеткам сохраняться [24–26].
На основании данных о структуре генома, полученных с использованием новых молекулярно-генетических методов, классификация дрожжевых грибов в последние годы активно пересматривается. В результате некоторые виды, ранее относившиеся к роду Candida, поменяли свою принадлежность, и в настоящее время их относят к другим родам (табл. 1). Соответственно, были изменены и названия этих видов (устаревшие названия приведены в скобках), которые уже не называются Сandida. Таким образом, в настоящее время «дрожжеподобные грибы рода Candida» представляют собой собирательное название дрожжевых грибов, относящихся к разным родам и не всегда являющихся Candida.
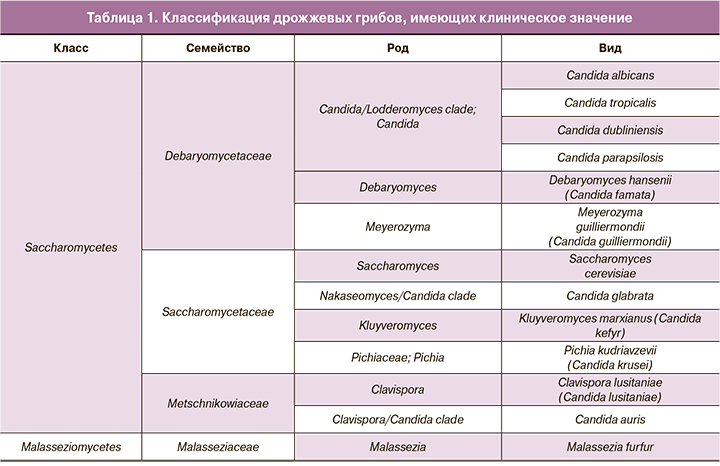
Однако в настоящее время появились новые диагностические возможности, в частности, в НМИЦ АГиП им. В.И. Кулакова проводится типирование грибов рода Candida для уточнения вида возбудителя при стертом течении инфекционного процесса. Компания «ДНК-Технология» разработала набор реагентов «МикозоСкрин» для выявления и типирования возбудителей грибковых инфекций родов Candida, Malassezia, Saccharomyces и Debaryomyces методом ПЦР в режиме реального времени. Состав набора не имеет аналогов в России и за рубежом. Исследование проводят при следующих показаниях: подозрение на кандидоз, кандидемию, кандидурию или кандидоносительство; видовая идентификация культур дрожжевых грибов; инфекционный контроль, в том числе у пациентов из групп риска; мониторинг динамики колонизации дрожжевыми грибами нестерильных в норме локусов пациентов, ран, катетеров. Набор реагентов «МикозоСкрин» имеет такие преимущества, как возможность идентифицировать трудно культивируемые дрожжевые грибы, комплексная диагностика расширенного перечня возбудителей микозов, исследование широкого спектра биологического материала для решения различных клинических задач в неонатологии/педиатрии/гинекологии/иммунологии.
Лечение ВВК
Несмотря на кажущуюся легкость, лечение ВВК – непростая задача для гинеколога. Противогрибковые препараты могут быть фунгицидными, когда противогрибковый агент вызывает гибель грибковых клеток, или фунгистатическими, когда лекарство останавливает пролиферацию клеток, но не убивает грибковые клетки [15]. Для лечения инфекций, вызываемых Candida, используют 3 основных класса противогрибковых препаратов: азолы, полиены и эхинокандины (табл. 2). Азолы (например, флуконазол, вориконазол и позаконазол) обладают фунгистатическим эффектом, блокируя синтез эргостерола, воздействуя на фермент ланостерин 14α-деметилазу (связанный с ERG11), что приводит к накоплению токсичных промежуточных продуктов метаболического пути стеролов. Полиены (например, амфотерицин B и нистатин) оказывают фунгицидное действие, внедряясь в мембраны, содержащие эргостерин, создавая поры, которые разрушают протонный градиент, что приводит к оттоку цитоплазмы и другого клеточного содержимого. Эхинокандины (например, каспофунгин, микафунгин и анидулафунгин) имеют фунгицидное действие, направленное на синтез 1,3-β-глюкана – компонента клеточной стенки видов Candida. Эхинокандины и полиены рекомендуются при тяжелом течении инфекционного процесса, вызванного C. glabrata, который считается очень устойчивым к азолам, или в случае лечения пациента азолами ранее. Эхинокандины являются первыми противогрибковыми препаратами в тяжелых случаях кандидемии [8].

Снижение толерантности к противогрибковым препаратам, являющееся предпосылкой к возникновению устойчивости, также требует разработки эффективных и специфичных для грибов адъювантов, которые будут использоваться в сочетании с системными противогрибковыми средствами. Разрабатываются новые лекарственные формы, такие как эхинокандины и липидные формы средств, ингибирующие грибковую биопленку как in vitro, так и in vivo. Одним из вариантов лечения является комбинация лекарств: использование более одного препарата может повысить эффективность из-за возможности воздействия на более чем одну мишень, кроме того, снижается токсичность, поскольку используется меньшее количество препарата. Комбинации лекарств могут привести к повышению активности, например синергической, или снижению антагонистического действия [15].
Для лечения острого ВВК применяются топические антимикотики в разовой дозе или коротким курсом. Для лечения хронического ВВК (РВВК, 4 и более эпизодов в год) лучше использовать комбинацию из местной и системной терапии курсом 6–14 дней. Во время беременности назначают производные имидазола для местного применения и натамицин [20, 27, 28].
Терапия местными и пероральными противомикотиками обеспечивает одинаково эффективное лечение ВВК [29], однако пациенты чаще выбирают локальные препараты с минимальной кратностью применения. Таким примером могут служить средства на основе сертаконазола.
В частности, триада средств представляет комплексный подход к лечению ВВК в 3 шага: первичная гигиена («Залагель интим»), лечение («Залаин» суппозиторий и/или «Залаин» крем), интимная гигиена («Залагель интим»). Основной компонент геля «Залагель интим» – масло чайного дерева Melaleuca alternifolia, обладающее антисептическими свойствами, ингибирует рост грибов и бактерий, способствует устранению зуда, жжения и воспаления. Среди компонентов масла чайного дерева выделяют: монотерпены (40–50%), дитерпены (до 40%) и цинеол (3–15%). Терпинен-4-ол – основной компонент, отвечающий за противогрибковые свойства геля, антибактериальную активность в отношении Gardnerella vaginalis, Staphylococcus и Streptococcus, противовирусную (действует на Herpes simplex) и aнтипротозойную (на Trichomonas vaginalis). А наличие в составе 1,8-цинеона обеспечивает дезодорирующее действие [30–35].
Действующим веществом суппозитория и крема «Залаин» является двойная молекула сертаконазола – это современный представитель азолов, благодаря которому сохраняется минимальный уровень рецидивов ВВК. Двойная молекула сертаконазола состоит из бензотиофена и азолового кольца (имидазола) и обладает тройным механизмом действия: фунгицидным, фунгистатическим и блокировкой перехода непатогенной формы гриба в патогенную (ингибированием диморфной трансформации). Сертаконазол угнетает синтез эргостерола (компонента клеточных мембран грибов) и увеличивает проницаемость клеточной мембраны грибов, что приводит к лизису клетки гриба. Он также обладает антибактериальной активностью в отношении грамположительных бактерий (стафило- и стрептококков), не действуя на лактобациллы, что является преимуществом данного препарата [36–39]. Streptococcus agalactiae и C. albicans часто совместно колонизируют женские половые пути и, при определенных условиях, вызывают воспаление слизистой оболочки. Зарубежные авторы установили, что взаимодействие между S. agalactiae и C. albicans может ослаблять иммунитет слизистой оболочки влагалища хозяина и способствовать ее колонизации C. albicans [40, 41]. Преимуществами лечения суппозиторием «Залаин» являются отсутствие системного эффекта, однократный прием и широкий спектр действия (действует как на C. albicans, так и на C. non-albicans), отсутствие перекрестной резистентности грибов к «Залаину», отсутствие воздействия на лактобациллы, сопутствующая антибактериальная активность и противовоспалительный эффект. Однократное интравагинальное введение суппозитория (300 мг) составляет полный курс лечения эпизода ВВК. Если клинические симптомы сохраняются, возможно повторное введение суппозитория через 7 дней [39, 42–44].
Часто очередное обострение РВВК у женщины сопряжено с менструальным кровотечением [41]. Многие антимикотики противопоказаны при менструации в отличие от суппозитория и крема «Залаин», а «Залагель интим» обеспечивает деликатный уход за наружными половыми органами в этот период. У больных СД кандидоз зачастую поражает не только область вульвы и влагалище, но и распространяется на паховые складки, перианальную область в виде кандидозного дерматита [41]. В таком случае в сочетании с суппозиторием «Залаин» возможно нанесение сертаконазола в виде крема на пораженные участки. ВВК редко передается половым путем. При необходимости мужчине можно рекомендовать крем «Залаин», который обладает теми же свойствами, что и суппозиторий, для лечения дерматомикозов. Стоит отметить, при беременности и лактации препарат следует применять в том случае, если польза для женщины превышает риск для плода (ребенка).
Субтерапевтические уровни лекарств повышают риск неэффективности лечения, они также могут увеличивать риск развития резистентности при инвазивных грибковых инфекциях у пациентов, получающих противогрибковую профилактику или продолжающих лечение. Например, многочисленные исследования показали, что субтерапевтические минимальные концентрации во время профилактики позаконазолом у пациентов с гематологическими злокачественными новообразованиями связаны с прорывными инвазивными грибковыми инфекциями. Исследования реципиентов трансплантата легких, принимавших вориконазол с профилактической целью, показали более высокие показатели грибковой колонизации и прорывных грибковых инфекций у пациентов с субтерапевтическим уровнем препарата вориконазол. Из-за повышения уровня резистентности к азолам и эхинокандинам инвазивные инфекции Candida стало труднее лечить, учитывая ограниченное количество классов противогрибковых средств, доступных в настоящее время. Это ограничение в противогрибковых вариантах лечения было спровоцировано появлением C. аuris, вида с множественной лекарственной устойчивостью, который был связан со вспышками во всем мире. В США 90% C. аuris устойчивы к флуконазолу, около 30% были устойчивы к амфотерицину B и менее чем 5% были устойчивы к эхинокандинам, при этом около 1/4 последних индийских изолятов C. auris были устойчивы к 2 или более классам противогрибковых средств. Следовательно, целесообразно применять препараты однократно в высоких дозах [45].
Профилактика ВВК
Несмотря на рост числа сообщений об успехах в противогрибковой терапии, количество случаев инфекции и устойчивости к антимикотикам еще велико. Многочисленные рекомендации по изменению образа жизни (например, избегать продуктов с высоким содержанием сахара, тесной одежды и изменить сексуальное поведение) не доказали свою целесообразность [7]. В первую очередь следует разумно применять антибиотики, т.к. их прием способствует росту грибов, уничтожая бактерии нормофлоры. При СД необходим контроль гликемии. Вопреки распространенному мнению, комбинированные оральные контрацептивы (КОК) не повышают шансы женщины заболеть ВВК, но при подборе КОК лучше остановиться на препарате с низким содержанием уровня эстрогена с точки зрения профилактики рецидива ВВК.
Клинический пример диагностики и лечения РВВК
На прием в НМИЦ АГиП им. В.И. Кулакова обратилась пациентка Т., 22 лет, с жалобами на частые обострения «молочницы» (до 10–12 раз в год). Неоднократно обращалась по месту жительства к гинекологу, проходила курсы лечения (название препаратов не помнит). Со слов: является «хроническим носителем грибов», контрацепция – презерватив, «молочница» с разными партнерами, партнеры в лечении не участвовали. Начало половой жизни с 15 лет, практикует промискуитет. При гинекологическом осмотре обращает на себя состояние слизистой влагалища: гиперемирована, отечна и болезненна, но не травмируется при заборе анализов. Выделения из половых путей: обильные, желтовато-зеленоватые, творожистые, плотно лежат по стенкам влагалища, снимаются с затруднением, подлежащие ткани не кровоточат (рис. 1).

Взят бактериологический посев на неспецифическую флору из заднего свода (рис. 2), а также ПЦР на «МикозоСкрин» (рис. 3).
Установлен диагноз: Острый вагинит, рецидив хронического ВВК. Пациентке рекомендовано временно исключить из рациона насыщенные углеводы: мучное, торты, лактазу (все молочные животные продукты) и кокосовое молоко, а также была предложена триада средств: «Залаин» суппозиторий 300 мг №1 во влагалище однократно; «Залаин» крем на область вульвы ежедневно 2 раза в день в течение 5 дней; «Залагель интим» для гигиенических процедур.
Показано обследование и лечение полового партнера. После окончания терапии рекомендован повторный прием у врача.
Заключение
Таким образом, ВВК – одна из актуальных проблем современного здравоохранения, которая значительно снижает качество жизни женщины. Candida albicans – представитель нормальной микрофлоры влагалища – при определенных условиях вызывает поражение слизистой, а также образует наиболее агрессивную форму – биопленку. Снижение токсичности, повышение биодоступности, улучшение противогрибкового спектра и борьба с резистентностью – это усилия, которые, как ожидается, увеличат эффективность доступных противогрибковых средств. В связи с ростом устойчивости Candida к системным антимикотикам для лечения ВВК предпочтительнее применять препараты для локальной терапии. Триада средств позволяет комплексно подойти к лечению ВВК в трех шагах: первичная гигиена («Залагель интим»), лечение («Залаин» суппозиторий и/или «Залаин» крем), интимная гигиена («Залагель интим»). Для профилактики рецидивов также важно соблюдать интимную гигиену, разумно проходить антибактериальную терапию, при наличии СД контролировать гликемию, при подборе КОК отдавать предпочтение препаратам с низким содержанием эстрогена. В целом новые стратегии в отношении противогрибковой терапии, идентификации целей и технологий рационального дизайна лекарств могут значительно ускорить процесс разработки новых противогрибковых средств, сокращая время на выздоровление или обеспечивая лучшее качество жизни пациентов.



