The effect of the Russian combined vector vaccine against the novel coronavirus infection caused by SARS-CoV-2 on ovarian reserve and menstrual function in reproductive-aged women
Relevance: Despite the widespread use of COVID-19 vaccination worldwide, the number of studies on the impact of various types of vaccines on women’s reproductive health is limited in the scientific literature. The preliminary results of the study on the negative effect of vaccination with the Russian Gam-COVID-Vac vaccine on the ovarian reserve and the level of antiphospholipid antibodies in reproductive-aged women were first published in 2021.Dolgushina N.V., Dovgan A.A., Drapkina Yu.S., Ivanets T.Yu., Vtorushina V.V., Gus A.I., Sukhikh G.T.
Objective: To evaluate the effect of the Russian combined vector vaccine against the novel coronavirus infection caused by SARS-CoV-2 on the parameters of ovarian reserve and menstrual function in reproductive-aged women.
Materials and methods: A prospective interventional study included 220 women vaccinated with a combined vector vaccine Gam-COVID-Vac for the prevention of a novel coronavirus infection caused by SARS-CoV-2. The inclusion criteria were age from 18 to 45, preserved menstrual function, no history of COVID-19, negative PCR test result for SARS-CoV-2 and negative SARS-CoV-2 IgG antibody test before vaccination, no pregnancy, and no history of serious illnesses. The patients were examined twice: immediately before vaccination and 90 days after the first dose was injected. Antral follicle count was determined during the ultrasound examination of the pelvic organs. Serum levels of AMH, FSH, estradiol on the 2nd–5th day of the menstrual cycle, as well as IgG antibodies to SARS-CoV-2 were measured using enzyme immunoassay.
Results: The efficacy and safety of the Russian combined vector vaccine against COVID-19 was high. The humoral immune response (specific IgG to SARS-CoV-2) was detected in 98.6% of vaccinated patients. There were no cases of severe side effects after vaccination. There were no significant changes in the hormone levels, antral follicle counts and menstrual function before and after vaccination; women of advanced reproductive age (≥37 years) did not show considerable changes either.
Conclusion: The results of the study indicate that vaccination with a combined vector vaccine Gam-COVID-Vac against a novel coronavirus infection caused by SARS-CoV-2 is effective and safe; it does not have a negative effect on ovarian reserve and menstrual function in reproductive-aged women.
Keywords
Various strains of coronaviruses have been identified and studied for several decades, but none of them has resulted in such significant complications and such a high mortality rate as SARS-CoV-2, and none of them has caused a pandemic. Nowadays, one of the most effective ways of primary prevention of a new coronavirus infection is vaccination. The number of vaccinated people varies greatly in different countries, for example, it is 94.9% in the United Arab Emirates (UAE), and 2.7% in South Sudan. The vaccination rate in Russia was 49.0% as at March 03, 2022 (https://yandex.ru/covid19/stat).
COVID-19 vaccines which are currently used are classified into combined vector vaccines containing recombinant adenovirus particles with the SARS-CoV-2 protein gene, mRNA vaccines, single-component adjuvant peptide vaccines and whole-virus inactivated vaccines. The first registered COVID-19 vaccine in the world was the Gam-COVID-Vac vaccine (Sputnik V) was approved by the Ministry of Health of the Russian Federation on September 11, 2020 after the results of clinical trials, phases I and II. The safety of Gam-COVID-Vac was also confirmed by the interim results of phase III studies, and then by the results of monitoring the health of the vaccinated population in Russia and in other countries (from several thousand to several million people) [1].
In spite of the increased availability of COVID-19 vaccinations across the world, there is a limited amount of research in the scientific literature on the effect of various types of vaccines on human reproductive health [2–8]. Previous studies did not reveal a negative effect of the COVID-19 vaccine on reproductive health, and there was no relationship between vaccination, a decrease in anti-muller hormone (AMH) or menstrual cycle disorders either. It should be noted that there are the results of studies on animal models and data from clinical studies on the possible negative effect of adjuvant vaccines on reproductive function due to autoimmune genesis [9–14].
The preliminary results on the effect of the Gam-COVID-Vac vaccine on the female reproductive function have been published as part of this study. The prospective study included 51 women who were vaccinated against COVID-19 with the Gam-COVID-Vac vaccine (Sputnik V). The results of the study in this sample of patients did not show a negative effect of vaccination on the indicators of ovarian reserve [8].
The study of the effect of COVID-19 vaccination on reproductive health is an important issue that requires understanding, discussion and the development of adequate and useful practical recommendations. Therefore, it was decided primarily to analyze the effect of the Gam-COVID-Vac vaccine on hormonal profile indicators which reflect the state of the ovarian reserve and the antral follicle count (AFC).
Thus, the objective of the study was to evaluate the effect of the combined vector vaccine against the novel coronavirus infection caused by SARS-CoV-2 on the parameters of ovarian reserve and menstrual function in reproductive-aged women.
Materials and methods
The prospective interventional study included 250 women vaccinated with a combined vector vaccine Gam-COVID-Vac for the prevention of a novel coronavirus infection caused by SARS-CoV-2. Among 250 women, 30 did not come for the second examination (point 2). Thus, 220 (88%) women underwent a complete examination. All patients included in the study signed an informed consent for the participation. The criteria for inclusion in the study were age from 18 to 49 years, preserved menstrual function, a negative RT-PCR result in SARS-CoV-2 testing, and negative SARS-CoV-2 IgG and IgM results prior to vaccination. no previous history of COVID-19 disease, no contact with COVID-19 patients for at least 14 days before the beginning of the study. The criteria for non-inclusion were contraindications to vaccination according to the instructions for the medication, pregnancy and lactation, acute inflammatory and infectious diseases, rheumatic diseases, oncological diseases of any localization, hormonal therapy affecting the menstrual cycle, immunomodulatory therapy, vaccination within 3 months before inclusion in the study. The women with a significant decrease in ovarian reserve (FSH>12 IU/L and AFC<6 follicles in both ovaries), as well as patients with morbid obesity (body mass index (BMI) ≥40.0 kg/m2) were not eligible for inclusion in the study. The exclusion criteria were COVID-19 during the vaccination period, a severe undesirable reaction resulting in the cancelled 2nd dose of the vaccine, and the patient’s refusal to continue vaccination.
The patients were examined twice: before the vaccination and 90–100 days after the first dose was injected. Gynecologic examination was performed, blood samples were taken on the 2nd –5th day of the menstrual cycle. Antral follicle count was determined during the ultrasound examination of the pelvic organs. All patients had a blood test for the beta subunit of human chorionic gonadotropin (β-hCG) prior to the beginning of the study, the positive result corresponded to the level of β-hCG greater than 35 IU/L.
On the 2nd-5th day of the menstrual cycle, the level of follicle-stimulating hormone (FSH), AMH and estradiol (E2) in the blood serum was analyzed using an electrochemiluminescent method. Ovarian reserve in women was assessed using AMH, FSH and AFC parameters in blood serum. Ovarian reserve was considered normal when the level of AMH ≥ 1.2 ng/mL, the level of FSH <12 IU/L and AFC ≥ 5 follicles in both ovaries.
SARS-CoV-2 from nasopharyngeal swabs was detected using reverse-transcription polymerase chain reaction (RT-PCR) assay.
Before vaccination, IgG and IgM antibodies to SARS-CoV-2 were determined using an immunochromatographic method. The quantitative assessment of the level of IgG antibodies to SARS-CoV-2 was performed in 90–100 days after vaccination of the patients included in the study using enzyme immunoassay.
The patients were vaccinated in a specially equipped vaccination room. Before each stage of vaccination, all women included in the study were examined by a doctor; such parameters as thermometry, saturation, heart rate (HR) and blood pressure (BP) were measured; the doctors listened to the patient’s heart and lungs and also examined the pharynx. The use of the vaccine was prepared strictly in accordance with the official instructions for the medication.
The study was approved by the Local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
Statistical analysis
Statistical processing of the obtained data was carried out using Microsoft Excel tables and the statistical software package Statistica V10 (USA). Proportions (%) were calculated for the evaluation of qualitative data. The McNemar’s test was used to compare correlated binary data in one group of patients before and after vaccination. The quantitative data in the comparison groups were analyzed according to the type of data distribution (Kolmogorov–Smirnov test, graphical data analysis). Since the data were not distributed normally, methods of nonparametric statistics were used. Medians with an interquartile range (Me(Q25–Q75)), as well as a sign test for comparing related nonparametric data in correlated groups, were determined. The differences between the statistical values were considered statistically significant at р˂0.05.
Results
The average age of the patients included in the study was 33 years, and every third woman was of advanced reproductive age (≥37 years). The average BMI was 22.4 kg/m2. All the patients included in the study met the inclusion criteria, the prevalence of bad habits was low, the incidence of gynecological diseases did not exceed 10%, allergic diseases were the most common among patients’ diseases (Table 1).
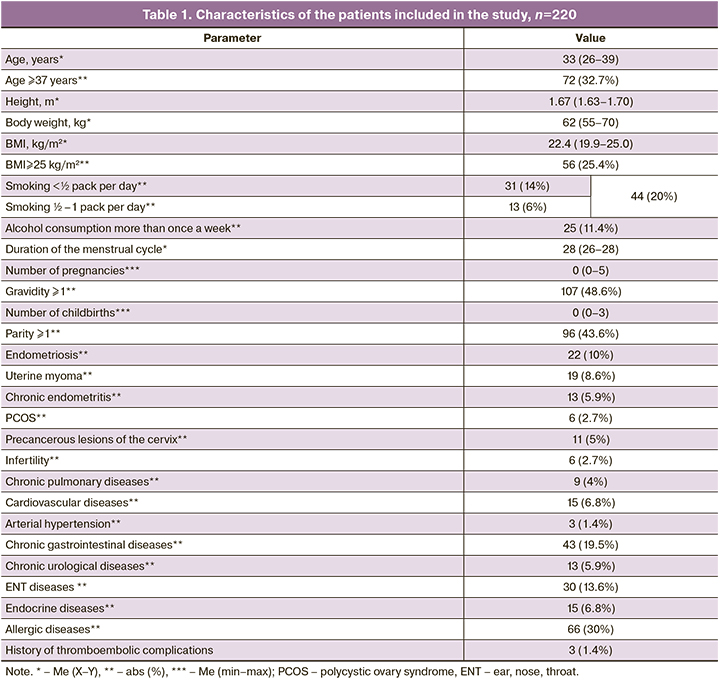
The vaccine was generally well tolerated by the patients, there were no serious adverse events associated with the injection of the vaccine. The reaction to the vaccine was more frequently observed after injecting the 2nd dose and was short-term (1–2 days).
SARS-CoV-2 IgG antibodies were detected in 98.6% of vaccinated patients.
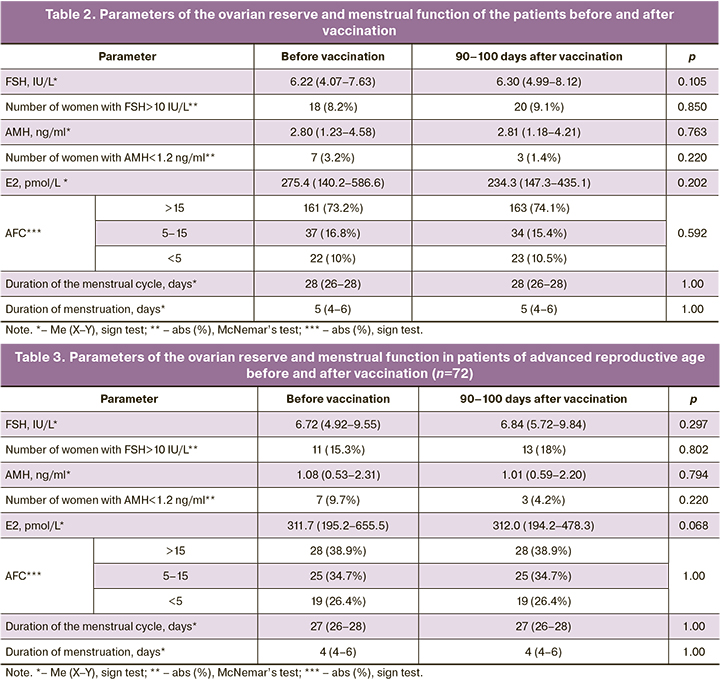
The ovarian reserve of the patients was analyzed twice: before vaccination and 90–100 days after the injection of the 1st dose of the vaccine (Table 2). The AFC value was converted to a categorical value, where 3 refers to CAF>15 in both ovaries (high ovarian reserve), 2 refers to CAF=5–15 in both ovaries (normal ovarian reserve), 1 refers to CAF<5 in both ovaries (poor ovarian reserve). The ovarian reserve was analyzed separately in patients of advanced reproductive age (≥37 years) and early reproductive age (<37 years) (Tables 3, 4).
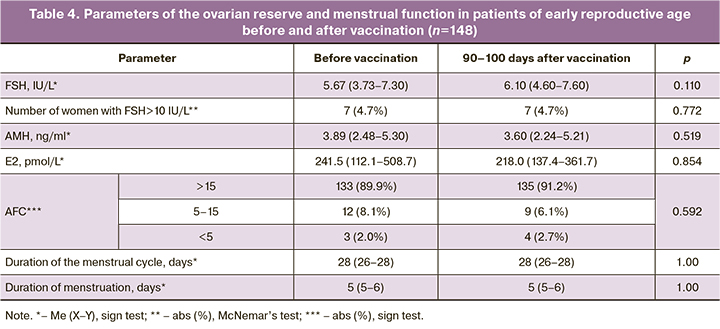
The patients did not show significant changes in hormonal parameters and AFC. The average values with an interquartile range of all the above hormones were within the reference values, and the number of patients with FSH level above reference values and AMH level below reference values did not differ in the groups of patients of early and advanced reproductive age before and after vaccination. A decrease in AMH level below reference values was noted in patients of advanced reproductive age more often before vaccination than after it.
A more detailed analysis of changes in the parameters of the ovarian reserve revealed that a decrease in AFC after vaccination was noted only in one patient (0.4%).
An increase in FSH level by more than 30% (a negative trend) was observed in 52/220 (23.6%) patients, among them there were 21/52 (9.5%) women of advanced reproductive age and 31/52 (14.1%) women of early reproductive age. A decrease in FSH level by more than 30% (a positive trend) was observed in 38/220 (17.3%) patients, among them there were 12/38 (5.4%) women of advanced reproductive age and 26/38 (11.9%) women of early reproductive age. A decrease in AMH level by more than 30% (a negative trend) was observed in 27/220 (12.3%) patients, among them there were 15/27 (6.8%) women of advanced reproductive age and 12/27 (5.5%) women of early reproductive age. An increase in AMH level by more than 30% (a positive trend) was observed in 35/220 (15.9%) patients, among them there were 20/35 (9.1%) women of advanced reproductive age and in 15/35 (6.8%) women of early reproductive age.
An increase in FSH level and a decrease in AMH level were observed simultaneously in 30/220 (13.6%) patients (a negative trend), but only 6/220 (2.7%) women showed a simultaneous increase in FSH level and a decrease in AMH level by more than 30%. All 6 patients were of advanced reproductive age. There was a simultaneous decrease in FSH level and an increase in AMH level (a positive trend) in 28/220 (12.7%) patients; a decrease in FSH level and an increase in AMH level by more than 30% was simultaneously observed in 9/220 (4.1%) patients. Among these 9 patients there were 6 (2.7%) patients of advanced reproductive age.
Thus, vaccination did not have a negative effect on the ovarian reserve of patients. There was an insignificant number of women in the vaccinated group whose hormonal parameters associated with the ovarian reserve deteriorated and improved in approximately equal proportions. A simultaneous negative change in FSH and AMH by more than 30% was noted in 2.7% of the patients of advanced reproductive age (6 women), while a simultaneous positive change in FSH and AMH by more than 30% was noted in the same proportion of women of the same age group. The average age of these women was 44 years which corresponds to the period of menopausal transition and the variability of hormonal function of the ovaries and pituitary gland. The study did not reveal any cases of menstrual cycle disorders in women after vaccination.
Discussion
As part of this study, an article was published in 2021 showing the absence of the effect of the Gam-COVID-Vac vaccine on the reproductive function of women, however, a study on a large sample of patients was required to confirm the preliminary obtained data [8]. To date, this study is the first scientific research confirming the absence of a negative effect of the Russian combined vector vaccine Gam-COVID-Vac on the ovarian function in reproductive-aged women.
It is necessary to know that COVID-19 can have a negative impact on the reproductive system of a woman. Thus, a number of studies have revealed a wide representation of SARS-CoV-2 receptors (angiotensin converting enzyme (ACE2), transmembrane serine protease 2 (TMPRSS2), CD147 (basigin) in human reproductive organs and tissues. The main cellular receptor for the SARS-CoV-2 S glycoprotein is ACE2, which is expressed mainly by type II pneumocytes, as well as cells of the endothelium, myocardium and intestinal mucosa [15]. It was also revealed that this receptor is present in the ovaries, uterus and placenta [16, 17]. According to a study by Ding T. et al., there was a decrease in the ovarian reserve (a decrease in AMH level) in patients who had COVID-19, especially in the group of women of advanced reproductive age [18]. Most likely, disorders in the female reproductive system that occur after infection with COVID-19 result from damage to the ovarian tissue, oocytes and endometrial cells by SARS-CoV-2. The above-described changes can lead to impaired ovulatory function, the production of infected or aneuploid oocytes with reduced fertility potential and impaired embryo implantation as well [19]. Given the potential negative impact of COVID-19 on ovarian reserve indicators, vaccination during the coronavirus pandemic once again confirms that it is appropriate and necessary.
Conclusion
This is the first study devoted to the analysis of the effect of the Russian combined vector vaccine Gam-COVID-Vac on the indicators of the ovarian reserve and menstrual function in women. The data obtained indicate that the vaccine does not have a negative effect on the ovarian reserve in women, including the patients of advanced reproductive age. Due to the absence of changes in the hormonal profile, AFC and parameters of the menstrual cycle after vaccination it is possible to conclude that there is no negative effect of Gam-COVID-Vac, a combined vector vaccine for the prevention of a new coronavirus infection caused by SARS-CoV-2, on the indicators of ovarian reserve and menstrual function in women.
References
- Logunov D.Y., Dolzhikova I., Shcheblyakov D., Tukhvatulin A.I., Zubkova O.V, Dzharullaeva A.S. et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021; 397(10275): 671-81. https://dx.doi.org/10.1016/S0140-6736(21)00234-8.
- Orvieto R., Noach-Hirsh M., Segev-Zahav A., Haas J., Nahum R., Aizer A. Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle? Reprod. Biol. Endocrinol. 2021; 19(1): 69. https://dx.doi.org/10.1186/s12958-021-00757-6.
- Bentov Y., Beharier O., Moav-Zafrir A., Kabessa M., Godin M., Greenfield C.S. et al. Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum. Reprod. 2021; 36(9): 2506-13. https://dx.doi.org/10.1093/humrep/deab182.
- Lifshitz D., Haas J., Lebovitz O., Raviv G., Orvieto R., Aizer A. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod. Biomed. Online. 2022; 44(1): 145-9. https://dx.doi.org/10.1016/j.rbmo.2021.09.021.
- Gonzalez D.C., Nassau D.E., Khodamoradi K., Ibrahim E., Blachman-Braun R., Ory J. et al. Sperm Parameters Before and After COVID-19 mRNA Vaccination. JAMA. 2021; 326(3): 273-4. https://dx.doi.org/10.1001/jama.2021.9976.
- Mohr-Sasson A., Haas J., Abuhasira S., Sivan M., Doitch Amdurski H., Dadon T. et al. The effect of Covid-19 mRNA vaccine on serum anti-Müllerian hormone levels. Hum. Reprod. 2022; 37(3): 534-41. https://dx.doi.org/10.1093/humrep/deab282.
- Драпкина Ю.С., Долгушина Н.В., Шатылко Т.В., Николаева М.А., Менжинская И.В., Иванец Т.Ю., Кречетова Л.В., Красный А.М., Гамидов С.И., Байрамова Г.Р., Сухих Г.Т. Вакцина Гам-КОВИД-Вак (Спутник V) не оказывает негативного влияния на сперматогенез у мужчин. Акушерство и гинекология. 2021; 7: 88-94. [Drapkina Yu.S., Dolgushina N.V., Shatylko T.V., Nikolaeva M.A., Menzhinskaya I.V., Ivanets T.Yu., Krechetova L.V., Krasnyi A.M., Gamidov S.I., Bairamova G.R., Sukhikh G.T. Gam-COVID-Vac (Sputnik V) vaccine has no adverse effect on spermatogenesis in men. Akusherstvo i Ginekologiya/ Obstetrics and Gynecology. 2021; 7: 88-94 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.7.88-94.
- Долгушина Н.В., Драпкина Ю.С., Кречетова Л.В., Иванец Т.Ю., Менжинская И.В., Гус А.И., Байрамова Г.Р., Сухих Г.Т. Вакцина Гам-КОВИД-Вак (Спутник V) не оказывает негативного влияния на овариальный резерв у женщин репродуктивного возраста. Акушерство и гинекология. 2021; 7: 81-6. [Dolgushina N.V., Drapkina Yu.S., Krechetova L.V., Ivanets T.Yu., Menzhinskaya I.V., Gus A.I., Bairamova G.R., Sukhikh G.T. Gam-COVID-Vac (Sputnik V) vaccine has no adverse effect on the ovarian reserve in reproductive-age women. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 7: 81-6 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.7.81-86.
- Guimarães L.E., Baker B., Perricone C., Shoenfeld Y. Vaccines, adjuvants and autoimmunity. Pharmacol. Res. 2015; 100(January): 190-209. https://dx.doi.org/10.1016/j.phrs.2015.08.003.
- Shoenfeld Y., Agmon-Levin N. “ASIA” – autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011; 36(1): 4-8. https://dx.doi.org/10.1016/j.jaut.2010.07.003.
- Watad A., Bragazzi N.L., McGonagle D., Adawi M., Bridgewood C., Damiani G. et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: Insights from an analysis of 500 cases. Clin. Immunol. 2019; 203(March): 1-8. https://dx.doi.org/10.1016/j.clim.2019.03.007.
- Carp H.J.A., Selmi C., Shoenfeld Y. The autoimmune bases of infertility and pregnancy loss. J Autoimmun. 2012; 38(2-3): J266-74. https://dx.doi.org/10.1016/j.jaut.2011.11.016.
- Cruz-Tapias P., Blank M., Anaya J.M., Shoenfeld Y. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr. Opin. Rheumatol. 2012; 24(4): 389-93. https://dx.doi.org/10.1097/BOR.0b013e32835448b8.
- Zivkovic I., Stojanovic M., Petrusic V., Inic-Kanada A., Dimitrijevic L. Induction of APS after TTd hyper-mmunization has a Different Outcome in BALB/c and C57BL/6 Mice. Am. J. Reprod. Immunol. 2011; 65(5): 492-502. https://dx.doi.org/10.1111/j.1600-0897.2010.00922.x.
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020; 46(4): 586-90. https://dx.doi.org/10.1007/s00134-020-05985-9.
- Vaz-Silva J., Carneiro M.M., Ferreira M.C., Pinheiro S.V.B., Silva D.A., Silva A.L. et al. The vasoactive peptide angiotensin-(1-7), its receptor mas and the angiotensin-converting enzyme type 2 are expressed in the human endometrium. Reprod. Sci. 2009; 16(3): 247-56. https://dx.doi.org/10.1177/1933719108327593.
- Valdés G., Neves L.A.A., Anton L., Corthorn J., Chacón C., Germain A.M. et al. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006; 27(2-3): 200-7. https://dx.doi.org/10.1016/j.placenta.2005.02.015.
- Ding T., Wang T., Zhang J., Cui P., Chen Z., Zhou S. et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: An observational study. Front. Med. 2021; 8: 635255. https://dx.doi.org/10.3389/fmed.2021.635255.
- Долгушин Г.О., Романов А.Ю. Влияние SARS-CoV-2 на репродукцию человека. Акушерство и гинекология. 2020; 11: 6-12. [Dolgushin G.O., Romanov A.Yu. Impact of SARS-CoV-2 on human reproduction. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 11: 6-12 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.6-12.
Received 16.03.2022
Accepted 26.03.2022
About the Authors
Nataliya V. Dolgushina, MD, PhD, Professor, Deputy Director – Chief of the Department for Scientific Projects Organization, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, n_dolgushina@oparina4.ru,https://orcid.org/0000-0003-1116-138X, 117997, Russia, Moscow, Academician Oparin str., 4.
Alina A. Dovgan, MD, Postgraduate Student, Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, +7(929)910-46-00, lina.dovgan@gmail.com,
https://orcid.org/0000-0002-4927-3590, 117997, Russia, Moscow, Academician Oparin str., 4.
Yuliya S. Drapkina, MD, PhD, Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, yu_drapkina@oparina4.ru, https://orcid.org/0000-0002-0545-1607,
117997, Russia, Moscow, Academician Oparin str., 4.
Tatyana Yu. Ivanets, MD, PhD, Head of the Clinical Diagnostic Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, t_ivanets@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Valentina V. Vtorushina, PhD, Doctor of Clinical Laboratory Diagnostics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, vtorushina@inbox.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Alexander I. Gus, MD, PhD, Professor, Chief Researcher at the Department of Ultrasound and Functional Diagnostics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, https://orcid.org/0000-0003-1377-3128,
117997, Russia, Moscow, Academician Oparin str., 4.
Gennady T. Sukhikh, MD, PhD, Academician of the Russian Academy of Sciences, Professor, Director of the Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation, g_sukhikh@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Authors’ contributions: Dolgushina N.V. – collection and analysis of the literature data, writing the article, statistical data processing; Dovgan A.A., Drapkina Yu.S. – writing the article, management of patients taking part in the study; Ivanets T.Yu. – measuring the level of AMH, FSH and estradiol in the patients included in the study; Vtorushina V.V. – assessment of the level of IgG and IgM antibodies to SARS-CoV-2; Gus A.I. – performing ultrasound examination, measuring antral follicle count; Sukhikh G.T. – editing and approval of the publication.
Conflicts of interest: The authors declare that there are no conflicts of interests.
Funding: The study was supported by the Charitable Foundation ‘Contribution to the Future’ within the framework of the special program ‘Let’s Stop the Coronavirus Together’.
Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Dolgushina N.V., Dovgan A.A., Drapkina Yu.S., Ivanets T.Yu.,
Vtorushina V.V., Gus A.I., Sukhikh G.T. The effect of the Russian combined vector vaccine against the novel coronavirus infection caused by SARS-CoV-2 on ovarian reserve and
menstrual function in reproductive-aged women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 4: 115-122 (in Russian)
https://dx.doi.org/10.18565/aig.2022.4.115-122



