Ovarian age – an early marker of premature ovarian insufficiency
Mashaeva R.I., Marchenko L.A., Gus A.I., Kostyukov K.V.
Objective: To assess the degree of ovarian aging in patients with occult, biochemical, and overt ovarian insufficiency (POI) using the composite marker "ovarian age" (OvAge), calculated using the regression model proposed by Venturella R. et al. (2015).
Materials and methods: This cross-sectional study included patients with various clinical forms of POI (n=82) and women with preserved ovarian function (n=36) aged 18–39 years (mean age 33.1 (5.59) years). Follicle-stimulating hormone (FSH) and anti-Müllerian hormone (AMH) levels were measured on days 2–3 of the menstrual cycle, antral follicle count (AFC) was determined, and Doppler ultrasound of intraovarian blood flow was performed to calculate the vascularization index (VI) and blood flow index (FI).
Results: The OvAge was significantly higher in the POI group than in the control group. An additional marker, "excess chronological age", was calculated to represent the difference between the ovarian and chronological age of the patient. This excess was 1.25 (0.71) years in the control group, 6.63 (1.39) years in the latent POI group, 12.6 (0.98) years in the early POI group, and 18.91 (1.32) years in the overt POI group. The excess ovarian age over chronological age increases on average by six years as individuals transition from the group of healthy women to each subsequent POI group.
Conclusion: A gradual increase in ovarian age during the transition from latent to early and then to overt POI indicates progressive morphofunctional failure of the ovaries during disease development. The degree of excess ovarian age over chronological age allows for an assessment of the severity of changes in the primary POI markers measured in patients, providing a clearer reflection of the process of ovarian "aging" at different stages of the disease.
Authors' contributions: Mashaeva R.I. – review of relevant literature, structuring, collection of materials, acquisition and analysis of data, statistical analysis, drafting of the manuscript; Marchenko L.A. – conception and design of the study, structuring and editing of the manuscript; Gus A.I., Kostyukov K.V. – collection of materials, acquisition and analysis of data.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Mashaeva R.I., Marchenko L.A., Gus A.I., Kostyukov K.V.
Ovarian age – an early marker of premature ovarian insufficiency.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (3): 120-127 (in Russian)
https://dx.doi.org/10.18565/aig.2024.271
Keywords
Unlike other organs, the biological functions of ovaries are confined to the reproductive period [1]. The onset of menopause typically occurs between the ages of 50 and 52 years; however, in 3.7% of women, the depletion of the follicular pool occurs before the age of 40 years, resulting in menstrual and reproductive disorders such as secondary amenorrhea and infertility [2–4]. The term "ovarian reserve," introduced over 35 years ago by Daniel Navotas [5], remains widely used today and has been cited in more than 5,556 articles. Modern perspectives suggest that the accelerated decline in ovarian reserve, which underlies premature ovarian insufficiency (POI), is characterized by a latent disease course (occult POI) [6, 7]. This condition features a regular menstrual cycle along with anti-Müllerian hormone (AMH) levels below 1.2 ng/mL and follicle-stimulating hormone (FSH) levels of up to 12 mIU/mL. In biochemical POI, patients experience periodic menstrual delays, with FSH levels rising above 12 mIU/mL and AMH levels continuing to decrease (below 1.2 ng/mL) [8]. According to ESHRE criteria, the overt POI is marked by significant clinical signs (secondary oligo/amenorrhea) and pronounced hormonal changes (FSH>25 mIU/mL). In contrast, the IMS recommendations set this indicator at a minimum of 40 mIU/mL. However, the precise threshold levels that define complete ovarian function shutdown remain undetermined [9, 10]. Additionally, FSH and AMH levels are significantly influenced by the age [11].
These observations provide the foundation for developing an accurate diagnostic model to assess ovarian reserve, while accounting for the chronological age of patients. Venturella R. et al. (2015) proposed a method involving a regression equation to estimate a woman's ovarian age (OvAge) based on markers of ovarian reserve. This model was derived from the examination of 645 healthy, fertile women with a regular menstrual rhythm and was based on five indicators of ovarian reserve: antral follicle count (AFC), AMH, FSH, and ultrasound parameters of intraovarian blood flow, including the vascularization index (VI) and blood flow velocity (FI) [12].
Ovarian age can be calculated using the following formula: OvAge = 48.05 - 3.14×AMH + 0.07×FSH ─ 0.77×AFC ─ 0.11×FI + 0.25×VI + 0.1×AMH×AFC + 0.02×FSH×AFC.
The findings of this study indicated that the ovarian age of the 645 healthy, fertile women corresponded significantly to the patients' chronological age for each marker included in the equation (p<0.001). The minimum and 10-fold cross-validated accuracies of this model were 79.865% and 79.832%, respectively.
If the age calculated from the morphological and functional characteristics of the ovaries exceeded the biological age of the woman by several years, this indicated that the morphology and functional state of the gonads corresponded to an older age, suggesting accelerated gonadal aging [12].
Based on the above, this study aimed to assess the degree of ovarian aging in patients with occult, biochemical, and overt POI using the composite marker "ovarian age" (OvAge), calculated using the regression model proposed by Venturella R. et al. (2015).
Materials and methods
A total of 118 women participated in this observational, cross-sectional study. The study group consisted of 82 women with a verified diagnosis of POI who presented with complaints of infertility of unclear origin, secondary oligomenorrhea, amenorrhea, slight variations in the menstrual cycle within its regularity, periodic abnormal uterine bleeding without endometrial pathology, and a history of recurrent functional ovarian cysts. The AMH levels in the study group were less than 1.2 ng/mL, which, according to the Bologna criteria, indicated a decrease in ovarian reserve [13].
To assess ovarian age as the clinical presentation of POI progresses, 82 patients were divided into three clinical groups based on FSH levels, which reflect the stage of disease development.
Occult POI: The FSH level in this group of patients was less than 12 mIU/ml [7.05; 10.65], and the AMH level was 0.65 ng/ml [0.3; 0.99].
Biochemical POI: The FSH level in this group was between 12 mIU/ml and 19.95 mIU/ml [16.6; 26], while the AMH level was 0.14 ng/ml [0.12; 0.3].
Overt POI: This group included 38 patients with secondary amenorrhea lasting six months or more. The FSH level in these patients was greater than 25 mIU/ml according to the ESHRE criteria [12].
The comparison group consisted of 36 women with regular menstrual cycles, showing no characteristic clinical signs of the study group. These women sought preventive examinations the Center and had FSH levels below 10 mIU/ml and AMH levels above 1.2 ng/ml.
The exclusion criteria included the presence of primary hyper-, normo-, or hypogonadotropic amenorrhea; severe hereditary diseases (such as galactosemia and blepharophimosis); iatrogenic causes of hypergonadotropic amenorrhea (including ovarian surgery and chemo/radiotherapy); oncological diseases; a history of thrombosis; impaired liver and kidney function; and hyperprolactinemia. To verify the various clinical forms of POI, the functional state of the hypothalamic-pituitary-ovarian system was assessed using hormonal testing and 2D/3D ultrasound studies.
Hormonal testing involved measuring FSH and AMH levels, which are traditionally considered markers of decreased ovarian reserve. FSH levels in blood serum were determined using the immunochemiluminescent method on automated analyzers (Immulite 2000, Immulite 1000; Siemens, USA) with reagents from the same companies. The AMH concentration was measured using an enzyme immunoassay or radioimmunoassay with appropriate test systems on a Cobar Core II automatic analyzer. Blood samples were collected on days 2–3 of the menstrual cycle or on any other day in cases of secondary amenorrhea. The morphofunctional state of the ovaries was evaluated based on echograms obtained during 2D scanning and 3D reconstruction using the VOLUSON-E8 device (GE Kretz, Zipf, Austria) and a transvaginal sensor (3.3–10.0 MHz) on days 4–5 of the menstrual cycle. The ovarian volume was determined, antral follicle count (AFC) was calculated, and intraovarian blood flow parameters, including vascularization indices (VI) and blood flow indices (FI), were measured.
3D ultrasound examinations were performed for all 118 women included in the study.
To calculate ovarian age based on the examination data of the 82 patients with different clinical forms of POI and the 36 women in the comparison group, we used the formula provided by Venturella et al. [12].
Statistical analysis
Statistical analysis was conducted using STATISTICA v.7, MedCalc v.22, and MS Excel. The Shapiro-Wilk test was used to assess the normality of continuous variable distributions. For normally distributed variables, the mean and standard deviation were reported in M (SD) format; otherwise, the median and interquartile range (Me [Q1; Q3]) were used. The Kruskal–Wallis test was employed to compare four independent groups for continuous variables, followed by pairwise comparisons using the Mann–Whitney U test. ROC analysis was used to evaluate the overall diagnostic accuracy of the test with continuous variables and to select the cutoff point, which was established based on the highest value of the Youden index, ensuring the best sensitivity and specificity ratio. The significance level for testing the statistical hypotheses was set at p<0.05. To correct for type I errors in multiple pairwise comparisons, Bonferroni correction was applied.
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; all patients provided informed consent to participate in the study.
Results
The results of all five parameters involved in the calculation of ovarian age, including FSH and AMH levels, AFC, vascularization, and intraovarian blood flow characteristics, are shown in Table 1.
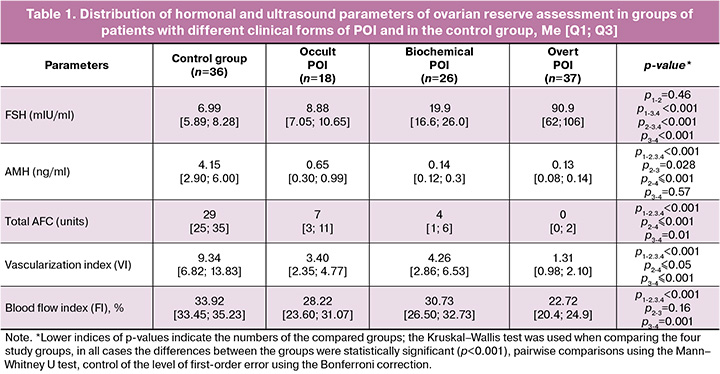
The results presented in Table 1 illustrate the monotonic change in all parameters accompanying the gradual development of POI, with a statistically significant decrease in AMH, AFC, and intraovarian blood flow characteristics in parallel with a monotonic increase in FSH levels.
In our study, we calculated the ovarian age in three groups of patients with different forms of POI as well as in the control group, based on the use of five key markers of the morphofunctional state of the ovarian reserve.
Note that since POI can occur at any age from 18 to 40 years and has different forms, including latent, it is not the ovarian age itself that is important for assessing the degree of ovarian aging, but the number of years it is ahead of the patient's chronological age. We calculated a new parameter ‘excess ovarian age’ over chronological age, obtained as the difference between the calculated ovarian age and the real (chronological) age of the patient. The results of calculating the indicators ‘ovarian age, chronological age’, and ‘excess ovarian age’ in each of the three groups of patients with consecutive forms of POI and the study group are shown in Table 2.
The results in Table 2 show that ovarian age increases monotonically and statistically significantly when moving from the group of healthy patients to each of the groups with different forms of POI and is 28.97 (0.43) years in healthy women and further 39.40 (1.22), 45.64 (0.28) and 52.3 (0.77) years in the groups of patients with occult, biochemical and overt POI, respectively.
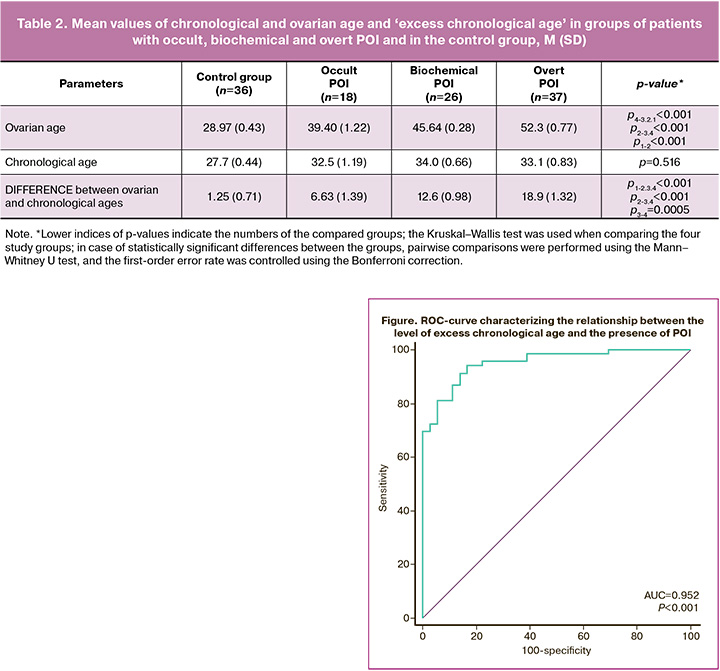
The excess of ovarian age over chronological age was 1.25 (0.71), 6.63 (1.39), 12.6 (0.98) and 18.9 (1.32) years in the comparison group and in the groups of patients with occult, biochemical and overt POI, respectively.
The monotonic and statistically significant increase in the difference between the ovarian age and chronological age of the patients in the compared groups indicated an increase in the morphofunctional failure of the ovaries during the gradual development of POI (from occult through biochemical to the overt POI).
In order to assess the overall diagnostic accuracy of the quantitative trait test and to select the cut-off point, ROC analysis was performed (Figure).
The area under the ROC curve was 0.952 (0.019), with a 95% CI 0.893–0.984. The threshold for exceeding chronological age at the cutoff point corresponding to the optimal ratio of sensitivity and specificity scores was ≥4.98 years. In the course of our study, it was determined that exceeding the ovarian age by 5 years can be considered as the value separating normal from POI, including the earliest ‘hidden’ form with a sensitivity of 92.7% (95% CI 83.9–97.6%) and specificity of 83.3% (95% CI 67.2–93.6%), indicating high diagnostic accuracy of the index.
Comparison of groups of patients with different sequential forms of POI showed that, on average, the ‘excess chronological age’ increases by 6 years when moving from occult to biochemical and from biochemical to overt POI.
Discussion
Over the last two decades, large-scale studies have been conducted worldwide to develop comprehensive methods that can be used to determine the biological age of a person or his or her organs and systems. These methods are based on the analysis of DNA methylation and allow estimation of how fast the organism ages at the cellular level [14]. Currently, several variants of techniques have been developed to estimate the biological age of a person compared to their chronological parameters. Each of these epigenetic clock variants is unique in their approach and scope of application. The Horvath's clock is the most widely used method for estimating an individual's biological age. PhenoAge is best used to predict the risk of chronic diseases and mortality accurately. Mammalian epigenetic clocks are valuable for studying general evolutionary mechanisms of aging, and clocks based on stochastic data (random, unpredictable events) provide a deeper understanding of random factors affecting aging. At present, these models are not widely used in gynecological practice because it is impossible to perform a functional assessment of the ovarian reserve and predict the age of menopause onset based on their use [14]. In the context of the present study, the work Xu W et al., published in PLoS One, is of interest, devoted to the development of an online calculator OvAge for predicting ovarian age based on an adapted model Venturella R. et al. [15]. The authors performed a comprehensive analysis comparing different regression approaches, including normal linear regression and generalized linear models (GLM) with logarithmic and identity relationships. Independent variables, such as body mass index, estradiol, prolactin (PRL), luteinizing hormone, FSH, and AMH, were used to construct the optimal model, with age acting as the dependent variable.
The key stage of the study was the step-by-step selection of predictors based on the Akaike Information Criterion (AIC) criterion, which allowed the authors to create three models (mod1a, mod2a, and mod3a) that take into account different data distributions. The most effective formula obtained using the GLM was OvAge=exp(3.5254−0.0001× PRL−0.0231×AMH).
The application of this model to Chinese population data demonstrated a high accuracy in predicting ovarian age in healthy women. However, for patients with polycystic ovary syndrome and reduced ovarian reserve, the results were less accurate, which is likely due to the peculiarities of the hormonal profile and heterogeneity of these groups. Since the first half of the 21st century, machine learning has become a real catalyst in the development of new formulas for assessing biological systems and, more recently, ovarian age, adding accuracy and innovation to this important area of medicine. Ding et al. [16] proposed an innovative approach to assess ovarian reserve using machine learning methods with the help of AMH, inhibin B, body mass index, FSH, estradiol, luteinizing hormone, and AFC markers. According to the results of the study, the best machine learning model was LightGBM (a gradient boosting algorithm developed to improve the speed and efficiency of machine learning models), which showed high accuracy in predicting ovarian age, especially in women aged 20 to 35 years. This model demonstrated the lowest average absolute error in women aged 20–35 years with a preserved reproductive function. However, it has not been validated in patients with POI or PCOS. In addition, the model does not allow us to estimate a critically important parameter, "excess ovarian age" (the difference between ovarian and chronological age), which quantitatively reflects the degree of "aging" of the ovaries. In contrast to the two models presented above, the formula of Venturella R. et al. integrates both hormonal markers (AMH, FSH) and modern sonographic indices, which provides a comprehensive assessment of the morphofunctional state of the ovaries (AFC, vascularization index VI, blood flow index FI), which are directly associated with the concepts of "reduced" or "poor" ovarian reserve.
Currently, we can only assess the functional ovarian reserve, which reflects the ability of ovaries to respond to stimulation and produce eggs [11]. Total ovarian reserve, which represents the total number of oocytes remaining in the ovaries, cannot be accurately measured by clinical methods since there is currently no direct way to quantify all immature eggs.
In the study by Venturella R. et al., not only was a formula developed to determine the ovarian age in women with functionally active ovaries, but a group of patients with the full form of POI (45 women) was also tested. The obtained results showed that for this category of patients, the ovarian age corresponds to 50.63 (3.80) years with an average chronological age of 37.90 (3.31) years [12]. In our study, the average OvAge value in the group of patients with the overt POI was 52.3 (0.77) years with an average chronological age of women in this group of 33.1 (0.83) years (p<0.001). Thus, our results were in the same age range as the data reported by Italian scientists.
To the best of our knowledge, this is the first study to use the "ovarian age" parameter to assess the degree of decrease in ovarian reserves during the development of POI (from occult to overt form). The analysis showed that ovarian age increases with POI progression: in the occult form – 39.40 (1.22) years, in the biochemical form – 45.64 (0.28) years, and in the overt form – 52.3 (0.77) years, which confirms the correlation between POI progression and ovarian age increase. It is particularly significant that our study calculated a new parameter, "excess ovarian age" over chronological age (the difference between ovarian and chronological age), which allows us to quantitatively assess the degree of ovarian "aging" at successive stages of disease development. The average value of the "excess ovarian age" parameter in the group of patients with the occult POI is statistically significantly higher than in the comparison group (6.63 (1.39) years in the occult form vs. 1.25 (0.71) years in the comparison group). In our study, the "excess ovarian age" was 6.63 (1.39), 12.6 (0.98) and 18.9 (1.32) years for occult, biochemical and overt POI, respectively. We have proven that exceeding the chronological age by five years can be considered a threshold value, that is, a value separating the norm from POI, including the earliest occult form. Using this threshold value, the sensitivity of POI diagnostics was 92.7% (95% CI 83.9–97.6%) and specificity was 83.3% (67.2–93.6%), which indicates high diagnostic accuracy of the indicator. The value of the “excess ovarian age” parameter increases on average by six years with a sequential transition from the occult, biochemical to the overt POI. Thus, the “excess ovarian age” parameter can be considered an additional early diagnostic marker of POI.
A number of studies have shown that the “ovarian age” parameter can be used not only to predict POI but also to analyze various clinical situations [17, 18].
The ovarian age assessment (OvAge) technique was employed to confirm the minimal impact of bilateral salpingectomy on the ovarian reserve in women who underwent hysterectomy. The results indicated that bilateral salpingectomy did not significantly affect ovarian age when examining women from 3 months to 5 years post-surgery, establishing it as an acceptable preventive measure to reduce the risk of ovarian cancer without substantially worsening ovarian reserve or increasing the risk of early menopause [19, 20]. This method has gained widespread acceptance and validated the feasibility and safety of the new paradigm for ovarian cancer prevention outlined in the 2015 ACOG recommendations [21]. Currently, OvAge should be utilized as a diagnostic tool for assessing functional ovarian reserve, facilitating the inclusion of patients in in vitro fertilization programs, and enabling the individualized selection of superovulation stimulation regimens. Such an interpretation of test results may enhance our understanding of both patients and doctors, clarifying the necessity for timely adjustments to reproductive plans. Furthermore, it may provide a rationale for long-term hormone therapy to prevent cardiovascular and cognitive diseases and mitigate the reduction in mineral density of skeletal muscles and bones [22, 23]. According to the ESHRE recommendations, long-term hormone replacement therapy is recommended for patients with POI until the age of natural ovarian function cessation (50–52 years) [17].
The limitations of the study include the small sample size, which may restrict the generalizability of the results, as well as the inability to assess the long-term consequences of the findings. Although 3D ultrasound technologies are informative, they are expensive and less accessible in general practice, limiting patient participation in studies.
Conclusion
To assess the degree of ovarian "aging" at various stages, it is not the "ovarian age" itself, which is crucial, but rather how many years it exceeds the patient's chronological age. We proposed the "excess ovarian age" parameter, defined as the difference between ovarian age and chronological age, which demonstrated a sensitivity of 92.7% (95% CI 83.9–97.6%) and a specificity of 83.3% (95% CI 67.2–93.6%). This parameter characterizes a decline in the morphofunctional state of the ovarian reserve in overt POI and, importantly, in the early stages of the disease. The "excess ovarian age" parameter increases by an average of 6 years during the transition from the occult form to the biochemical and from the biochemical to the overt POI, allowing us to consider this measure as a marker for POI in general and its individual forms. The "excess ovarian age" parameter that we developed provides a clearer reflection of the ovarian "aging" process.
References
- Polonio A.M., Chico-Sordo L., Córdova-Oriz I., Medrano M., García-Velasco J.A., Varela E. Impact of ovarian aging in reproduction: from telomeres and mice models to ovarian rejuvenation. Yale J. Biol. Med. 2020; 93(4): 561-9.
- Mishra G.D., Chung H.F., Cano A., Chedraui P., Goulis D.G., Lopes P. et al. EMAS position statement: Predictors of premature and early natural menopause. Maturitas. 2019; 123: 82-8. https://dx.doi.org/10.1016/j.maturitas.2019.03.008.
- Golezar S., Ramezani Tehrani F., Khazaei S., Ebadi A., Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019; 22(4): 403-11. https://dx.doi.org/10.1080/13697137.2019.1574738.
- Чернуха Г.Е., Табеева Г.И., Рштуни С.Д., Машаева Р.И., Черных В.Б., Марченко Л.А. Гены, вовлеченные в развитие преждевременной недостаточности яичников. Акушерство и гинекология. 2021; 11: 71-80. [Chernukha G.E., Tabeeva G.I., Rshtuni S.D., Mashaeva R.I., Chernykh V.B., Marchenko L.A. Genes involved in premature ovarian failure. Obstetrics and Gynecology. 2021; (11): 71-80 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.71-80.
- Navot D., Rosenwaks Z., Margalioth E.J. Prognostic assessment of female fecundity. Lancet. 1987; 2(8560): 645-7. https://dx.doi.org/10.1016/s0140-6736(87)92439-1.
- Марченко Л.А., Машаева Р.И. Клинико-лабораторные критерии оккультной формы преждевременной недостаточности яичников. Гинекология. 2018; 20(6): 73-6. [Marchenko L.A., Mashaeva R.I. Clinical and laboratory criteria for occult form of premature ovarian failure. Gynecology. 2018; 20(6): 73-6. (in Russian)]. https://dx.doi.org/10.26442/20795696.2018.6.180069.
- Cohen J., Chabbert-Buffet N., Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder--a plea for universal definitions. J. Assist. Reprod. Genet. 2015; 32(12): 1709-12. https://dx.doi.org/10.1007/s10815-015-0595-y.
- Машаева Р.И., Марченко Л.А., Олимпиева С.П., Гус А.И., Костюков К.В., Чернуха Г.Е. Анализ морфофункционального состояния яичников при различных клинических формах преждевременной недостаточности яичников с использованием энергетической допплерометрии в режиме 2D/3D. Акушерство и гинекология. 2020; 12: 129-36. [Mashaeva R.I., Marchenko L.A., Olympieva S.P., Gus A.I., Kostyukov K.V., Chernukha G.E. Morphofunctional characteristics of ovaries determined by 2D and 3D power Doppler sonography in different clinical forms of premature ovarian failure. Obstetrics and Gynecology. 2020; (12): 129-36 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.129-136.
- Yeganeh L., Boyle J.A., Wood A., Teede H., Vincent A.J. Menopause guideline appraisal and algorithm development for premature ovarian insufficiency. Maturitas. 2019; 130: 21-31. https://dx.doi.org/10.1016/j.maturitas.2019.09.009.
- Baber R.J., Panay N., Fenton A.; IMS Writing Group. 2016 IMS Recommendations on women's midlife health and menopause hormone therapy. Climacteric. 2016; 19(2): 109-50. https://dx.doi.org/10.3109/13697137.2015.1129166.
- Марченко Л.А., Машаева Р.И. Клинико-лабораторная оценка овариального резерва с позиции репродуктолога. Акушерство и гинекология. 2018; 8: 22-5. [Marchenko L.A., Mashaeva R.I. Clinical and laboratory assessment of ovarian reserve from a reproductologist’s point of view. Obstetrics and Gynecology. 2018; (8): 22-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.22-25.
- Venturella R., Lico D., Sarica A., Falbo M.P., Gulletta E., Morelli M. еt al. OvAge: a new methodology to quantify ovarian reserve combining clinical, biochemical and 3D-ultrasonographic parameters. J. Ovarian Res. 2015; 8: 21. https://dx.doi.org/10.1186/s13048-015-0149-z.
- Mashayekhy M., Barabi F., Arabipoor A., Zolfaghari Z. Live birth rates in different subgroups of poor ovarian responders according to Bologna and POSEIDON group classification criteria. J. Gynecol. Obstet. Hum. Reprod. 2021; 50(7): 102169. https://dx.doi.org/10.1016/j.jogoh.2021.102169.
- Meyer D.H., Schumacher B. Aging clocks based on accumulating stochastic variation. Nat. Aging. 2024; 4(6): 871-85. https://dx.doi.org/10.1038/s43587-024-00619-x.
- Xu W., Wang H., Han L., Zhao X., Chen P., Zhao H. et al. Development, promotion, and application of online OvAge calculator based on the WeChat applet: Clinical prediction model research. PLoS One. 2023; 18(2): e0279633. https://dx.doi.org/10.1371/journal.pone.0279633.
- Ding T., Ren W., Wang T., Han Y., Ma W., Wang M. et al. Assessment and quantification of ovarian reserve on the basis of machine learning models. Front Endocrinol (Lausanne). 2023; 14: 1087429. https://dx.doi.org/10.3389/fendo.2023.1087429.
- Panay N., Anderson R.A., Bennie A., Cedars M., Davies M., Ee C. et al.; ESHRE, ASRM, CREWHIRL, and IMS Guideline Group on POI. Evidence-based guideline: premature ovarian insufficiency. Hum. Reprod. Open. 2024; 2024(4): hoae065. https://dx.doi.org/10.1093/hropen/hoae065.
- Younis J.S., Ben-Ami M., Ben-Shlomo I. The Bologna criteria for poor ovarian response: a contemporary critical appraisal. J. Ovarian Res. 2015; 8: 76. https://dx.doi.org/10.1186/s13048-015-0204-9.
- Venturella R., Lico D., Borelli M., Imbrogno M.G., Cevenini G., Zupi E. et al. 3 to 5 years later: long-term effects of prophylactic bilateral salpingectomy on ovarian function. J. Minim. Invasive Gynecol. 2017; 24(1): 145-50. https://dx.doi.org/10.1016/j.jmig.2016.08.833.
- Nassif A., Elnory M.A. Impact of prophylactic bilateral salpingectomy on ovarian reserve in women undergoing vaginal hysterectomy: A randomized controlled trial. Evidence Based Women’s Health Journal. 2020; 10(2): 150-61. https://dx.doi.org/10.21608/ebwhj.2020.22949.1074.
- ACOG Committee Opinion No. 774: Opportunistic salpingectomy as a strategy for epithelial ovarian cancer prevention. Obstet. Gynecol. 2019; 133(4): e279-e284. https://dx.doi.org/10.1097/AOG.0000000000003164.
- Pinelli S., Artini P.G., Basile S., Obino M.E.R., Sergiampietri C., Giannarelli D. et al. Estrogen treatment in infertile women with premature ovarian insufficiency in transitional phase: a retrospective analysis. J. Assist. Reprod. Genet. 2018; 35(3): 475-82. https://dx.doi.org/10.1007/s10815-017-1096-y.
- Dragojevic Dikic S., Vasiljevic M., Jurisic A., Vujovic S. Resumption of ovarian function and successful pregnancy in a patient with premature ovarian insufficiency after a long-term hormone replacement therapy. Gynecol. Reprod. Endocrinol. Metab. 2020; 1(4): 223-7.
Received 29.10.2024
Accepted 12.02.2025
About the Authors
Roza I. Mashaeva, PhD student, Department of Endocrinological Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,117997, Russia, Moscow, Ac. Oparina str., 4, +7(909)933-64-76, i@mdrose.ru, SPIN: 6780-3831, https://orcid.org/0000-0001-5518-1572
Larisa A. Marchenko, Dr. Med. Sci., Professor, Department of Endocrinological Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)438-85-40, l_marchenko@yandex.ru
Aleksandr I. Gus, Dr. Med. Sci., Professor, Chief Researcher at the Department of Ultrasound and Functional Diagnostics, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)438-11-77, a_gus@oparina4.ru, https://orcid.org//0000-0003-1377-3128
Kirill V. Kostyukov, Dr. Med. Sci, Head of the Department of the Ultrasound and Functional Diagnosis, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-11-77, k_kostyukov@oparina4.ru, https://orcid.org/0000-0003-3094-4013



