Вульвовагинальный кандидоз (ВВК) является одним из наиболее распространенных инфекционных заболеваний, поражающих женщин фертильного возраста. Согласно результатам опросов, подавляющее большинство женщин встречаются на протяжении жизни минимум с одним эпизодом ВВК: по данным Farr A. (2021), их доля достигает 70-75% [1], по итогам исследования Yano J. (2019) - 77,5% [2]. Рецидивирующая форма ВВК занимает одно из первых место среди всех микозов в Российской Федерации, по данным Klimko N. et al. (2015), 2 487 215 российских женщин страдают данной патологией (3481/100 000) [3].
Грибы рода Candida широко распространены в качестве компонента оппортунистической микрофлоры. Избыточный рост, приводящий к развитию ВВК, может отмечаться на фоне состояний, приводящих к изменению морфофункционального состояния слизистой в вульвовагинальной области, а также состояний, характеризующихся выраженным изменением состава микрофлоры. Физиологическим фактором, провоцирующим в ряде случаев избыточный рост Candida, является беременность. По данным Leli C. (2013), достоверно более высокий уровень колонизации Candida spp. характерен для беременных в сравнении с небеременными женщинами (31,4% против 19,9%;/2=5,59;/>=0,018) [4]. Избыточный рост грибов рода Candida в слизистой оболочке влагалища и вульвы приводит к появлению типичной клинической картины, включающей такие симптомы, как вагинальный зуд (91,2%), жжение (68,3%), боль и покраснение (58,1%), выделения из влагалища (55,6%), боль во время полового акта (40,5%), сухость влагалища (29,3%) [2]. Типичная для ВВК симптоматика является причиной значимого снижения качества жизни женщины, в особенности в случае рецидивирующих форм [5, 6]. Провоцировать развитие ВВК могут лечение антибиотиками широкого спектра действия, использование контрацептивов с высоким содержанием эстрогена, повышенная сексуальная активность, беременность и неконтролируемый сахарный диабет [2, 7]. В ретроспективном исследовании, включавшем 950 000 женщин [8], в качестве факторов риска развития ВВК были определены следующие: применение антибиотиков в гинекологии (ОШ=2,88), системных антибиотиков (ОШ=1,45), пероральных контрацептивов (ОШ=1,74), вагинальных контрацептивов (ОШ=1,84), наличие рака (ОШ=1,20) и состояние беременности (ОШ=1,59). Присутствие представителей Lactobacilli spp. во влагалище противодействует росту Candida, в частности, фунгицидная активность против C. albicans и C. lusitaniae была продемонстрирована для L. crispatus и L. vaginalis [9]. Также подавление роста Candida было отмечено в присутствии ацетата, бутирата, пропионата, возможное ингибирующее воздействие было продемонстрировано для низкого уровня рН (4-4,5) и высокого уровня лактата. Напротив, стимулирующий эффект в отношении роста Candida могут вызывать глюкоза, простагландин Е2 и тромбоксан В2 [10].
Роль Candida в развитии инфекций слизистых невозможно переоценить. В исследовании [11] было продемонстрировано, что дрожжевые грибы доминировали в мазках из влагалища (40,2%), на 2-м месте по частоте обнаружения находились мазки из ротоглотки (24,9%). В мокроте определялось 20,1% всех изолятов, в соскобах с роговицы - 9,1%, в соскобах с ногтевых пластинок - 5,7% [11]. Среди всех изолятов 49,8% приходилось на долю C. albicans, 43,1% составляли прочие штаммы Candida, 7,2% - дрожжевые грибы других видов. Прочие штаммы Candida включали C. krusei (15,6%), C. famata (14,4%), C. rugosa (11,1%) и C. lusitaniae (10,0%). Среди дрожжевых грибов, не относящихся к Candida, основным был Cryptococcus laurentii (66,7%) [11]. Абсолютное доминирование C. albicans в структуре возбудителей ВВК подтверждается данными Moreira D. (2021): при первичных эпизодах ВВК частота выделения C. albicans составляет 68,5%, значительно реже встречаются прочие штаммы (C. tropicalis (7,5%) и С. parapsilosis (5,5%)). C. albicans остается основным возбудителем и в случае рецидивирующих форм ВВК (38,5%), другие штаммы включают C. parapsilosis (17%), C. glabrata (4%) и C. tropicalis (6%) [12]. В практике отечественного здравоохранения отмечается тенденция к снижению доли C. albicans в общей структуре грибов рода Candida, выделенных от пациентов с урогенитальным кандидозом, с 90,2% в 2010 г. до 66,7% в 2020 г. (анализ 1927 штаммов дрожжевых грибов) на фоне возрастания распространения C. glabrata (с 6,5 до 16,7%), C. krusei (с 2,2 до 7,4%), C. parapsilosis (с 0 до 4,6%), C. tropicalis^ 1,1 до 2,8%) и C. kefyr(с 0 до 1,8%) [13].
Стандартные режимы фармакотерапии ВВК включают применение противогрибковых препаратов, относящихся преимущественно к группам азолов и полиенов [1, 14, 15]. Современные данные свидетельствуют о том, что полное излечение в случае ВВК является редко достижимым исходом, несмотря на подавление активной симптоматики; применение многих распространенных противогрибковых препаратов, в частности флуконазола, не всегда позволяет достичь полной эрадикации возбудителя [16]. Рост резистентности дрожжевых грибов - возбудителей ВВК связан с высокой частотой применения основ
ных групп противогрибковых препаратов на фоне рецидивирующих форм заболевания, частота которых достигает 8% [17]. Отмеченные тенденции к росту резистентности возбудителей ВВК диктуют необходимость динамического мониторинга эффективности имеющихся в арсенале врачей-гинекологов инструментов фармакотерапевтического воздействия.
Основные группы противогрибковых препаратов и их мишени
Существующие на фармацевтическом рынке противогрибковые препараты включают полиеновые антибиотики, азолы, эхинокандины, аллиламины и препараты различного происхождения (гризеофуль- вин, 5-флуцитозин, циклопирокс). Строение и состав мембраны грибковой клетки имеют значительные различия в сравнении с клетками млекопитающих и человека, в частности (рисунок, А). Согласно локализации мишени действия, все препараты можно условно разделить на действующие в зоне клеточной мембраны и действующие на различные внутриклеточные мишени и процессы (рисунок, В). Этот факт лежит в основе создания многочисленных препаратов, нарушающих строение мембраны грибов и не оказывающих аналогичного эффекта в отношении клеток макроорганизма.

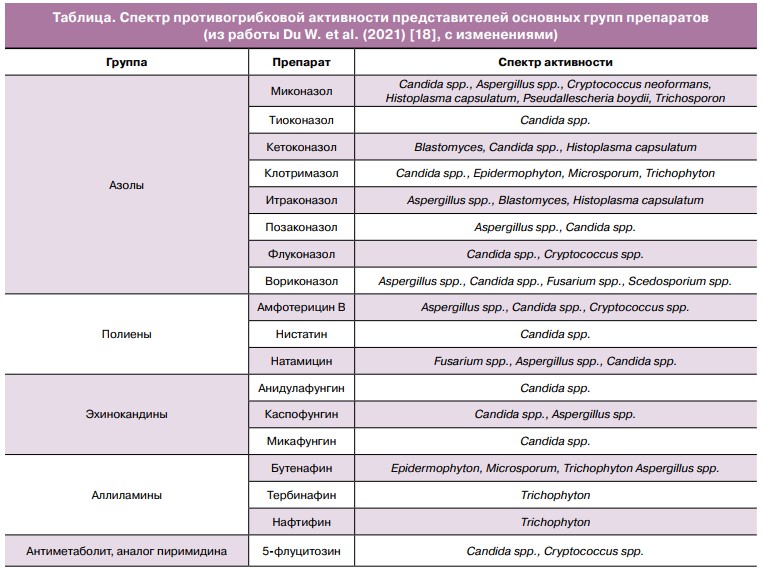
Активность против дрожжевых грибов рода Candida в различной степени характерна для представителей описанных выше групп препаратов. Таблица содержит данные о спектре противогрибковой активности полиенов, азолов, аллиламинов, эхинокандинов и 5-флуцитозина.
Резистентность к основным группам противогрибковых препаратов
Высокий уровень распространенности ВВК в популяции и рецидивирующий характер течения заболевания лежат в основе роста резистентности грибов рода Candida к наиболее применяемым препаратам. Исследование Yassin M.T. (2020) обнаружило максимальный уровень резистентности, включая множественную лекарственную резистентность, в случае C. glabrata. Изоляты C. albicans характеризовались резистентностью к флуконазолу, клотримазолу и нистатину, наиболее чувствительными оказались изоляты C. tropicalis, у которых резистентность была отмечена только к нистатину [19]. При анализе чувствительности C. albicans, выделенных у пациенток с рецидивирующим ВВК, доля резистентных изолятов составила 94% [20]. В исследовании, включавшем 150 изо- лятов C. albicans и 89 прочих штаммов дрожжевых грибов, общий уровень резистентности к флуконазолу составил 76%, итраконазолу — 62%, кетоконазолу — 72%, клотримазолу — 55%, вориконазолу — 6%, позаконазолу — 7%, нистатину — 1%, амфотерицину B — 0% [21]. Анализ чувствительности Candida spp., выполненный Bitew A. et al. (2018) [22], обнаружил, что наибольшая общая лекарственная устойчивость видов Candida наблюдалась в отношении флуконазола (17,2%) и флу- цитозина (5,7%). Все 100% изолятов Candida были восприимчивы к вориконазолу, каспофунгину и микафунгину. Изоляты C. albicans продемонстрировали 100% чувствительность ко всем протестированным препаратам, за исключением флуконазола и флуцитозина (98% чувствительность для каждого). Изоляты C. krusei имели резистентность, равную 100%, в случае флуконазола и 33,3% - в случае флуцитозина. В работе Anh D.N. (2021) 100% чувствительность C. albicans была продемонстрирована в случае микафунгина, каспофунгина и микона- зола. К амфотерицину B уровень чувствительности составил 95,65%, 5-флуцитозину - 91,3%, флуконазолу - 91,3%, итраконазолу - 82,61%, вориконазолу - 86,95% [23]. По данным Seyoum E. (2020), общее число резистентных к флуконазолу штаммов среди Candida составляло 10% [11]. По данным Hashemi S.E. et al. (2019), C. albicans характеризуются абсолютной резистентностью к итраконазолу (100%) и чрезвычайно высокой (85,7%) - к флуконазолу, резистентность к каспофунгину
составила 42,8% [24]. В работе Мальбаховой Е.Т. (2012) оценивалась динамика изолятов дрожжевых грибов за период 2008-2010 гг. [25]. Среди изолятов, полученных от пациенток с ВВК за анализируемый период, снизилось число штаммов, абсолютно чувствительных к азолам: к флуконазолу — в 2,2 раза, к клотримазолу — в 1,9, к кетоконазолу - в 2,7, к итраконазолу — в 1,7 раза. Число штаммов, чувствительных к натамицину, повысилось в 3,4 раза. Анализ параметров динамики резистентности дрожжевых грибов - возбудителей урогенитальных кандидозов в РФ за период 2010— 2020 гг. продемонстрировал, что уровень чувствительности C. albicans к флуконазолу снизился со 100% до 73,6%, к миконазолу - с 98,8% до 79,2%. Резистентность к итраконазолу возросла в случае C. albicans с 14,5 до 52,9%, C. krusei - с 0 до 37,5%, C. parapsilosis - с 0 до 60,0%. Изоляты С. glabrata, C. tropicalis, C. kefyr характеризовались более чем 50% уровнем резистентности к итраконазолу [13].
Особенности формирования резистентности к противогрибковым лекарственным средствам различаются в зависимости от групповой принадлежности препарата. Чем больше этапов включает механизм действия, тем потенциально большее число факторов может привести к развитию устойчивости грибов. Полиены реализуют действие посредством прямого связывания с эргостеролом клеточной мембраны гриба, в связи с чем основным механизмом приобретенной
устойчивости является модификация структуры стеролов клеточной мембраны грибов вследствие мутаций в ERG генах грибов рода Candida (ERG11, ERG3, ERG2 и ERG6) [26-29]. В сравнении с азолами полиены характеризуются более низким уровнем устойчивости со стороны грибов, в частности возникающей в ответ на длительное применение препарата, что подтверждается работой Streekstra H. et al. (2016), исследовавшей феномен формирования резистентности в ответ на сверхдлительное применение натамицина в условиях эксперимента in vitro [30]. Длительность нагрузки натамицином, в зависимости от вида гриба, составила от 12 до 46 недель. После окончания эксперимента минимальная подавляющая концентрация для натамицина в ряде случаев была увеличена, тем не менее, чувствительность была сохранена. Максимальный рост минимальной подавляющей концентрации и, соответственно, резистентности отмечался в случае Colletotrichum musae (в 3,25 раза) и Aspergillus ochraceus (в 2,85 раза).
Снижение чувствительности к азолам является тревожной тенденцией, характерной как для международной клинической практики, так и для практики отечественного здравоохранения. В основе данного явления лежат комплексные процессы, вовлекающие как нарушения структуры и функции ферментов, необходимых для образования ланостерола (предшественника эргостерола), так и фено
мен эффлюкса - обратного выброса молекул препарата из клетки гриба [31-34]. Опубликованы данные, свидетельствующие о повышении устойчивости изолятов грибов к азолам на фоне повышения образования в клеточной мембране сфинголипидов [35].
Устойчивость грибов рода Candida к 5-флуцито- зину является следствием снижения его внутриклеточного содержания и нарушения процессов метаболизма нуклеотидов, приводящих к уменьшению токсичности препарата [36].
Фермент глюкансинтаза служит основной мишенью для эхинокандинов, связывание, в частности, происходит через белок Fks1. Изменение структуры фермента путем аминокислотных замен приводит к невозможности реализации действия препаратов данной группы [37-39]. Парадоксальный рост грибов в присутствии каспофунгина также может быть объяснен компенсаторным увеличением содержания хитина в клеточной стенке на фоне снижения глюкана [40].
Клиническая эффективность препаратов, активных против Candida spp.
Клиническая эффективность противогрибковых препаратов определяется уровнями их концентрации в тканях, которые, в свою очередь, зависят от молекулярной массы, пути введения, продолжительности контакта и способности соединения преодолевать клеточные барьеры. В терапии ВВК противогрибковые препараты могут применяться как системно (внутрь), так и местно, интравагинально. Эффективность обоих путей сопоставима: по данным Кохрановского обзора, местное применение противогрибковых препаратов характеризуется не меньшей эффективностью, но большей безопасностью, связанной со снижением риска системных нежелательных реакций [41]. Фармакотерапия урогенитального кандидоза, включая ВВК, основана на применении азолов и полиенов [1, 14, 15]. Проект Федеральных клинических рекомендаций по ведению пациентов с урогенитальным кандидозом, разработанный Российским обществом дерматовенерологов и косметологов (2020), в качестве препаратов выбора для интравагинального применения содержит клотримазол, натамицин, миконазол, бутоконазол, итраконазол; для перорального приема - флуконазол и итраконазол [42]. Более высокий уровень роста резистентности к азолам в сравнении с полиенами делает последние более привлекательной опцией для фармакотерапии ВВК. Фармакокинетические и фармакодинамические характеристики натамицина свидетельствуют о его высокой эффективности и безопасности при местном применении на коже и слизистых оболочках. В группе полиеновых антибиотиков натамицин характеризуется меньшей токсичностью в сравнении с нистатином при местном применении, системная абсорбция обоих препаратов близка к 0 [43]. Применение натамицина в гинекологии имеет достаточно длительную историю, подтверждающую клиническую эффективность препарата. Большое число работ было опубликовано в 80-е годы ХХ в., в частности, работа Ainsworth J.W. et al. (1980), демонстрировавшая результаты проспективного исследования, включавшего 50 женщин с вагинальной инфекцией, вызванной C. albicans, получавших вагинальные таблетки, содержащие натамицин, в течение 10 дней. Уровень излечения через 2 недели составил 76% и поддерживался в течение последующих 4 недель [44]. В исследовании Buch A. ct al. (1982) оценивали эффективность натамицина у 33 пациенток с вагинальным кандидозом (интравагинальные таблетки, 10 дней), чьи партнеры получали либо крема c натамицином, либо плацебо. Последующие осмотры проводились через неделю после прекращения лечения и примерно через месяц. Частота излечения среди пациенток, у которых активно лечились партнеры, составляла 94%, что достоверно не отличалась от частоты 88% для пациенток, чьи партнеры получали плацебо [45]. Christensen E.S. (1982) опубликовал данные эффективности применения натамицина у 88 пациенток с влагалищным инфицированием C. albicans (вагинальный крем, 6 дней, по 5 мл (концентрации: 25 мг натамицина/5 мл, 50 мг ната- мицина/5 мл и 100 мг натамицина/5 мл)). Частота излечения, оцененная по отрицательным культурам мазков через 4-7 дней после прекращения лечения, составила 89,3, 85,7 и 96,9% соответственно для каждой концентрации. Мазки, взятые через 2 недели после следующего менструального периода, показали показатели излечения 67,9, 67,9 и 78,1% соответственно [46]. Результаты трехэтапного рандомизированного контролируемого исследования, включившего 423 пациентки с ВВК, опубликованы Wiedey K.D. et al. (1984). На первом этапе 168 пациенток были рандомизированы в 3 группы, получавшие 3-, 6- или 10-дневный курс лечения суппозиториями, содержащими 100 мг натамицина. На втором этапе 138 женщин были рандомизированы в 3 группы, получавшие либо 1 суппозиторий, содержащий 100 мг натамицина, однократно, либо в 1-й день лечения - суппозиторий 100 мг натамицина, а в 2 последующих - суппозитории, не содержащие активного вещества, либо по одному суппозиторию 100 мг натамицина в течение 3 дней. На третьем этапе 117 женщин были рандомизированы в 3 группы, получавшие либо 300 мг натамицина (по 1 суппозиторию 100 мг натамицина в течение 3 дней), либо суппозитории, содержащие 500 мг клотримазола однократно, либо 600 мг изоконазола однократно. Эффективность применения натамицина была высокой (85-96%) во всех группах, было отмечено положительное влияние натамицина на микробиоценоз влагалища [47]. Сравнение натамицина и миконазола было продемонстрировано в простом слепом рандомизированном контролируемом клиническом исследовании (n=52: женщины детородного возраста с ВВК), методом рандомизации пациентки были распределены в 2 группы: 1-я получала суппозитории «Пимафуцин» (100 мг натамицина) 1 раз в день в течение 3 дней, 2-я - вагинальные капсулы «Гино-Дактарин» (400 мг миконазола нитрата) в течение 3 дней. Результаты свидетельствовали о высокой эффективности обоих препаратов на фоне отсутствия значимых различий. Элиминация Candida spp. отмечалась у 81% пациенток в группе натамицина и у 85% пациенток, получавших миконазол (p>0,7). Полное купирование симптомов отмечалось у 42% пациенток 1-й группы и у 35% пациенток 2-й группы (p>0,5). Нежелательных лекарственных реакций в группе натамицина не было зарегистрировано, в группе миконазола у 1 пациентки был отмечен аллергический контактный дерматит [48]. Масштабное открытое многоцентровое исследование (n=2248, женщины с ВВК, 490 медицинских центров), оценивавшее эффективность 3-дневного курса интравагинального натамицина (суппозитории, 100 мг, 1 раз в день), обнаружило, что показатели эффективности лечения, оцениваемые как «очень хорошие» или «хорошие», были достигнуты в 90% случаев. Переносимость была оценена как «очень хорошая» или «хорошая» в 97% случаев. В 3% случаев было отмечено чувство жжения в вульвовагинальной области [49].
Среди работ, выполненных после 2010 г., стоит остановиться на следующих. Мальбахова Е.Т. и соавт. (2012) представили результаты проспективного исследования эффективности натамицина у пациенток с ВВК различных вариантов течения (1 группа — острое течение (n=11) — свечи вагинальные, 100 мг, 6 дней; 2 группа — пациентки с хроническим течением, разделены на 2 подгруппы: подгруппа 1 (n=15, 100 мг натамицина в день, 9 дней), подгруппа 2 (n=15, суппозитории 100 мг в день плюс таблетированная форма натамицина, по 1 таблетке 2 раза в сутки, 10 дней с 1-го дня использования свечей с целью элиминации возбудителя из кишечника, а также натамицин в форме крема при симптомах дерматита (2 раза в сутки)). Контрольные визиты и обследование выполнялись через 3 и 6 недель после терапии. В подгруппе 1 повторный курс терапии ВВК в связи с рецидивом потребовался 4 пациенткам (26,7%); в подгруппе 2 рецидив ВВК наблюдался у 1 пациентки (6,7%) (р<0,001). При остром течении ВВК была продемонстрирована достаточность назначения натамицина в виде свечей циклом 6 дней, при хроническом рецидивирующем ВВК — необходимость удлиненного курса терапии в течение 9 дней с добавлением таблетированной формы и крема [25].
Высокая эффективность 6-дневного курса натамицина в лечении кандидозного вагинита была представлена в работе Карапетян Т.Э. и соавт. (2014). В рамках проспективного исследования (n=70, средний возраст 27,4±1,3 года) пациентки с лабораторно подтвержденным диагнозом «острый кандидозный вагинит» получали натамицин по 1 вагинальной свече (100 мг) в сутки в течение 6 дней. Контроль эффективности терапии осуществляли через 7—10 дней и через 1 месяц от начала терапии. Результаты продемонстрировали уровень элиминации дрожжевых грибов из вагинального отделяемого, равный 98,6%. При обследовании через 28—31 день было выявлено 0 рецидивов; побочных реакций, связанных с местным применением натамицина, не было отмечено [50].
Согласно данным Мозговой Е.В. и соавт. (2014), применение натамицина характеризовалось более высокой эффективностью на фоне добавления преи пробиотиков. В исследование были включены пациентки с первичным эпизодом кандидоноси- тельства (n=24, концентрация Candida <105 КОЕ/ мл) и минимальными клиническими проявлениями, получавшие 6-дневный курс препаратом «Примафунгин» («Авексима», Россия) интраваги- нально (монотерапия), а также пациентки с рецидивирующим ВВК с выраженной симптоматикой (n=36, концентрация Candida <105 КОЕ/мл, микроскопически большое количество лейкоцитов, присутствовали клинические признаки кольпита), получавшие 9-дневный курс в комбинации с пероральным приемом пробиотиков и пребиоти- ков [51].
Высокий уровень эффективности короткого курса натамицина (3 дня) приводится в результатах исследования Доброхотовой Ю.Э. и соавт. (2018). В рамках проспективного клинического исследования 30 пациенток с острым ВВК получали натамицин в течение 3 дней (Примафунгин («Авексима», Россия)). Положительный клинический результат лечения был отмечен у 91%, микологическая эради- кация — у 96%; был выявлен высокий уровень приверженности терапии [52].
Результаты применения натамицина у пациенток с хроническим рецидивирующим ВВК (проспективное наблюдательное исследование, n=150, средний возраст 30,5±3,5 года): группа 1 (n=50) - хронический рецидивирующий ВВК + кандидозный дисбиоз кишечника, грибы рода Candida выявлены в высоких титрах (t 104 КОЕ/мл) из влагалища и кишечника - получали натамицин в форме вагинальных свечей по 100 мг (1 свеча) 1 раз в день в течение 6 дней + натамицин в кишечнорастворимых таблетках по 100 мг (1 таблетка) 4 раза в день перорально в течение 10 дней + поддерживающая терапия 6 месяцев; группа 2 (n=100) — хронический рецидивирующий ВВК, грибы рода Candida выявлены в высоких титрах (t 104 КОЕ/мл) только из влагалища — получали натамицин в форме вагинальных свечей по 100 мг (1 свеча) 1 раз в день в течение 6 дней, из них подгруппа 2а (n=50) — дополнительно получали натамицин в форме вагинальных свечей по 100 мг 1 раз в сутки в течение 3 дней перед ожидаемым обострением ежемесячно; подгруппа 2б (n=25) — дополнительно получали флуконазол по 150 мг перорально 1 раз в неделю; подгруппа 2в (n=25) — дополнительно получали клотримазол в форме вагинальной таблетки по 500 мг 1 раз в неделю. Клиническое выздоровление было отмечено у 100% пациенток в группах 1 и 2; через 10 дней после окончания терапии у 86% пациенток рост грибов во влагалище отсутствовал, у 24% титр Candida spp. из влагалища составил менее 103 КОЕ/мл. Исследование материала из кишечника пациенток группы 1 обнаружило отсутствие роста в 100% случаев. Эффективность поддерживающей терапии натамицином составила 98% и достоверно превосходила таковую в отношении флуконазола (^=0,008) и клотримазола (р=0,024) [53].
Интересны результаты открытого сравнительного рандомизированного многоцентрового клинического исследования по изучению эффективности и безопасности препаратов «Таржифорт», суппозитории вагинальные» (метронидазол 500 мг, хлорамфеникол 200 мг, натамицин 150 мг, гидрокортизона ацетат 15 мг; ОАО «Авексима», Россия) и «Тержинан», таблетки вагинальные» (тернида- зол 200 мг, неомицина сульфат 100 мг, нистатин 100 000 МЕ, преднизолона метасульфобензоат натрия 4,7 мг; «Лаборатории Бушара-Рекордати», Франция) в лечении вагинитов различной этиологии (женщины, «=360, 2 параллельные группы, возраст от 18 до 45 лет с клиническим диагнозом «вагинит в острой стадии», уточненный лабораторно). В работе было продемонстрировано отсутствие боли у 98,3%, жжения - у 97,8% и зуда - у 98,9% пациенток группы «Таржифорт» и 98,3, 97,8 и 95,4% пациенток группы «Тержинан»; скорость исчезновения симптомов «боль» и «жжение» - 4,3 и 4,8 дня соответственно, что статистически значимо выше, чем у пациенток, получавших «Тержинан» (5,6 и 5,8 дня) [54].
Эффективность применения натамицина у беременных оценивалась в проспективном когортном исследовании («=173, из них у 77 — бактериальный вагиноз (БВ), у 37 — аэробный вагинит, у 39 — кан- дидозный вагинит (КВ), у 20 — БВ + КВ), в зависимости от времени обращения 76 беременных наблюдались с I, 45 — со II и 52 — c III триместра беременности. Пациентки с КВ получали натамицин, 1 вагинальная свеча (100 мг) в сутки в течение 6 дней. Эффективность натамицина составила 94,1%. На 22—23-й неделе рецидив был выявлен у 29,4%, на 33—34-й неделе — у 5,9% пациенток [55].
В работе Николаевой О.А. и соавт. (2016) также представлены данные о применении натамицина у беременных с ВВК («=80, группа 1 (n=18): натамицин, 3 вагинальных суппозитория; группа 2 (n=20): натамицин, 6 вагинальных суппозиториев; группа 3 (n=24): натамицин, 3 вагинальных суппозитория + натамицин перорально в течение 3 дней (100 мг 4 раза в сутки); группа 4 («=18): натамицин вагинальные суппозитории + натамицин перорально (100 мг 4 раза в сутки) в течение 6 дней). Лечение полового партнера (натамицин, 2% крем) осуществлялось во всех группах. Максимальная эффективность терапии ВВК была продемонстрирована в группе 4 (0% рецидивов на протяжении всей беременности). Режим, использованный в данной группе, также характеризовался минимальным числом гестационных, родовых и послеродовых осложнений среди всех прочих групп [56].
Эффективность и безопасность применения натамицина у беременных также подтверждаются результатами проспективного рандомизированного слепого исследования, включавшего 60 беременных с ВВК, возраст — от 18 до 37 лет (группа, получавшая Примафунгин («Авексима», Россия) — 30 человек, группа, получавшая Пимафуцин (Temmler Italia S.r.L., Италия) — 30 человек). Результаты продемонстрировали высокую эффективность натамицина в эрадикации C. albicans и C. non-albicans, а также высокий профиль безопасности применения на всех сроках беременности и лактации, даже при отягощенном гинекологическом анамнезе [57].
Обсуждение
Уникальный механизм действия натамицина характеризуется минимальным числом мишеней для возможного развития резистентности, что выгодно отличает его от многих других антимико- тиков. Фунгицидный эффект и широкий спектр действия препарата, наряду с оптимальными фармакокинетическими характеристиками (минимальный уровень системной абсорбции при интрава- гинальном применении), лежат в основе высокого уровня эффективности и безопасности, продемонстрированного при применении натамицина у женщин репродуктивного возраста и беременных. Натамицин используется 1 раз в сутки на ночь, большинство клинических исследований обнаружило стойкое устранение острой симптоматики и эрадикацию возбудителя у пациенток при применении препарата в течение 6 дней [46, 47, 50—52, 54, 56], ряд работ приводит близкие данные в случае применения препарата коротким, 3-дневным курсом [47—49, 52]. Профиль безопасности препарата, согласно полученным данным, высокий, возникновение нежелательных лекарственных реакций нетипично не только для интравагинальных форм натамицина (суппозитории, крем), но и для системных (таблетки для приема внутрь).
Манифестация ВВК наиболее характерна для II и III триместров беременности [58], в связи с чем оптимальный препарат для местной терапии должен характеризоваться отсутствием рисков для плода. Опубликованные данные клинических исследований продемонстрировали практическое отсутствие нежелательных лекарственных явлений при применении натамицина у беременных. Важным является отмеченное в исследованиях отсутствие нежелательного эффекта натамицина на плод в случае его системного применения [56]. В целом группа полиенов характеризуется достаточно низким уровнем рисков применения у беременных в сравнении с прочими антимикотиками, включая азолы. Тератогенный потенциал натамицина при интравагинальном применении на 2-м и 3-м месяце беременности (критическом периоде для возникновения большинства серьезных врожденных аномалий) не был выявлен (парный анализ случай-контроль, 38 151 беременных женщин, родивших здоровых детей (контрольная группа) и 22 843 беременных, родивших детей с анома- лиями/дефектами (скорректированное ОШ 0,9; 95% ДИ 0,4—1,8)). Средний вес при рождении был выше (на 72 г) у новорожденных в контрольной группе, рожденных от матерей, получавших лечение натамицином, по сравнению с данными для новорожденных в контрольной группе (^=0,01), несмотря на меньший гестационный возраст [59].
Анализ масштабной когорты беременных женщин (Квебек, Канада, 1998-2015) позволил оценить риски применения перорального флуконазола в низких и высоких дозах, <150 мг и >150 мг соответственно. Исследователями было продемонстрировано, что любое воздействие флуконазола на мать во время беременности приводило к увеличению риска самопроизвольного выкидыша, флуконазол в дозе <150 мг, примененный в течение I триместра, увеличивал риск аномалий закрытия перегородки сердца [60]. В работе Zhu Y. (2020) было продемонстрировано, что использование перорального флуконазола в I триместре провоцирует пороки развития опорно-двигательного аппарата. В исследовании анализировались 1 969 954 беременностей, из них 37 650 (1,9%) включали применение флуконазола внутрь и 82 090 (4,2%) - применение местных азолов в течение I триместра. Риск пороков опорно-двигательного аппарата составил 52,1 (95% ДИ 44,8-59,3) на 10 000 беременностей, подвергшихся воздействию флуконазола, по сравнению с 37,3 (95% ДИ 33,1-41,4) на 10 000 беременностей, подвергшихся воздействию местных азолов. Скорректированный относительный риск после точной стратификации оценки предрасположенности составил 1,30 (от 1,09 до 1,56) для скелетномышечных пороков развития [61].
Эффективность и безопасность натамицина делают его перспективным препаратом для дальнейшего использования в терапии ВВК, что подтверждается активным поиском новых лекарственных форм, обеспечивающих максимальный эффект при его интравагинальном введении. Высокий уровень эффективности и низкий риск развития резистентности со стороны дрожжевых грибов при использовании натамицина могут также объясняться наличием дополнительного фармакодинамического эффекта, состоящего в возможном противодействии образованию биопленок Candida spp. [62], являющихся одним из факторов резистентности и, как правило, включающих клетки грибов, гифы и псевдогифы, образующие подобие сети, покрытой внеклеточным матриксом. Дополнительным положительным терапевтическим эффектом натамицина является его возможное положительное влияние на микробиоценоз влагалища [47], что способствует снижению рисков дальнейшего активного роста Candida spp. и вносит вклад в снижение частоты рецидивов данного заболевания, в том числе у такой уязвимой категории больных, как беременные, для популяции которых было продемонстрировано достижение нулевого уровня обострений хронического рецидивирующего ВВК на протяжении всего периода беременности.
Заключение
Высокий уровень эффективности натамицина в отношении различных дрожжевых грибов, наряду с низким потенциалом развития резистентности, а также положительным влиянием на микробиоценоз влагалища, позволяет обеспечить быстрое устранение симптоматики при остром эпизоде ВВК и снизить риск рецидивов при хроническом рецидивирующем ВВК. Удобный режим использования (1 раз в сутки) предполагает высокий уровень приверженности фармакотерапии. Продемонстрированные в клинических исследованиях результаты применения интравагинальных форм натамицина (суппозитории) свидетельствуют о достаточности в большинстве случаев 6-дневного курса приема препарата. Токсические реакции при использовании препарата не характерны, что обеспечивается минимальной системной абсорбцией при интравагинальном применении вследствие высокой гидрофобности молекул. Отсутствие тератогенного потенциала натамицина, наряду с лучшими показателями новорожденных (более высокий вес, несмотря на меньший срок гестации), делает препарат одним из оптимальных фармакотерапев- тических инструментов для ведения беременных пациенток с ВВК. Продемонстрированная в исследованиях безопасность системного приема натамицина косвенно свидетельствует о наличии высокого профиля безопасности применения интравагиналь- ного натамицина на фоне лактации. Доступные на фармацевтическом рынке РФ отечественные препараты натамицина для интравагинального применения включают монопрепарат «Примафунгин» (суппозитории вагинальные, 100 мг; «Авексима», Россия), а также комбинированный препарат «Таржифорт» (суппозитории вагинальные, метронидазол 500 мг, хлорамфеникол 200 мг, натамицин 150 мг, гидрокортизона ацетат 15 мг; «Авексима», Россия).



