BRCA1 pathogenic variants in women with premature ovarian failure
The role of BRCA1 pathogenic variants in the genesis and development of premature ovarian aging has been discussed in the literature in recent years.Rshtuni S.D., Chernukha G.E., Bystritskiy A.A., Tabeeva G.I., Krasheninnikova R.V., Marchenko L.A.
Objective: To identify the proportion of female carriers of BRCA1 pathogenic variants among patients with premature ovarian failure (POF).
Materials and methods: This was an observational longitudinal study which included 142 women with POF who underwent BRCA testing. The patients were aged from 18 to 39 years (median age is 35 years (Q1–Q3 29–38). The control group consisted of 150 women with timely menopause (median age is 54 years (Q1–Q3 46–71); they underwent BRCA testing optionally.
Results: During the study we identified two cases of the most frequent BRCA1 pathogenic variants (loci 3819del GTAAA and 5382insC) which constitute 1.4% (95% CI: 0.4–5.0%) in POF pathology.
Conclusion: BRCA1 pathogenic variants are associated not only with an increased risk of oncopathology, but also with a diminished ovarian reserve; therefore, the principles of cancer prevention in patients with POF should be revised. In case of diminished ovarian reserve, it is necessary to carry out not only primary standard cancer screening, but one should also carefully take a family history of cancer with the subsequent administration of BRCA genetic testing.
Keywords
The decline in ovarian reserve is nonlinear under physiological conditions and it accelerates with age. Given the initial total ovarian reserve (TOR) within 600,000–1 million primordial follicles, physiological menopause occurs at the age of 49–51 [1]. Most of the mechanisms of age-related follicle pool depletion are unknown; if they are identified and controlled, a woman’s reproductive period could be extended by at least 20 years. This would be especially important for patients with a congenital decrease in ovarian reserve, whose TOR is 10 times lower (60,000–75,000 primordial follicles) and secondary hypergonadotropic amenorrhea occurs on average at the age of 27–33 [2]. Such a strategy may be implemented in the future with the help of Yamanaka cocktail (proteins Oct4, Sox2, KLF4 and c-Myc) [3].
The aging of the organism was previously explained by the replicative theory (telomere shortening resulting from terminal chromosome underreplication); on the basis of this theory, irreversible loss of proliferative activity occurs when the Hayflick limit is reached (50–52 divisions) [4]. The length of the telomeric sections determines the age of the cell, namely, the shorter the telomeric tail, the older it is. However, telomeres are restored during gametogenesis. Currently, stress-induced cellular aging is considered to be no less important than replicative aging, which is also characterized by a block of the cell cycle leading to a stop of proliferation mechanisms and DNA damage. As a result, a cascade of intracellular signaling events aimed at DNA repair is triggered [5, 6].
In recent years, the use of next-generation sequencing (NGS) has made it possible to identify several groups of genes involved in intrauterine and postnatal follicular and steroidogenesis, processes of apoptosis, hormonal signaling and cellular metabolism. Special attention is paid to the genes regulating the mechanisms of meiosis and DNA repair (BRCA1, STAG3, MCM8 and MCM9) [7]. Among the presented genes, the attention of researchers has been recently attracted by the BRCA1 gene localized on chromosome 17 in the 17q21 locus. This gene is involved in the processes of meiotic and mitotic cell division, as well as the repair of double-stranded DNA breaks that contribute to chromosome instability. According to one theory, oocytes may die prematurely in carriers of pathogenic variants of the BRCA1 gene due to the failure of DNA repair and accelerated process of apoptosis; therefore, their death may lead to premature depletion of the ovarian reserve [8, 9]. According to another theory, the accumulation of double-stranded DNA breaks in the primordial follicles prevents their normal growth and maturation, resulting in accelerated apoptosis and subsequent premature ovarian failure (POF) [10].
Pathogenic variants of the BRCA1 gene are extremely rare (from 0.1 to 1%) in the population of European women. They play an important role not only in carcinogenesis, but they are also responsible for a third of cases of diminished ovarian reserve which may result in subclinical forms of POF (occult and biochemical) [11, 12]. The study by Wang E.T. et al. showed that the relative risk of detecting the value of Anti-Muller hormone (AMH) <1 ng/ml in carriers of the pathogenic variant of the BRCA1 gene was increased by 4 times (OR=4.22, 95% CI: 1.48–12.0) [8].
All of the above suggests that pathogenic variants of the BRCA1 gene play a certain role in premature ovarian aging which results in POF. However, the rate of these genetic disorders as the genetic causes of POF has not been presented in the previous studies.
The aim of the study was to identify the proportion of female carriers of BRCA1 pathogenic variants among patients with premature ovarian failure.
Materials and methods
This was a cross sectional study which included 142 women with POF aged from 18 to 39 years (median age 35 years (Q1–Q3 29–38). The patients were examined and/or treated at the Department of Gynecological Endocrinology of the National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia. The diagnosis was made according to the criteria of the European Society of Human Reproduction and Embryology (ESHRE) [13]. The control group consisted of 150 women with timely menopause (median age is 54 years (Q1–Q3: 46–71); they underwent BRCA testing optionally. The study was approved by the Ethical Review Board of the Centre.
There were the following criteria for inclusion in the study: the age of women at the time of diagnosis ranging from 18 to 39 years; karyotype 46 XX; absence of menstruation for 4 months or more; the level of follicle stimulating hormone (FSH) exceeding 25 mIU/L after two measurements.
The patients who had primary or iatrogenic hyper-, normo- and hypogonadotropic amenorrhea were excluded from the study.
In order to verify the diagnosis of POF, we evaluated the functional state of the hypothalamic-pituitary-ovarian axis using the assessment of the levels of FSH and estradiol (E2) in blood serum with an electrochemiluminescent method on an automatic Cobas e411 immunoassay analyzer (Roche Diagnostics GmbH, Germany). AMH was determined using the AMH Gen II ELISE kit (Beckman Coulter, USA).
The ultrasound examination of the pelvic organs was performed with 2000 Toshiba SSA-240 (Japan) with a 7.5 MHz transvaginal curvilinear probe. During the examination, we determined the location, size of the uterus, and structure of the myometrium. We also studied the state of the median M-echo. While examining the ovaries, we measured their size, location, and assessed the condition of the follicular apparatus and stroma. Special attention was paid to the volume of the ovaries (V), which was calculated with the help of the formula:
V=0.523× (L×S×H),
where L is length, S is the width, H is height, 0.523 is the constant coefficient.
The most common pathogenic variants of the BRCA1 gene, namely, 185delAG, 4153delA, 5382insC, 3819delGTAAA, 3875delGTCT, 300T>G (Cys61Gly), 2080delA, which are associated with the risk of breast and ovarian cancer, were identified with the help of BRCA mutations REAL-TIME PCR Genotyping Kit manufactured by “DNA-Technology Research & Production”, LLC, Russia. Genetic pathogenic variants in the BRCA1 gene were detected by polymerase chain reaction (PCR) with real-time detection of the results and subsequent analysis of the melting curves of amplification products.
Statistical analysis
Statistical analysis was carried out using the StatTech v. 2.8.8 software program (Stattech LLC, Russia).
Quantitative indicators were checked for normal distribution using the Shapiro–Wilk test (when the number of subjects was less than 50) or the Kolmogorov–Smirnov test (when the number of subjects exceeded 50).
In the absence of a normal distribution, quantitative data were described using median, lower and upper quartiles – Me (Q1; Q3).
The confidence interval for the fraction was checked for the possibility of approaching the normal distribution. Since the observed fraction turned out to be too small, exact confidence intervals for the fractions were used. For this purpose, we used binomial distribution based on the Wilson method.
Results
During BRCA testing of 142 women with POF we identified two cases of the BRCA gene pathogenic variants (loci 3819del GTAAA and 5382insC) which constitute 1.4% (95% CI: 0.4–5.0%) in POF pathology. The detected variants are classified as pathogenic ones according to the criteria of the American College of Medical Genetics and Genomics (ACMG) [14]. The above pathogenic variants were not detected in the control group.
The results of clinical and hormonal examination of 142 women with POF showed that the median age of menarche was 13 years (Q1–Q3: 12–14). Most women (88%, 125/142) had a regular menstrual cycle before the onset of the disease. The median age of the onset of the disease was 31 years (Q1–Q3: 23–36). The duration of secondary amenorrhea is from 8 months to 23 years.
When the patients were diagnosed with POF, there was not any case of a previous history of cancer among them. There was a family history of breast cancer in 7.7% (11/142) of cases. Fibrocystic disease was detected in 14.8% (21/142) of patients.
The hormonal profile data are presented in the Table.
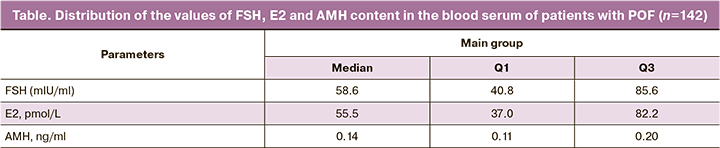
The median volume of the right ovary was 1.8 cm3 (Q1–Q3: 1.3–2.73), the volume of the left ovary was 1.7 cm3 (Q1–Q3: 1.2–2.6).
In order to identify the characteristics of the reproductive history of two carriers of the BRCA1 gene pathogenic variants, we conducted a detailed analysis of their clinical and hormonal status. In the first case, a 36-year-old patient had a pathogenic variant which was detected in the locus 3819del GTAAA in a heterozygous state; it was maternally inherited pathogenic variant. Siblings and first- and second-degree relatives had a burdened family history of cancer (mammary gland, ovaries). The analysis of the patient’s reproductive status revealed that menarche occurred at the age of 14, the cycle was regular until the age of 34, after which there were delays of menses up to 3 months. She has a history of two pregnancies, two births, and she did not receive hormonal contraception. The disease started at the age of 35. The level of FSH was 44 mIU/L, AMH was less than 0.14 ng/ml, E2 was 77 pg/ml. The ultrasound investigation of the pelvic organs showed that the volume of the right ovary was 1.5 cm3, the left ovary was 3.2 cm3, and the follicles were not detected. Due to the severe bilateral fibrocystic mastopathy confirmed by magnetic resonance imaging of the mammary glands, the patient underwent a bilateral preventive subcutaneous mastectomy with immediate allomammoplasty. Histological examination of breast tissue showed fibrosis of the stroma of the right breast without cell atypia. There was a focal typical ductal proliferation of the left mammary gland. According to the recommendation of the mammologist, she did not receive hormone replacement therapy.
In the second case, the BRCA1 gene pathogenic variant was detected in the locus 5382insC in a 44-year-old female patient. The level of FSH was 25 mIU/L, AMH was less than 0.14 ng/ml, E2 was 8 pg/ml. The ultrasound assessment of the pelvic organs showed that the volume of the right ovary was 2.7 cm3, left ovary was 1.6 cm3, and there were single follicles. The first- and second-degree relatives had a burdened history of breast cancer. The analysis of the patient’s reproductive status revealed that menarche occurred at the age of 13, the cycle was regular until the age of 38, then persistent amenorrhea occurred. She had a history of infertility for 10 years with regular sexual activity, she did not receive hormonal contraception. Due to persistent infertility which was accompanied by POF for 4 years, the patient underwent a transfer of a donor embryo into the uterine cavity, according to the recommendations of ESHRE. The patient had a miscarriage at 18 weeks gestation. Four months after the miscarriage, the ultrasound of the mammary glands revealed BIRADS4b, and therefore, a trepan biopsy of tumor of the external localization of the breast and lymph nodes was performed. The histological examination of the biopsy showed invasive breast cancer of a non-specific type, Grade 3 (atypical medullary cancer). It was a triple-negative breast cancer subtype, basal-like phenotype. The patient underwent positron emission and computer tomography which revealed the presence of active tumor tissue in the right mammary gland, no distant metastases were detected. At the first stage of treatment, the patient had a six-month course of chemotherapy followed by a bilateral radical subcutaneous mastectomy with immediate mammoplasty of the pectoralis major muscle in combination with a lower de-epidermized skin flap and an endoprosthesis. The histological examination of the mammary gland tissue showed oncocytic adenocarcinoma of the right breast, Grade 3 (1 cm). It was secretory cancer of the right breast, Grade 1 (0.7 cm), ypT1b(m)N0 (0/12).
Discussion
During the BRCA testing of 142 patients in our study we identified two cases of the pathogenic variants of this gene which constitute 1.4% in POF pathology.
Due to the absence of available studies covered in the literature on the frequency of the BRCA1 gene pathogenic variants in patients with POF, it is not possible to conduct a comparative analysis of frequency indicators. The total prevalence of the BRCA1 gene pathogenic variants in the population is extremely low and amounts to 0.1–0.5%, however, it reaches 5–10% and 5–17% in patients with breast and ovarian cancer, respectively [15, 16]. We revealed a heterozygous pathogenic variant 3819del GTAAA in one patient with POF, and a pathogenic variant 5382insC in another patient with POF, the latter variant occurs in 90% of all cases of pathogenic variants [17]. The women who had these genetic disorders did not differ from other women in the average age of menarche, the nature of the menstrual cycle and hormonal profile. It should be noted that patients with pathogenic variants of the BRCA1 gene had a later onset of the disease (35 and 38 years old versus 31 years, average age of onset of the disease in the main group); therefore, their period of estrogenic deprivation was shorter in comparison with women who did not carry this pathogenic variant. These patients had a burdened history of breast cancer in first- and second-degree relatives; however, burdened family history was detected in 6.3% of cases in the group of women who were not carriers of pathogenic variants of the BRCA1 gene (9/140).
One can suggest that the BRCA1 gene contributes to the mechanism of POF development as it plays a role in the reduction of the primordial pool. It has been experimentally proved that a limited number of oocytes ripen in the multifollicular ovarian stimulation program in BRCA1 knockout mice and the number of offspring is significantly reduced. This can be explained not only by the accelerated process of apoptosis, but also by the accumulation of meiotic errors leading to aneuploidies. A phenotype of premature ovarian aging is subsequently formed in mice carrying heterozygous pathogenic variants of the BRCA1 gene. Long-term consequences of persistent estrogen deficiency such as osteoporosis and endothelial dysfunction develop in mice as well [10, 18]. The hypothesis expressed by Oktay K. et al. about the possible role of the BRCA1 gene pathogenic variants in the genesis of the formation of the subclinical form of premature ovarian aging (FSH levels are less than 12 mIU/L) has been confirmed by the results of numerous morphological and clinical-hormonal studies [12].
Phillips K.A. et al. showed that AMH level was 25% lower in carriers of pathogenic variants of the BRCA1 gene compared to non-carriers (95% CI: 5–41%, p=0.02), and it was more likely to be in the lowest age quartile (OR 1.84, 95% CI: 1.11–303, p=0.02). The use of the quadratic model showed that the biological age of patients with pathogenic variants is two years ahead of their chronological age (37 versus 35 years). Therefore, the authors made a suggestion about an increased risk of early menopause in carriers of pathogenic variants of the BRCA1 gene [19]. Even more convincing data were obtained in a study that demonstrated that carriers of this mutation over 35 years had a 10–fold higher risk of having a decrease in AMH levels to less than 0.5 than carriers under 35 years (OR 10.8, p=0.001, 95% CI: 2.53–45.9) [20].
The contribution of the BRCA1 gene to the genesis of POF development was finally confirmed and demonstrated in the study of Ben-Aharon I. et al., where women with pathogenic variants of the BRCA1 gene had a twofold decrease in the density of primordial, primary, secondary and antral follicles in the cortical layer of the ovary, which can confirm a decrease in the ovarian reserve; besides, the level of AMH was statistically significantly decreased in such women. For the first time, the authors additionally determined biomarkers of systemic aging in this category of patients: interleukin 1a (IL1A), fibroblast growth factor 23 (FGF23) and Klotho protein. This study revealed a tendency to a decrease in the Klotho index and an increase in the levels of IL1A and FGF23 (p=0.006). IL1A is produced by endothelial cells, and since it is a pro-inflammatory cytokine, it contributes to the development of cardiovascular pathology. Morphogenetic protein Klotho, expressed mainly by the vascular plexus in the brain, as well as partially by the ovarian tissue, activates FGF23. Changes in these indicators are associated with the progression of cardiovascular pathology (arterial hypertension and atherosclerosis), and a decrease in the level of Klotho protein can be considered as a predictor of a decrease in life expectancy. The obtained results allowed us to conclude that aging of the whole organism in women carrying pathogenic variants of the BRCA1 gene can be considered as a “mirror image” of ovarian aging [21].
The mechanisms of DNA damage and repair play an essential role not only in carcinogenesis, but also in the aging processes associated with cellular and oxidative stress. The women carrying pathogenic variants of the BRCA1 gene may be predisposed to premature aging of the body resulting in endothelial dysfunction and associated cardiovascular pathology (heart attack, stroke, ischemic heart disease), when the POF phenotype is only one of the manifestations of systemic aging. However, an alternative option cannot be excluded, when pathogenic variants in the BRCA1 gene lead to functional damage to the ovaries due to premature aging of germinal cells, which, in its turn, causes typical clinical manifestations characteristic of menopause, namely osteoporosis and cardiovascular diseases. Moreover, both mechanisms described above seem most likely to exist, and each of them can be implemented due to some modifying factors. All these findings allow us to formulate a new paradigm about the relationship between pathogenic variants of the BRCA1 gene, accelerated aging of the gonads and aging of the whole organism.
Conclusion
BRCA1 pathogenic variants are associated not only with an increased risk of oncopathology, but also with a diminished ovarian reserve and estrogen deprivation. Therefore, the principles of cancer prevention in patients with POF should be revised, since it was previously believed that this category of patients has an extremely low risk of developing breast, ovarian and endometrial cancer. The clinicians should remember that in case of diminished ovarian reserve it is necessary to carry out not only primary standard cancer screening, but one should also carefully take a family history of cancer with the subsequent administration of BRCA genetic testing. On the other hand, BRCA testing is gradually becoming a common screening procedure, especially among patients with a burdened family history of cancer; this procedure allows medical specialists to be wary of the risk of premature ovarian aging in this category of patients when receiving a positive examination result.
References
- Российское общество акушеров-гинекологов. Менопауза и климактерическое состояние у женщины. Клинические рекомендации. М.; 2021. 86 c. [Russian Society of Obstetricians-Gynecologists. Menopause and climacteric state in women. Clinical guidelines. M.; 2021. 86p. (in Russian)].
- Табеева Г.И., Позднякова А.А., Марченко Л.А. Эволюция диагностических и лечебных подходов при преждевременной недостаточности яичников. Акушерство и гинекология: новости, мнения, обучение. 2013; 2(2): 31-6. [Tabeeva G.I., Pozdnyakova A.A., Marchenko L.A. The evolution of diagnostic and therapeutic approaches in premature ovarian failure. Obstetrics and Gynecology: News. Opinions. Training. 2013; 2(2): 31-6. (in Russian)].
- Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem.Cell. 2012; 10(6): 678-84. https://dx.doi.org/10.1016/j.stem.2012.05.005.
- Martens U.M., Chavez E.A., Poon S.S., Schmoor C., Lansdorp P.M. Accumulation of short telomeres in human fibroblasts prior to replicative senescence. Exp. Cell Res. 2000; 256(1): 291-9. https://dx.doi.org/10.1006/excr.2000.4823.
- Bakkenist C.J., Kastan M.B. Initiating cellular stress responses. Cell. 2004; 118(1): 9-17. https://dx.doi.org/10.1016/j.cell.2004.06.023.
- Faddy M.J., Gosden R.G., Gougeon A., Richardson S.J., Nelson J.F. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum. Reprod. 1992; 7(10): 1342-6. https://dx.doi.org/10.1093/oxfordjournals.humrep.a137570.
- França M.M., Mendonca B.B. Genetics of ovarian insufficiency and defects of folliculogenesis. Best Pract. Res. Clin. Endocrinol. Metab. 2022; 36(1): 101594. https://dx.doi.org/10.1016/j.beem.2021.101594.
- Wang E.T., Pisarska M.D., Bresee C., Chen Y.D., Lester J., Afshar Y. et al. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil. Steril. 2014; 102(6): 1723-8. https://dx.doi.org/10.1016/j.fertnstert.2014.08.014.
- Finch A., Valentini A., Greenblatt E., Lynch H.T., Ghadirian P., Armel S. et al..; Hereditary Breast Cancer Study Group. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil. Steril. 2013; 99(6): 1724-8. https://dx.doi.org/10.1016/j.fertnstert.2013.01.109.
- Titus S., Li F., Stobezki R., Akula K., Unsal E., Jeong K. et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013; 5(172): 172ra21. https://dx.doi.org/10.1126/scitranslmed.3004925.
- Ford D., Easton D.F., Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am. J. Hum. Genet. 1995; 57(6): 1457-62.
- Oktay K., Kim J.Y., Barad D., Babayev S.N. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J. Clin. Oncol. 2010; 28(2): 240-4. https://dx.doi.org/10.1200/JCO.2009.24.2057.
- Webber L., Davies M., Anderson R., Bartlett J., Braat D., Cartwright B. et al.; European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 2016; 31(5): 926-37. https://dx.doi.org/10.1093/humrep/dew027.
- Nykamp K., Anderson M., Powers M., Garcia J., Herrera B., Ho Y.Y. et al.; Invitae Clinical Genomics Group; Topper S. Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet. Med. 2017; 19(10): 1105-17. https://dx.doi.org/10.1038/gim.2017.37.
- Hu C., Hart S.N., Gnanaolivu R., Huang H., Lee K.Y., Na J. et al. A population-based study of genes previously implicated in breast cancer. N. Engl. J. Med. 2021; 384(5): 440-51. https://dx.doi.org/10.1056/NEJMoa2005936.
- Malander S., Ridderheim M., Måsbäck A., Loman N., Kristoffersson U., Olsson H. et al. One in 10 ovarian cancer patients carry germ line BRCA1 or BRCA2 mutations: results of a prospective study in Southern Sweden. Eur. J. Cancer. 2004; 40(3): 422-8. https://dx.doi.org/10.1016/j.ejca.2003.09.016.
- Грудинина Н.А., Голубков В.И., Тихомирова О.С., Брежнева Т.В., Хансон К.П., Васильев В.Б., Мандельштам М.Ю. Преобладание широко распространенных мутаций в гене BRCA1 у больных семейными формами рака молочной железы Санкт-Петербурга. Генетика. 2005; 41(3): 405-10. [Grudinina N.A., Golubkov V.I., Tikhomirova O.S., Brezhneva T.V., Hanson K.P., Vasilyev V.B., Mandelshtam M.Y. Prevalence of widespread BRCA1 gene mutations in patients with familial breast cancer from St. Petersburg]. Genetika. 2005; 41(3): 405-10.
- Turan V., Oktay K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum. Reprod. Update. 2020; 26(1): 43-57.https://dx.doi.org/10.1093/humupd/dmz043.
- Phillips K.A., Collins I.M., Milne R.L., McLachlan S.A., Friedlander M., Hickey M. et al. Anti-Mullerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations. Hum. Reprod. 2016; 31(5): 1126-32. https://dx.doi.org/10.1093/humrep/dew044.
- Giordano S., Garrett-Mayer E., Mittal N., Smith K., Shulman L., Passaglia C. et al. Association of BRCA1 mutations with impaired ovarian reserve: connection between infertility and breast/ovarian cancer risk. J. Adolesc. Young Adult Oncol. 2016; 5(4): 337-43. https://dx.doi.org/10.1089/jayao.2016.0009.
- Ben-Aharon I., Levi M., Margel D., Yerushalmi R., Rizel S., Perry S. et al. Premature ovarian aging in BRCA carriers: a prototype of systemic precocious aging? Oncotarget. 2018; 9(22): 15931-41. https://dx.doi.org/10.18632/oncotarget.24638.
Received 19.07.2022
Accepted 10.10.2022
About the Authors
Sandra D. Rshtuni, postgraduate student, Department of Endocrinological Gynecology, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, rshtunisandra@gmail.com, 117997, Russia, Moscow, Ac. Oparina str., 4.Galina E. Chernukha, Dr. Med. Sci., Professor, Department of Endocrinological Gynecology, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, g_chernukha@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Andrey A. Bystritskiy, Leading researcher at the Laboratory of Molecular Genetic Methods, Institute of Reproductive Genetics, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare of Russian Federation, a_bystritskiy@oparina4.ru 117997, Russia, Moscow, Ac. Oparina str., 4.
Gyuzyal I. Tabeeva, Senior Researcher at the Department of Gynecological Endocrinology, V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology, and Perinatology, Ministry of Health of Russia, +7(903)199-72-82, doctor.gtab@gmail.com, 117997, Russia, Moscow, Ac. Oparina str., 4.
Regina V. Krasheninnikova, molecilar genetic lab pathologist, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology,
Ministry of Health of Russia, krv82@yandex.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Larisa A. Marchenko, Dr. Med. Sci., Professor, Department of Endocrinological Gynecology, V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology, and Perinatology, Ministry of Health of Russia, l_marchenko@yandex.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors’ contributions. Rshtuni S.D. – literature review; Marchenko L.A., Rshtuni S.D. – developing the design and concept of the study, writing the text; Bystritskiy A.A., Krasheninnikova R.V., Rshtuni S.D. – collecting and conducting the analysis of the material; Chernukha G.E., Bystritskiy A.A., Tabeeva G.I. – editing the article.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The research was carried out within the framework of the State Program “Development of innovative approaches to the prediction and preclinical diagnosis of premature ovarian failure based on the identification of molecular genetic and clinical hormonal markers in the Russian women of different ages” No. 121032500121–8, extension 17-А21.
Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Rshtuni S.D., Chernukha G.E., Bystritskiy A.A., Tabeeva G.I.,
Krasheninnikova R.V., Marchenko L.A. BRCA1 pathogenic variants
in women with premature ovarian failure.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 10: 76-82 (in Russian)
https://dx.doi.org/10.18565/aig.2022.10.76-82



