Markers of permeability and functional state of the placental barrier in pregnant women with chlamydial infection
Objective: To investigate the permeability of the placental barrier and the functional state of the placenta in pregnant women with chlamydial infection. Materials and methods: The study analyzed amniotic fluid and serum from 124 pregnant women at 38 to 40 weeks' gestation who had different courses of Chlamydia trachomatis infection. Group 1 included 83 pregnant women with acute infection, asymptomatic course (PCR+; IgM+; IgG titer <1:60), of whom 39 had children born with congenital infectious disease (CID) and 44 had healthy children. Group 2 included 41 pregnant Chlamydia trachomatis carriers (PCR-, IgM-, IgG titer >1:60) of whom 22 had CID babies and 19 had healthy babies. The control group comprised 37 healthy pregnant women of similar gestational age. High-molecular-weight alpha2-macroglobulin (α2-MG, 720 kDa) and low-molecular-weight albumin (68 kDa) were measured by enzyme immunoassay and quantitative rocket immunoelectrophoresis, respectively. Serum concentrations of α2-MG were determined by quantitative rocket immunoelectrophoresis, albumin by biochemical method. Placental proteins [pregnancy-associated protein A (PAPP-A) and trophoblastic β1-globulin (TBG)] were analyzed by immunoassay. Results: Women who gave birth to babies with CID had elevated levels of amniotic fluid albumin regardless of the course of the infection [5.5 (2.3–11.5) g/L vs 1.8 (1.2–2.9) g/L for carriers and 3.3 (2.3–7.4) g/L vs 1.8 (1.0–2.2) g/L for acute form]. The α2-MG level in the amniotic fluid was elevated only in chlamydia infection carriers; its concentration was highest in those who gave birth to babies with CID [55.0 (25.4–111.4) mg/L vs 13.8 (5.5–37.2) mg/L in Chlamydia trachomatis carriers who gave birth to children without CID]. In women with acute chlamydia infection, serum levels of α2-MG was increased among those in Group 1 who gave birth to babies with CID [3.3 (2.9–3.6) g/L vs 2.5 (2.3–2.7) g/L in women who gave birth to children without CID]. Pregnant Chlamydia trachomatis carriers showed a significant reduction in blood placental protein PAPP-A levels compared with controls and women who gave birth to children without CID. Conclusion: Determination of α2-MG and albumin in the amniotic fluid of pregnant Chlamydia trachomatis carriers and patients with acute chlamydial infection can be used to evaluate placental barrier permeability. Elevated blood levels α2-MG in pregnant women with acute chlamydia infection are indicative of fetal/newborn CID.Renge L.V., Zorina V.N., Grebneva V.S., Grigoryeva E.Yu., Vlasenko A.E.
Keywords
Maternal Chlamydia trachomatis infections are one of the causes of congenital infectious disease (CID) in newborns. Maternal Chlamydia trachomatis infection has several stages (chronic, persistent, latent, acute) that determine different variants of the clinical course of the disease, including Chlamydia trachomatis carrier (IgM-, IgG titer >1:60, PCR-) or acute asymptomatic with PCR+, IgM+ and/or IgG titer <1:60 [1–5]. The leading role in the protection of the fetus that becomes infected during pregnancy belongs to the placental barrier, which consists of the trophoblast epithelium, syncytium covering the placental chorionic villi, the connective tissue of the villi and the endothelium of their capillaries. The trophoblast cells that make up the placental chorionic villi generate a subpopulation of invasive extravillous cells that separate fetal blood by placental membrane from maternal blood. The fusion of the differentiated cytotrophoblast forms a syncytiotrophoblast that completely envelopes the developing placenta and forms an effective barrier that selectively regulates gas exchange and the transport of nutrients and exogenous agents (drugs, toxins, viruses, etc.). The dense polarized epithelial monolayer contains compounds that prevent lateral and paracellular diffusion of substrates to protect the developing fetus. However, a number of viruses and bacteria are able to cross the placental barrier, the permeability of which is determined by the protein levels in amniotic fluid synthesized by the pregnant liver [6–9].
Chlamydia trachomatis is detected in all tissue structures of the placenta of women with genital Chlamydia trachomatis infection. Chlamydia-infected cells are found in the capillary lumen of the chorion villi, indicating a possible hematogenous route of transmission from mother to fetus (the least studied route, compared to the ascending infection through the amnion membranes). In the placentas of women with genital Chlamydia trachomatis infection, immune homeostasis is affected by the formation of pathogenic immune complexes (PIC), including IgM, IgG, IgA and fixing the complement C3 fraction as a marker of pathogenicity. Fixation of PIC fixation on the membrane structures of the placenta has been shown to the destruction of syncytial membranes and the placental barrier [10]. An important role in maintaining the density and integrity of placental trophoblasts and endothelial cells is played by adhesive intercellular compounds (occlusin, VE-cadherin and β-catenin), which are destroyed by excess pro-inflammatory cytokines, including interleukin (IL)-1β and transforming growth factor (TGF) and promote chorioamnionitis development, penetration of infectious pathogens to the fetus [11]. An increased concentration of pro-inflammatory cytokines in the amniotic fluid is, according to some authors, a marker of placental barrier failure [12]. In addition, Chlamydia trachomatis is capable of manipulating multiple processes to ensure its developmental cycle, activating signaling pathways and promoting the release of tissue-damaging proteins, cytokines and other biologically active compounds, and self-modeling epithelial surfaces by reorganizing intercellular adhesion, reprogramming membrane transport, and immune response signals [11].
It should be noted that not all placental lesions are accompanied by intrauterine infection, congenital infectious disease (CID) of the fetus and the newborn. Most babies are born at term, viable, and even healthy. At the same time, more than 90% of infected children have no symptoms of the disease immediately after birth [13]. Therefore, it is very important to study the compensatory reactions of the placental barrier, its protective functions, including specific immunity [10]. Data on this issue are lacking, and therefore the search for methods to assess the functional state and permeability of the placenta in infected pregnant women before delivery to predict fetal and neonatal CID, to guide the delivery management strategy remains relevant.
The content of serum proteins of different molecular weights in the amniotic fluid offers information on the degree of permeability of the placental barrier, and the levels of placental proteins synthesized during pregnancy in the blood are associated with its functional state.
Alpha2-macroglobulin (α2-MG) and albumin are synthesized mainly by liver cells. The mean concentration of α2-MG in the blood ranges from 2–3 g/l, with a molecular weight of 720 kDa. The protein can bind to pathogen proteinases, preventing their invasion, and to bind to pathogen toxins, provoking cell apoptosis. α2-MH complexes with cytokines have immunomodulatory effects, while complexes of oxidized (damaged) α2-MH with proteinases and autoantibodies that partially retain lytic activity can destroy cells [14]. The concentration of albumin in the blood is 40–60 g/L, with a molecular weight of 67 kDa. These proteins diffuse across intact tissue barriers such as placenta and spinal cord in insignificant amounts or are synthesized in minimal concentrations (nanograms, picograms). The role of proteins synthesized by the trophoblast and placenta in the mechanisms of fetal growth and the evaluation of the functional state of the placental barrier is actively being investigated [15, 16]. The most studied of them are trophoblast-specific β1-globulin (TBG) and pregnancy-associated placental protein A (PAPP-A).
The present study is aimed to investigate the markers of permeability of the placental barrier during pregnancy complicated by Chlamydia trachomatis infection with different variants of its course and the state of newborns.
Materials and methods
The study was carried out at the Novokuznetsk Perinatal Center (2015–2020) and the immunological laboratory of the Novokuznetsk State Institute for Advanced Medical Education, branch of the RMAPE. We examined 125 pregnant women admitted to the obstetric hospital at 38–40 weeks of pregnancy complicated by Chlamydia trachomatis infection. Group 1 consisted of 83 pregnant women with laboratory evidence of acute chlamydial infection (PCR+, IgM antibodies, IgG titer <1:60 for Chlamydia trachomatis). Pregnant women in this group did not receive antibiotic therapy during antenatal care. Of these, 39 women gave birth to babies with CID. In 44 pregnant women with acute infection, the newborns were healthy.
Follow-up group 2 comprised 42 pregnant women with a titer > 1:60 for class G antibodies to Chlamydia trachomatis and negative PCR without clinical manifestations of the disease during pregnancy; of these, 22 pregnant women gave birth to babies with CID and 20 gave birth to healthy infants.
The parameters in 37 healthy women who gave birth to healthy children were considered as control values.
All participants provided signed informed consent to take part in the study. The study was approved by the Research Ethics Committee of the Novokuznetsk State Institute for Advanced Medical Education – branch of the RMAPE.
Pregnant women with a history of cancer, autoimmune and chronic pelvic inflammatory disease, decompensated cardiovascular, respiratory, hepatic, or renal failure, pregnancy with ABO- and Rh factor isosensitization and HIV infection, as well as pelvic inflammatory disease of non-chlamydial etiology were excluded from the study.
Amniotic fluid was collected from women in labor at the time of spontaneous effusion or instrumental opening of the fetal membrane. All women gave birth at 38–40 weeks of gestation.
Pregnant women's blood was collected upon admission to the maternity hospital. The obtained serum was frozen and used simultaneously to perform analyses under the same conditions.
Serum α2-MH concentration was measured by quantitative low-voltage rocket immunoelectrophoresis using monospecific polyclonal rabbit antiserum against this protein. Blood albumin values were examined by colorimetric method in the presence of bromcresol green (Spinreact test systems, Spain).
The concentrations of placental proteins, RARR-A and TBG were determined by enzyme immunoassay (ELISA) (Vector-Best test system, Novosibirsk). Levels of α2-MG in amniotic fluid were studied by ELISA, albumin by quantitative rocket immunoelectrophoresis using test systems designed in the Research Laboratory of Immunology of the Novokuznetsk State Institute for Advanced Medical Education.
Statistical analysis
The normality of the distribution was tested by the Shapiro–Wilk test. Quantitative variables were expressed as median (Me) with an interquartile range (Q1; Q3). The Kruskal–Wallis test was used to compare numerical data between the 4 groups, followed by pairwise comparison using the Dunn test. Adjustment for multiple comparisons was performed under the FDR control (false rejection rate) using the Benjamini, Krieger, and Yekutieli correction algorithm. Comparison of the variables in each group with the medians measured in healthy women was made using the Wilcoxon one-sample test. Categorical variables were described as counts with percentages and comparisons in different groups using the χ2 test. Differences were considered statistically significant at p≤0.05. Statistical analysis was performed using GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA).
Results
Among pregnant women in Group 1 with laboratory signs of acute chlamydial infection (PCR+, presence of IgM or IgG antibodies in titer <60), 39 women gave birth to babies with Apgar score of 7/8. The following nosological forms of chlamydial infection were diagnosed in the early neonatal period: generalized chlamydial infection, type 2 respiratory distress syndrome, pneumonia, conjunctivitis, dermatitis, carditis, and media otitis. Forty-four pregnant women with signs of acute infection gave birth to healthy infants with a high Apgar score (8–10 points).
The second group of pregnant women were carriers of Chlamydia trachomatis (PCR-, IgG titer>1:60). Twenty-two babies were born with a low Apgar score (6/7b) and signs of CID, whether of chlamydial or non-chlamydial etiology including congenital bacterial pneumonia, generalized candida infection, conjunctivitis, vesicular dermatitis, bacterial meningitis, and chlamydial cardiopathy. In 19 women, the newborns were healthy, with Apgar score of 8-10.
Histological examination of the placentas of mothers infected with Chlamydia trachomatis both in acute infection and in the presence of the pathogen and in the birth of CID revealed morphological signs of grade I–III infection: 1) ascending infection of grade 1 (serous chorioamnionitis) – 21/61 (35%); 2) ascending stage 2 infection (purulent chorioamnionitis, choriodeciduitis) – 10/61 (16%); 3) ascending stage 3 ascending infection (purulent chorioamnionitis, phlebitis, arteritis of umbilical vessels, exudative funiculitis) - 4/61 (6%). In pregnant women in observation groups that gave birth to healthy children, histological examination revealed ascending infection grade 1 – 3/64 (5%); serous chorioamnionitis, pathological immaturity of the differentiated intermediate chorionic villi variant – 4/64 (6%); placental hyperplasia, secondary chronic placental insufficiency, hypertensive form (obliterative angiopathy), compensation – 8/64 (13%), mature placenta – 35/64 (55%).
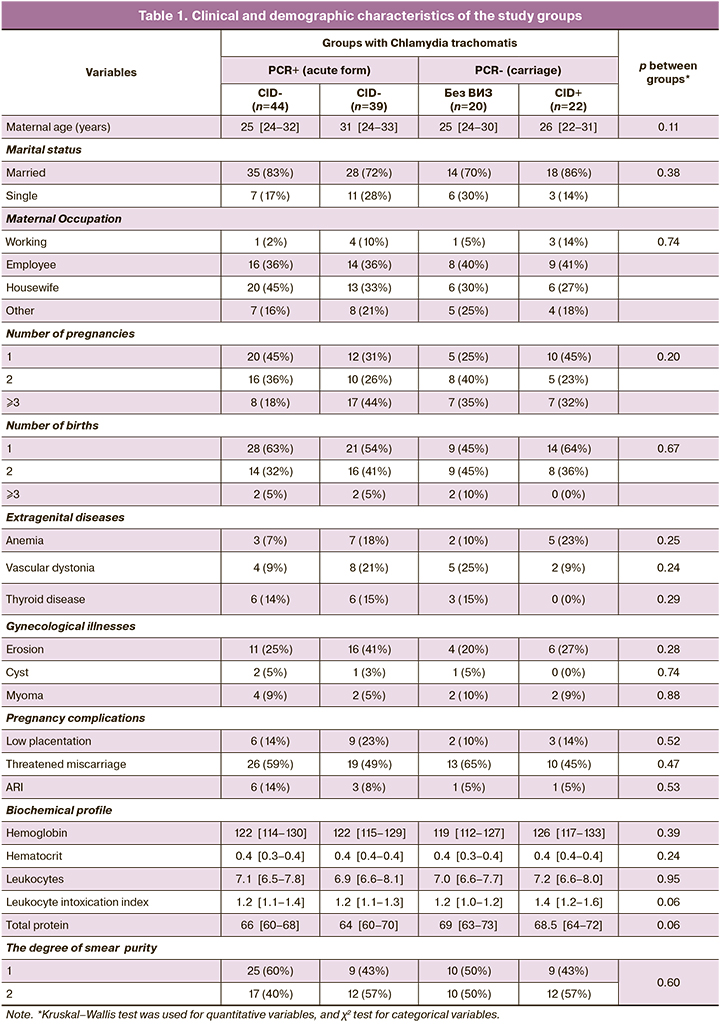
The study groups were comparable with each other in terms of age, comorbidities, and blood biochemical parameters (Table 1).
The findings on serum proteins in the composition of amniotic fluid allowed us to identify certain patterns and conclude about the permeability of the placental barrier in babies born with or without CID in pregnant women with Chlamydia trachomatis infection in different variants of its course. The mean concentration of high molecular weight α2-MH (molecular weight=720 kDa) in the amniotic fluid of pregnant women in the first group (PCR+) with the birth of children with CID was comparable to controls and did not differ statistically significantly from those who gave birth to children without CID (Table 2). However, infection carriers had statistically significantly higher levels of this protein in the CID group compared to women who gave birth to children without CID [55.0 (25.4–111.4) mg/L vs 13.8 (5.5–37.2) mg/L, p=0.03]. They were also statistically significantly higher than control values (p=0.001 in the CID group, p<0.001 in the non-CID group) and higher than in the women with acute infection group (p=0.005 in the CID group, p<0.001 in the non-CID group).
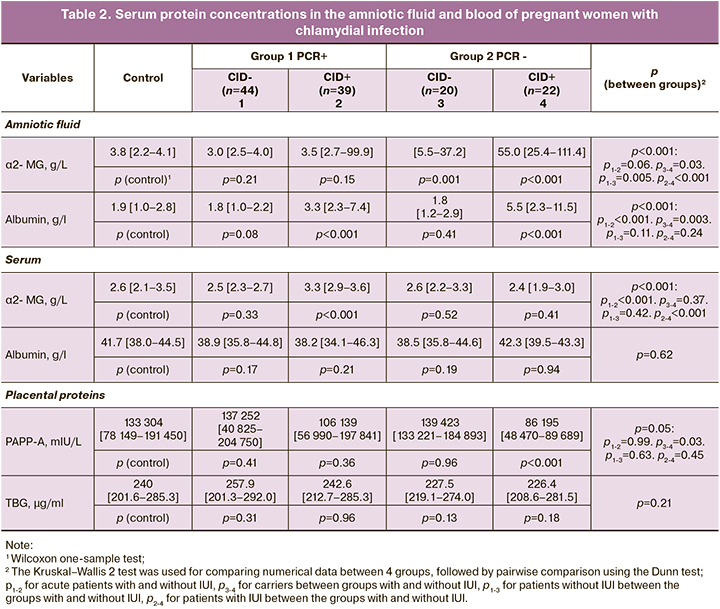
A somewhat different picture was observed in amniotic fluid parameters of another protein of serum origin – low-molecular weight albumin (molecular weight=67 kDa). There was a statistically significant increase in albumin levels at birth in children with congenital infection, regardless of the variant of the infectious process, both compared to controls and groups without CID in newborns. The presence of chlamydial infection in pregnancy and the birth of sick babies was associated with elevated albumin levels in the amniotic fluid: 5.5 (2.3–11.5) g/L versus 1.8 (1.2–2.9) g/L in the non-CID group when carrying Chlamydia trachomatis (p=0.003) and 3.3 (2.3–7.4) g/L versus 1.8 (1.0–2.2) g/L in mothers who had children without CID for acute infection (p<0.001).
There were no associations between albumin levels in pregnant women and the course of the infection process and the condition of the newborn. These parameters were not significantly different between the groups and did not differ from those of healthy pregnant women. At the same time, the α2-MH concentrations in pregnant women with acute chlamydial infection, who gave births to newborns with CID, were the highest. They were statistically significantly different from the control values (p<0.001) and those in the other groups: 3.3 (2.9–3.6) g/L versus 2.5 (2.3–2.7) g/L in the group without CID who had acute infection and versus 2.4 (1.9–3.0) g/L in the group of Chlamydia trachomatis carriers with CID.
Blood levels of TBG in pregnant women with Chlamydia trachomatis infection were stable and did not differ statistically significantly from those of healthy controls. At the same time, blood PAPP-A levels in pregnant Chlamydia trachomatis carriers were significantly decreased in those who gave birth to neonates with CID, both compared to controls (p<0.001) and in comparison to women who gave births to neonates without CID: 86195 (48470–89689) mU/l versus 139423 (133221–184893) mU/l (p=0.03).
Discussion
To study the nature of placental barrier permeability, we chose a methodical approach consisting in the determination of two proteins of serum origin (high-molecular α2-MG and low-molecular albumin) in amniotic fluid. The tissues of the fetal-placental unit synthesize them in negligible quantities, while blood concentrations of these proteins are rather high. Besides, high-molecular-weight α2-MG does not penetrate intact blood-tissue barriers. Therefore, the presence of high concentrations of low- and high-molecular-weight proteins in the amniotic fluid indicates increased nonselective permeability of the placental barrier.
According to our findings, during pregnancy complicated by chlamydial infection and birth of children with CID, non-selective placental permeability increases, which is accompanied by a significant (from 2 to 20 times) increase in serum proteins in the composition of amniotic fluid. Elevation of α2-MG was observed only in Chlamydia trachomatis carriage; maximum values of this parameter were observed at the birth of children with CID. Elevated albumin was observed in the amniotic fluid at birth of babies with CID in both Chlamydia trachomatis carriage and acute chlamydial infection. These findings are consistent with previously published clinical observations [17].
We believe that the increase in the blood concentration of α2-MH in pregnant women with the acute course of the disease and the birth of children with infection is due to the prolonged circulation of immune complexes. These immune complexes are formed with molecules of this proteinase inhibitor (including bacterial hydrolases) damaged by inflammation, due to impaired utilization rate [18, 19], which is not observed in Chlamydia trachomatis carriage.
Reduced blood PAPP-A in pregnant women carrying Chlamydia trachomatis and giving birth to children with CID indicates a lesion of placental tissue and disruption of its functional status, which, in turn, confirms the assumption about the leading role of the placenta in the development of CID in newborns and its compensatory capabilities [10].
Conclusions
All of the above allows us to conclude:
1. High and low molecular weight serum proteins in amniotic fluid may be markers of placental barrier permeability. Nonselective placental barrier permeability is increased in pregnancies complicated by chlamydial infection and births of newborns with CID. This process is particularly pronounced in the carriage of Chlamydia trachomatis, indicating deep damage to the placental tissue, despite the absence of clinical signs of the disease in pregnant women, and is accompanied by more severe forms of CID of the newborn.
2. In pregnancy complicated by chlamydial infection and its acute course, the blood concentration of α2-MH is increased, which may affect its functions as an immune modulator and proteinase inhibitor. In the carriage of Chlamydia trachomatis, the level of this protein does not differ from the control values of healthy pregnant women.
3. The lowest blood levels of the placental protein PAPP-A were detected in pregnant women carrying Chlamydia trachomatis, who gave birth to newborns with CID, indicating a compensatory protective function of the placenta in infected pregnant women.
References
- Кулаков В.И., Орджоникидзе Н.В., Тютюнник В.Л. Плацентарная недостаточность и инфекция. М.; 2004. 494с. [Kulakov V.I., Ordzhonikidze N.V., Tyutyunnik V.L. Placental insufficiency and infection. M.;2004. 494 p. (in Russian)].
- Каганова М.А., Спиридонова Н.В. Воспалительные изменения плаценты в аспекте экспрессии мРНК генов транскрипционных факторов при доношенной беременности. Акушерство и гинекология. 2022; 3: 49-58. [Kaganova M.A., Spiridonova N.V. Placental inflammatory changes associated with mRNA expression of transcription factor genes in term pregnancy. Obstetrics and Gynecology. 2022; 3: 49-58. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.3.49-58.
- Howie S.E.М., Horner P.J., Horne A.W. Chlamydia trachomatis infection during pregnancy: known unknowns. Discov. Med. 2011; 12(62): 57-64.
- Taylor B.D., Haggerty C.L. Management of Chlamydia trachomatis genital tract infection: screening and treatment challenges. Infect. Drug Resist. 2011; 4: 19-29. https://dx.doi.org/10.2147/IDR.S12715.
- Прилепская В.Н., Довлетханова Э.Р. Хламидийная инфекция в акушерстве и гинекологии. Эффективная фармакотерапия. 2014; 35: 28-33. [Prilepskaya V.N., Dovletkhanova E.R. Chlamydial infection in obstetrics and gynecology. Effective Pharmacotherapy. 2014; 35: 28-33. (in Russian)].
- Hernandez-Trejo М., Herrera-Gonzalez N.E., Escobedo-Guerra M.R., de Jesus de Haro Cruz M., Moreno-Verduzcod E.R., Lopez-Hurtado M. et. al. Reporting detection of Chlamydia trachomatis DNA in tissues of neonatal death cases. J. Pediatr. (Rio J). 2014; 90(2): 182-9. https://dx.doi.org/10.1016/j.jped.2013.09.002.
- Сидорова И.С., Макаров И.О., Матвиенко Н.А. Внутриутробная инфекция: ведение беременности, родов и послеродового периода. М.: МЕДпресс-информ; 2012. 160с. [Sidorova I.S., Makarov I.O., Matvienko N.A. Intrauterine infection: management of pregnancy, childbirth and postpartum period. M.: MEDpress-inform; 2012. 160 p. (in Russian)].
- Delorme-Axford E., Sadovsky Y., Coyne C.B. The placenta as a barrier to viral infections. Annu. Rev. Virol. 2014; 1(1): 133-46. https://dx.doi.org/10.1146/annurev-virology-031413-085524.
- Григорьева Е.Ю., Ренге Л.В., Зорина В.Н., Власенко А.Е., Филимонов С.Н. Неселективная проницаемость плацентарного барьера при преждевременном разрыве плодных оболочек и внутриутробной инфекции новорожденного. Мать и дитя в Кузбассе. 2020; 2: 38-43. [Grigoryeva E.Yu., Renge L.V., Zorina V.N., Vlasenko A.E., Filimonov S.N. Non-selective permeability of the placental barrier with premature rupture of the membranes and intrauterine infection of the newborn. Mother and Child in Kuzbass. 2020; 2(81): 38-43. (in Russian)]. 10.24411/2686-7338-2020-10020.
- Буданов П.В. Актуальные проблемы лечения беременных с рецидивирующей хламидийной инфекцией. Лечащий врач. 2007; 10: 21-5. [Budanov P.V. Actual problems of treatment of pregnant women with recurrent chlamydial infection. Lvrach.ru. 2007; 10: 21-5. (in Russian)].
- Цинзерлинг В.А. Внутриутробные инфекции: современный взгляд на проблему. Журнал инфектологии. 2014; 6(4): 13-8. [Tsinzerling V.A. Intrauterine Infections: Modern View upon the Problem. Journal Infectology. 2014; 6(4): 13-8. (in Russian)]. https://dx.doi.org/10.22625/2072-6732-2014-6-4-13-18.
- O'Connell C.M., Ferone M.E. Chlamydia trachomatis genital infections. Microb. Cell. 2016; 3(9): 390-403. https://dx.doi.org/10.15698/mic2016.09.525.
- Друккер Н.А., Линде В.А., Зенкина З.В., Авруцкая В.В., Некрасова М.Г. Участие провоспалительных цитокинов амниотической жидкости в регуляции уровня оксида азота при преждевременных родах. Российский вестник акушера-гинеколога. 2013; 13(6): 16-8. [Drukker N.A., Linde V.A., Zenkina Z.V., Avrutskaia V.V., Nekrasova M.G. Involvement of proinflammatory cytokines of amniotic fluid in the regulation of nitric oxide levels during preterm labor. Russian Bulletin of Obstetrician-Gynecologist. 2013;13(6):16 8. (in Russian)].
- Жетписбаев Г.А., Сагидуллина Л.С., Абдрахманова Г.Е. Хламидийная инфекция у новорожденных (обзор литературы). Вестник КазНМУ. 2014; 1: 113-6. [Zhetpysbaev G.A., Sagidullina L.S., Abdrakhmanova G.E. Chlamydial infection of nerwborns (Review of literature). Bulletin of KazNMU. 2014; 1: 113-6. (in Russian)].
- Зорина В.Н., Зорин Н.А. Белковые компоненты врожденного иммунитета в защите от патогенной инвазии. ЖМЭИ. 2013; 3: 111-7. [Zorina V.N., Zorin N.A. Protein components of innate immunity in protection from pathogenic invasion (Literature review). Journal of Microbiology (Moscow). 2013; 3: 111-7. (in Russian)].
- Michelsen T.M., Henriksen T., Reinnold D., Powell T.L., Jansson T. The human placental proteome secreted into the maternal and fetal cicenlation in normal pregnancy based on 4-vessel sampling. FASEB J. 2019; 33(2): 2944-56. https://dx.doi.org/10.1096/fj.201801193R.
- Morris R.K., Bilagi A., Devani P., Kilby M.D. Association of serum PAPP-A levels in first trimester with small for gestational age and adverse pregnancy outcome: systematic review ahd meta-analysis. Rev. Prenat. Diagn. 2017; 37(3): 253-6. https://dx.doi.org/10.1002/pd.5001.
- Зорина В.Н., Ботвиньева И.А., Ренге Л.В., Зорина Р.М., Чирикова Т.С., Зорин Н.А. Полифункциональные белки-иммуномодуляторы и цитокины у беременных женщин при антителоносительстве к Сhlamydia trachomatis. Иммунология. 2013; 34(6): 323-6. [Zorina V.A., Botvin’eva I.A., Renge L.V., Zorina R.M., Chirikova T.S., Zorin N.A. Polifunctional proteins with immunomodulatory functions at pregnancy with antibodies to Chlamydia trachomatis in circulation. Immunology. 2013; 34(6): 323-6. (in Russian)].
- Franke G.C., Böckenholt A., Sugai M., Rohde H., Aepfelbacher M. Epidemiology, variable genetic organization and regulation of the EDIN-B toxin in Staphylococcus aureus from bacteraemic patients. Microbiology. 2010;156(Pt 3): 860-72. https://dx.doi.org/10.1099/mic.0.030304-0.
Received 24.02.2022
Accepted 24.05.2022
About the Authors
Lyudmila V. Renge, Dr. Med. Sci., Associate Professor, Novokuznetsk State Institute for Advanced Medical Education – branch of the RMAPE, 7(3843)454-873,l.renge@mail.ru, https://orcid.org/0000-0002-7237-9721, 654005, Russia, Novokuznetsk, Stroiteley str., 5.
Veronika N. Zorina, Dr. Bio. Sci., Scientific Secretary, Leading Researcher at the Laboratory of Applied Toxicology and Pharmacology of the Department of Toxicology, Golikov Research Center of Toxicology, +7(921)315-37-50, v.n.zorina@hpb.spb.ru, https://orcid.org/0000-0001-9183-7663,
192019, Russia, Saint Petersburg, Bekhtereva str., 1.
Veronika S. Grebneva, Teaching Assistant at the Department of Obstetrics and Gynecology, Novokuznetsk State Institute for Advanced Medical Education –
branch of the RMAPE, +7(3843)454-873, veronika071988@yandex.ru, https://orcid.org/0000-0003-4546-0046, 654005, Russia, Novokuznetsk, Stroiteley str., 5.
Ekaterina Yu. Grigoryeva, PhD, Associate Professor at the Department of Obstetrics and Gynecology, Novokuznetsk State Institute for Advanced Medical Education –
branch of the RMAPE, +7(3843)454-873, prutovykh@icloud.com, https://orcid.org/0000-0002-8623-729Х, 654005, Russia, Novokuznetsk, Stroiteley str., 5.
Anna E. Vlasenko, PhD, Senior Lecturer at the Department of Medical Cybernetics and Informatics, Novokuznetsk State Institute for Advanced Medical Education –
branch of the RMAPE, +7(3843)454-873, vlasenkoanna@inbox.ru, https://orcid.org/0000-0001-6454-4216, 654005, Russia, Novokuznetsk, Stroiteley str., 5.
Authors' contributions: Renge L.V. – conception and design of the study, manuscript editing; Zorina V.N. – literature analysis, manuscript drafting; Grebneva V.S. – material collection, statistical analysis, manuscript drafting, literature collection and analysis; Grigoryeva E.Yu. – material collection, literature search and analysis; Vlasenko A.E. – medical statistics, mathematical methods of research results processing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The work was conducted within the framework of the planned PhD thesis of V.S. Grebneva, Teaching Assistant at the Department of Obstetrics and Gynecology, Novokuznetsk State Institute for Advanced Medical Education – branch of the RMAPE (state funding).
Ethical Approval: The study was approved by the Research Ethics Committee of the Novokuznetsk State Institute for Advanced Medical Education – branch of the RMAPE.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Renge L.V., Zorina V.N., Grebneva V.S., Grigoryeva E.Yu., Vlasenko A.E.
Markers of permeability and functional state of the placental barrier in pregnant women with chlamydial infection.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 6: 59-66 (in Russian)
https://dx.doi.org/10.18565/aig.2022.6.59-66



