Morphofunctional characteristics of ovaries determined by 2D and 3D power Doppler sonography in different clinical forms of premature ovarian failure
Aim. To investigate the hormonal profile and morphofunctional status of the ovaries in women with different clinical forms of premature ovarian failure (POF) and evaluate diagnostic value of new ovarian reserve (OR) markers using 2D and 3D power Doppler sonography.Mashaeva R.I., Marchenko L.A., Olympieva S.P., Gus A.I., Kostyukov K.V., Chernukha G.E.
Materials and methods. Baseline evaluation included medical history and clinical assessment. Laboratory testing consisted of measurements of FSH and AMG levels by ELISA. 3D pelvic ultrasound was used to measure antral follicle count (AFC) and intraovarian blood flow, including calculation of vascularization index (VI) and blood flow index (FI).
Results. Patients with the initial (biochemical) form of POF had statistically significantly higher FSH and lowered AMH levels than patients with the occult and complete POF forms. Patients with different forms of POF had statistically significantly lower values of all three 3D ultrasound parameters (VI, FI, and AFC) than control subjects. There was a sharp decrease in AFC, VI, and FI in patients with occult POF, suggesting that these characteristics may be promising for early diagnosis of the disease.
Conclusion. A comprehensive morphofunctional assessment of ovaries, based on hormonal indicators (AMH and FSH) and ovarian reserve parameters (ovarian volume, AFC, and vascularization and blood flow indexes), determined by three-dimensional ultrasound and Doppler studies may provide a more accurate diagnosis of early forms of POF and, first of all, its occult form.
Keywords
Premature ovarian failure (POF) occurs in 3.7% of the female population under 40, characterized by a diminished ovarian reserve (OR). Ultimately, it progresses to secondary hypergonadotropic amenorrhea and persistent anovulatory infertility similar to physiological menopause, which, according to EMAS, occurs between 50 and 52 years [1, 2].
Since POF is accompanied by the adverse pleiotropic effects of sex hormone deficiency, it is imperative to diagnose and start treating this disease at an early stage [3].
Staged depletion of the ovarian reserve (OR) was first described by Cameron IT and colleagues in 1988 as the triad of infertility, regular menses, and elevated plasma FSH concentrations, highlighting the occult form of POF [4]. In 2002, Welt C. et al. expanded the classification, highlighting three successive stages of the disease development: occult, biochemical and overt forms of POF [5]. Patients with occult POF often lack clinical manifestation of estrogen deprivation, such as menstrual irregularities and infertility, which makes clinical diagnosis extremely difficult [6].
The problem of identifying occult POF may be addressed by the search for more informative clinical and anamnestic signs or by the use of new diagnostic methods. Along with hormonal markers, ultrasound examination (US) is considered a highly informative non-invasive imaging technique to evaluate the ovaries and adnexa [7, 8]. The latest computer technologies and related transformations of classical two-dimensional echography into three-dimensional scanning (3D/4D) allows most accurate in real-time assessment of echographic parameters of the ovaries and OR, including linear characteristics of the ovaries and their volume, as well as the number of antral follicles (AFC) in the ovaries. The total AFC can be evaluated by transvaginal ultrasound at the beginning of the follicular phase of the menstrual cycle between day two and day 5 of periods. The AFC correlates with the histologically determined number of primordial follicles and can be used in the early assessment of the OR [7, 8].
The advent of three-dimensional ultrasound systems (Voluson E8 and Voluson-I) has led to the feasibility of evaluating AFC in the ovary using automated programs (SonoAVC). Also, it allows for more accurate measurement of the volume and velocity of ovarian blood flow parameters, including calculation of vascularization index (VI) and blood flow index (FI) [7, 8]. For 20 years, the technology for assessing OR included counting antral follicles in a section, but nowadays, it is customary to estimate their total count in two ovaries [7].
Existing literature provides no studies investigating the feasibility of calculating AFC, measuring blood flow in the ovaries using energy Doppler in 2D/3D mode, and evaluating the morphofunctional state of OR in patients with the occult, initial and overt forms of POF.
The present study aimed to investigate the hormonal profile and morphofunctional status of the ovaries in women with different clinical forms of POF and evaluate the diagnostic value of new OR markers using 2D and 3D power Doppler sonography.
Materials and methods
This cross-sectional study included 190 women aged 18 to 39 years [32 (26; 37)] who were managed at the Department of Gynecologic Endocrinology, V.I. Kulakov NMRC for OG & P, Ministry of Health of Russia. The study group (group 1) included 132 women who had infertility of unknown origin, oligomenorrhea, slightly shortened or lengthened menstrual cycle or oligomenorrhea, periodic abnormal uterine bleeding in the absence of endometrial pathology, and history of functional ovarian cysts. In patients of group 1, the level of anti-Müllerian hormone (AMH) was < 1.1 ng/ml, which is diagnostic of diminished OR according to the Bologna criteria [9]. The comparison group consisted of 58 women with a regular menstrual cycle who received preventive health examinations at the Center.
The exclusion criteria were the presence of primary hyper-, normal- and hypogonadotropic amenorrhea, severe hereditary diseases (galactosemia, blepharophimosis), iatrogenic hypergonadotropic amenorrhea (ovarian surgery, chemotherapy/radiation therapy), malignancies, a history of thrombosis, impaired liver and kidney function, and hyperprolactinemia.
To identify POF's clinical forms, the functional status of the hypothalamic-pituitary-ovarian system was assessed based on the determination of FSH levels by the chemiluminescent immunoassay on Immulite 2000, Immulite 1000 automatic analyzers (Siemens, USA) using reagents of the same companies. AMH levels were determined by enzyme immunoassay or radioimmunoassay using appropriate test systems on an automated Cobar Core II analyzer. Blood sampling was performed on days 2–3 of the menstrual cycle; in secondary amenorrhea cases, they were chosen arbitrarily.
The morphological and functional status of the ovaries was assessed based on the analysis of echograms obtained by 2D scanning and 3D reconstruction on the VOLUSON-E8 systems (GE Kretz, Zipf, Austria) according to the standard technique using a transvaginal transducer (3.3–10.0 MHz) at 4-5 day of the menstrual cycle. When ovaries were identified, a mean ovarian volume and follicle number was calculated, and the indicators of intraovarian blood flow were measured.
At the first stage of ovarian volume determination using the VOCAL (Virtual Organ Computer-aided AnaLysis) software, the image was selected using the Zoom regulator, which allows for increasing the image (rotation angle 9°, slice thickness 1.5 mm).
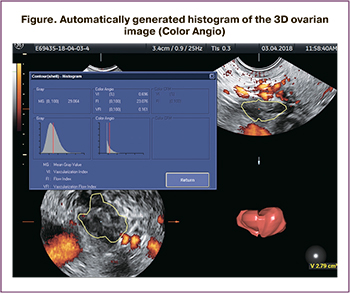 The VOCAL TM imaging program included the Color Angio function, which allowed for calculating the following indicators: the vascularization index (VI), which is the percentage of vessels in a specific volume of tissue; blood flow index (FI), which determines the volume of blood cells passing through the vessels during the study (Figure).
The VOCAL TM imaging program included the Color Angio function, which allowed for calculating the following indicators: the vascularization index (VI), which is the percentage of vessels in a specific volume of tissue; blood flow index (FI), which determines the volume of blood cells passing through the vessels during the study (Figure).
AFC was calculated manually on days 4–5 of the menstrual cycle with 3D ultrasound in the ovaries' entire volume, using the SonoAVC program, which allows for inverting hypoechoic structures into anechoic ones and automatically generate their quantitative values.
3D ultrasound was performed on 117 study participants (36, 18, 26, and 37 patients from the comparison group and groups of patients with the occult, initial and overt forms of POF, respectively).
Statistical analysis
The statistical analysis and table preparation were performed using the STATISTICA v.7 and MS Excel software. Quantitative variables were expressed as the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format.
Comparing numerical data between independent groups were performed with the nonparametric Mann– Whitney test. For multiple comparisons of independent groups, the Kruskal–Wallis ANOVA rank test was performed. For pairwise post-hoc comparison of groups, Dunn's nonparametric test was used. The relation between variables was assessed with a nonparametric Spearman correlation coefficient. The critical level of significance when testing statistical hypotheses were considered at p <0.05. In the correlation analysis, the Bonferroni correction was used and with the critical level of significance 0.001.
The study was approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG & P, Ministry of Health of Russia; all patients signed informed consent to participate in the study.
Results
Based on clinical and anamnestic data and hormonal profiles (FSH and AMH), a comparison group and three clinical groups were formed, reflecting the POF forms.
The comparison group consisted of 58 women with a regular menstrual cycle and FSH levels <12 mIU/ ml, who received preventive health examinations at the Center.
Group 2 consisted of patients with occult POF, which included 34 patients with a regular menstrual cycle (82.3%). A history of AUB was reported by 14.7% of patients, and 41.1% had recurrent functional cysts. The FSH level in the patients of this group was <12 mIU/ml.
Group 3 included 46 patients with POF's initial form, who had a regular menstrual cycle (43.5%) or oligomenorrhea (56.5%). A history of AUB was reported by 26.1% of patients, and 47.8% had recurrent functional cysts. The FSH level in the patients of this group ranged from 12 to25 mIU/ml.
Group 4 comprised 52 patients with overt POF and secondary amenorrhea for six months or more. The FSH level in group 4 patients was > 25 mIU/ml, according to the ESHRE criteria [10].
Hormonal measurements included FSH and AMH on days 2–4 of the menstrual cycle, traditionally considered markers of decreased ovarian reserve (Table 1). For both indicators, analysis of variance revealed group heterogeneity (p <0.001).
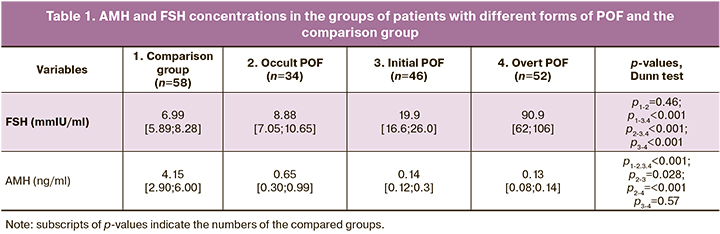
Analysis of hormonal profiles of patients with different clinical forms of POF and the comparison group (Table 1) showed that AMH and FSH concentrations in patients with POF and patients in the comparison group were statistically significantly different.
There was a statistically significant increase in FSH levels and a decrease in AMH levels when comparing occult and initial and initial and overt forms of POF.
It should be noted that the FSH levels in patients with occult POF were within the physiological range (up to 12 mIU/ml). In all patients in groups with initial and overt forms of POF, FSH levels were statistically significantly higher than normal, suggesting that an increase in FSH levels is a significant marker of POF's initial and overt forms.
We also compared groups of patients by the ovarian reserve's ultrasound characteristics, obtained by volumetric reconstruction of the ovaries using three-dimensional power Doppler ultrasound imaging (Table 2).
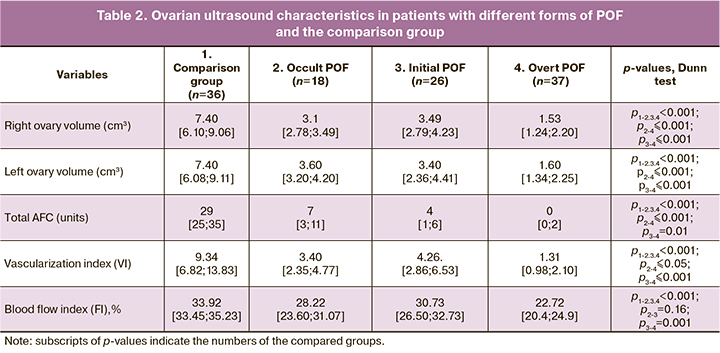
Patients in the study groups had gonadal hypoplasia unusual for their age. Those with occult POF and normal FSH levels had a twofold statistically significant decrease in mean ovarian volumes (p <0.001). In the initial POF, the mean ovarian volumes were similar to that in occult POF. Ovarian volumes in patients with overt POF were even lower, and this decrease was also statistically significant, compared with the group of patients with the initial form of POF.
In patients with occult POF, the total AFC in two ovaries was statistically significantly lower than in the comparison group. At the later stages of POF (initial and overt POF), AFC was statistically significantly lower than in patients with occult POF.
As an additional ultrasound marker for assessing the ovarian reserve, we analyzed the ovarian stromal blood flow parameters evaluated by three-dimensional power Doppler ultrasound imaging.
Vascularization index (VI) in the compared groups of patientsindicateditsmonotonousdecrease, accompanying POF development. Already in the group of patients with occult POF, the VI values were statistically significantly lower than in the comparison group. Patients with occult and initial forms of POF had similar VI values. In patients with occult POF, VI was statistically significantly lower than in those with occult and initial forms of POF.
The pattern of changes in the blood flow index was similar to that of the vascularization index (VI).
Changes in the ovarian reserve's ultrasound characteristics reflect the process of follicular depletion, starting with a statistically significant decrease in AMH, AFC, VI, and FI already in patients with an occult form of POF and continue to decrease during the transition from occult through the initial to the overt POF.
Of significant interest is how consistently these characteristics change during successive stages (forms) of POF and their relationship with FSH.
The correlation analysis results confirmed a stable and characteristic relationship between the main morphological and functional characteristics of the ovarian reserve. Of greatest interest is a statistically significant relationship between the functional characteristics and the AFC observed according to 3D ultrasound findings. Correlation coefficients were calculated between AFC and other features: AFC and VI (r=0.74, P <0.005, n=117), AFC and FI (r=0.67, P <0.005, n=117), AFC and AMG (r=0.72, P <0.005, n=168),) and AFC and FSH (r= -0.76, P <0.005, n=168),). The relationship with AFC of all characteristics (except FSH) is positive; that is, large AFC values correspond to large values of ovarian circulation indices and AMH levels. The relationship between AFC and FSH, as well as the relationship between the rest of the ultrasound characteristics of the ovarian reserve and FSH, is negative, which reflects the nature of the link between the ovaries and the hypothalamic-pituitary-ovarian system (the smaller the ovarian reserve, the greater the level of FSH due to a decrease in feedback between the ovaries and pituitary gland).
It should be noted that in overt POF, antral follicles often were not visualized. In our study, the non-follicular type of ovarian structure was detected in 34.8% of cases (in 44 out of 132 patients with different forms of POF). For the first time, we found that the progression of POF stages was accompanied by an increase in detection rates of the non-follicular type of ovarian structure, which was 5.9%, 23.9%, and 61.8% in the groups of patients with the occult, initial and overt forms of POF, respectively.
A comparative analysis of 3D ultrasound indicators of the morphofunctional status of the ovaries (VI, FI and AFC) in patients with different forms of POF and the comparison group revealed a statistically significant decrease in all three 3D ultrasound indicators (AFC, VI, and FI) in the groups of patients with different forms of POF. Moreover, a sharp and statistically significant decrease in these characteristics was found already in the group of patients with an occult POF, which indicates the potential of their use for early diagnosis of the disease.
Our results of comparing patients with different forms of POF and a group of women with preserved reproductive function (comparison group) indicate that, as follicular depletion progresses, changes in AMH levels and ultrasound characteristics of ovarian reserve (AFC and indices of vascularization and blood flow) have a unidirectional tendency towards a decrease in their values. A significant statistically significant reduction is found already in patients with occult POF. A statistically significant decrease in these ovarian reserve characteristics continues in patients with the initial and overt POF.
Discussion
The study findings revealed that the FSH levels were above the threshold (>12 mIU/ml) only in patients with initial and overt POF. Concentrations of FSH in patients with occult POF were statistically significantly higher than in the comparison group but remained within the normal range.
Vet A. et al. noted that at the initial stages, ovarian reserve depletion was accompanied by a decrease in AMH levels without significant clinical manifestations. However, an increase in FSH levels usually occurred somewhat later [10–12]. Our data indicate that the AMH level may provide more accurate identification of an early decrease in ovarian reserve. At the same time, FSH is a significant marker of later forms of POF (initial and overt) [1, 5, 10].
An essential step in diagnosing each of the POF forms is their detailed description using an ever-expanding set of new parameters. Analysis of ovarian morphological and functional parameters together with biomarkers of the ovarian reserve in patients with various clinical forms of POF showed a gradual decrease in the levels of AMH and 2D/3D ultrasound parameters (ovarian volume, AFC, VI, FI), which is characteristic not only for overt and initial but also for the occult form of POF. In our study, we found a positive statistically significant correlation between AFC and AMH (r=0.72; p<0.001) and a negative correlation between AMH and FSH (r = -0.76; p <0.001).
Similar results were presented in a study by Barbakadze L. et al., in which they noted a statistically significant positive relationship between AMH and AFC levels (r=0.69) and a statistically significant negative relationship between AMH and FSH levels (r= -0.69) [13], which is consistent with the results of our study. In a study comparing ultrasound and hormonal methods for assessing ovarian reserve, Fleming R. et al. also showed that AFC directly correlated with ovarian reserve, which is confirmed by histological examination of ovarian biopsy [14]. The authors concluded that AFC and AMH levels reflect the number of recruited follicles in a given cycle and can estimate the ovarian reserve.
Three-dimensional ultrasound scanning allows automatic assessment of AFC in the entire ovarian volume, thereby increasing the diagnostic value of this imaging modality [7, 8]. Fagundes P. et al. in 2017 studied the morphological and functional status of the ovaries in 42 women under 40 suffering from infertility. As a result of their study, they concluded that the predictive value of AFC measured with 3D ultrasound was significantly higher than with 2D. They also found a positive correlation between AFC and AMH levels [15]. Our results, demonstrating characteristic changes in the levels of three key markers of the ovarian reserve state (AMH, FSH, and AFC) in occult POF, are consistent with the results of Izhar R. et al.. They reported a significant reduction in the mean levels of AMH to 0.51 ng/ml with a concurrent decrease in AFC to 2 follicles (in the ovarian section) and a simultaneous increase in FSH levels up to 10 mIU/ml (≤ threshold of 12 mIU/ml) [16].
So far, no studies have reported using 3D ultrasound characteristics, including AFC and features of ovarian blood flow (VI, FI) in patients with the occult, initial and overt POF to assess ovarian reserve.
Overt POF is characterized by very low AFC or the absence of antral follicles. However, Kawamura N. et al. showed that even in overt POF pathomorphological examination of ovarian tissue samples found residual follicles in 48.1% of cases [17]. Thus, the absence of antral follicles in patients with overt forms of POF (complete and initial) indicates follicle recruitment inhibition [18].
In our study, we showed for the first time that as the POF stages progressed, detection rates of the non-follicular type of ovaries increased, which was found in 5.9%, 23.9%, and 61.8% in groups of patients with occult, initial and overt POF, respectively, which is consistent with the results of Kawamura N. et al. [17].
Age and FSH are considered traditional markers of ovarian reserve [19]. There is an inverse relationship between ovarian vascularization and chronological age, which is due to a decrease in the ovaries' functional activity [20]. However, based on our results, age may not always be an objective marker for assessing ovarian reserve. In our study, the patients included in the research and the comparison groups had similar chronological ages, corresponding to the STRAW+10 staging system, to the heyday of reproductive function. However, their morphofunctional and perfusion parameters of the ovaries differed sharply [21]. Venturella R. et al. in 2015 showed that as the AMH levels decreased in patients under the age of 40, there was a parallel blood flow reduction in the perifollicular vasculature, which utterly consistent with our data [22]. Thus, it can be concluded that intraovarian blood flow indicators, along with AMH and AFC, can be used as additional diagnostic markers of the early stages of ovarian aging.
Conclusion
A comprehensive morphofunctional assessment of ovaries, including hormonal indicators (AMH and FSH) and ovarian reserve parameters (ovarian volume, AFC in both ovaries, vascularization, and blood flow indexes), determined by three-dimensional ultrasound and Doppler studies may provide a more accurate diagnosis of occult POF. This can help develop an algorithm for diagnosing early stages of POF, allowing a patient-tailored approach to preserving the patient's reproductive potential.
References
- Webber L., Davies M., Anderson R., Dartlett J., Braat D., Cartwright B. et al.; European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 2016; 31(5): 926-37. https://dx.doi.org/10.1093/humrep/dew027.
- Mishra G.D., Chung H.F., Cano A., Chedraui P., Goulis D.G., Lopes P. et al. EMAS position statement: predictors of premature and early natural menopause. Maturitas. 2019; 123: 82-8. https://dx.doi.org/10.1016/j.maturitas.2019.03.008.
- Golezar S., Ramezani Tehrani F., Khazaei S., Ebadi A., Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019; 22(4): 403-11. https://dx.doi.org/10.1080/13697137.2019.1574738.
- Cameron I.T., O'Shea F.C., Rolland J.M., Hughes E.G., de Kretser D.M., Healy D.L. Occult ovarian failure: a syndrome of infertility, regular menses, and elevated follicle-stimulating hormone concentrations. J. Clin. Endocrinol. Metab. 1988; 67(6): 1190-4. https://dx.doi.org/10.1210/jcem-67-6-1190.
- Welt C.K. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin. Endocrinol. (Oxf). 2008; 68(4): 499-509. https://dx.doi.org/10.1111/j.1365-2265.2007.03073.x.
- Марченко Л.А., Машаева Р.И. Клинико-лабораторные критерии оккультной формы преждевременной недостаточности яичников. Гинекология. 2018; 20(6): 73-6. [Marchenko L.A., Mashaeva R.I. Clinical and laboratory criteria for occult forms of premature ovarian insufficiency. Gynecology. 2018; 20 (6): 73-6. (in Russian)].
- Coelho Neto M.A., Ludwin A., Borrell A., Benacerraf B., Dewailly D., da Silva Costa F. et al. Counting ovarian antral follicles by ultrasound: a practical guide. Ultrasound Obstet. Gynecol. 2018; 51(1): 10-20. https://dx.doi.org/10.1002/uog.18945.
- Hansen K., Massey J., Craig L. Antral follicle counts obtained by transvaginal ultrasound and histological examination are correlated with ovarian non-growing follicle number. Fertil. Steril. 2007; 88(1); S79-S80. https://dx.doi.org/10.1016/j.fertnstert.2007.07.266.
- Younis J.S., Ben-Ami M., Ben-Shlomo I. The Bologna criteria for poor ovarian response: a contemporary critical appraisal. J. Ovarian Res. 2015; 8: 76. https://dx.doi.org/10.1186/s13048-015-0204-9.
- De Vet A., Laven J.S.E., de Jong F.H., Themmen A.P.N., Fauser B.C.J.M. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil. Steril. 2002; 77(2): 357-62. https://dx.doi.org/10.1016/s0015-0282(01)02993-4.
- Tal R., Seifer D.B. Ovarian reserve testing: a user’s guide. Am. J. Obstet. Gynecol. 2017; 217(2): 129-40. https://dx.doi.org/10.1016/j.ajog.2017.02.027.
- La Marca A., Sighinolfi G., Radi D., Argento C., Baraldi E., Artenisio A.C. et al. Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum. Reprod. Update. 2010; 16(2): 113-30. https://dx.doi.org/10.1093/humupd/dmp036.
- Barbakadze L., Kristesashvili J., Khonelidze N., Tsagareishvili G. The correlations of anti-müllerian hormone, follicle-stimulating hormone and antral follicle count in different age groups of infertile women. Int. J. Fertil. Steril. 2015; 8(4): 393-8. https://dx.doi.org/10.22074/ijfs.2015.4179.
- Fleming R., Seifer D.B., Frattarelli J.L., Ruman J. Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reprod. Biomed. Online. 2015; 31(4): 486-96. https://dx.doi.org/10.1016/j.rbmo.2015.06.015.
- Peres Fagundes P.A., Chapon R., Olsen P.R., Schuster A.K., Mattia M.M.C., Cunha-Filho J.S. Evaluation of three-dimensional SonoAVC ultrasound for antral follicle count in infertile women: its agreement with conventional two-dimensional ultrasound and serum levels of anti-Müllerian hormone. Reprod. Biol. Endocrinol. 2017; 15(1): 96. https://dx.doi.org/10.1186/s12958-017-0314-x.
- Izhar R., Husain S., Tahir S., Husain S. Occult form of premature ovarian insufficiency in women with infertility and oligomenorrhea as assessed by poor ovarian response criteria. J. Reprod. Infertil. 2017; 18(4): 361-7.
- Kawamura K., Kawamura N., Hsueh A.J.W. Activation of dormant follicles: a new treatment for premature ovarian failure? Curr. Opin. Obstet. Gynecol. 2016; 28(3): 217-22. https://dx.doi.org/10.1097/GCO.0000000000000268.
- Gleicher N., Barad D.H. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod. Biol. Endocrinol. 2011; 9: 67. https://dx.doi.org/10.1186/1477-7827-9-67.
- Bishop L.A., Richter K.S., Patounakis G., Andriani L., Moon K., Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil. Steril. 2017; 108(6): 980-7. https://dx.doi.org/ 10.1016/j.fertnstert.2017.09.011.
- Jayaprakasan K., Al-Hasie H., Jayaprakasan R., Campbell B., Hopkisson J., Johnson I. et al. The three-dimensional ultrasonographic ovarian vascularity of women developing poor ovarian response during assisted reproduction treatment and its predictive value. Fertil. Steril. 2009; 92(6): 1862-9.https://dx.doi.org/10.1016/j.fertnstert.2008.09.031.
- Harlow S.D., Gass M., Hall J.E., Lobo R., Maki P., Rebar R.W. et al. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012; 19(4): 387-95. https://dx.doi.org/10.1097/gme.0b013e31824d8f40.
- Venturella R., Lico D., Sarica A., Falbo M.P., Gulletta E., Morelli M. et al. OvAge: a new methodology to quantify ovarian reserve combining clinical, biochemical and 3D-ultrasonographic parameters. J. Ovarian Res. 2015; 8: 21. https://dx.doi.org/10.1186/s13048-015-0149-z.
Received 16.09.2020
Accepted 20.10.2020
About the Authors
Roza I. Mashaeva, Ph.D. Student at the Department of Gynecologic Endocrinology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.E-mail: mashaevarosa@gmail.com. 4, Oparina str., Moscow, 117997, Russia.
Larisa A. Marchenko, Dr.Med.Sci., Professor, Leading Researcher at the Department of Gynecologic Endocrinology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-85-40. E-mail: l.a.marchenko@yandex.ru. 4, Oparina str., Moscow, 117997, Russia.
Sophia P. Olimpieva, Ph.D. (Bio. Sci.), Senior Lecturer, Department of Medical Cybernetics and Informatics, Faculty of Medicine and Biology, RNRMU, Ministry of Health of Russia. Tel.: + 7(905)514-41-42. E-mail: vkilikov@yandex.ru. ORCID: 0000-0001-5188-8052. 1, Ostrovityanova str., Moscow, 117997, Russia.
Alexander I. Gus, Dr.Med.Sci., Head of the Department of Ultrasound and Functional Diagnostics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-11-77. E-mail: a_gus@oparina4.ru. 4, Oparina str., Moscow, 117997, Russia.
Kirill V. Kostyukov, M.D., Ph.D., Senior Researcher at the Department of Fetal Medicine, Institute of Obstetrics; Physician at the Unit of Functional and Ultrasound Diagnostics, Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(926)214-97-84. E-mail: kostyukov_k@yahoo.com.
4, Oparina str., Moscow, 117997, Russia.
Galina E. Chernukha, Professor, Dr.Med.Sci., Head of the Department of Gynecologic Endocrinology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(916)311-05-21. E-mail: g_chernukha@oparina4.ru. ORCID: 0000-0002-9065-5689. 4, Oparina str., Moscow, 117997, Russia.
For citation: Mashaeva R.I., Marchenko L.A., Olympieva S.P., Gus A.I., Kostyukov K.V., Chernukha G.E. Morphofunctional characteristics of ovaries determined by 2D and 3D power Doppler sonography in different clinical forms of premature ovarian failure.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 12: 129-136 (in Russian)
https://dx.doi.org/10.18565/aig.2020.12.129-136



