The influence of immunosuppressive therapy during pregnancy on the immune system of the mother and the newborn
Aim. The comparative analysis of study results of newborn immune system in women who received immunosuppressive chemotherapy during pregnancy.Vanko L.V., Zubkov V.V., Krechetova L.V., Makieva M.I., Baybarina E.N., E.A.Shatalova, Zhukova A.С., Klimenchenko N.I., Shmakov R.G.
Material and methods. Physical examination of pregnant women with transplanted organs, oncological or autoimmune diseases and their newborns (n=106) was carried out. The method of flow cytometry was used for phenotyping lymphocytes in the umbilical cord blood of newborns (n=82), as well as in the venous blood of their mothers. The serum immunoglobulin’s concentration had determined of turbidimetric method.
Results. We revealed the decreasing in the ratio CD3+CD4+/CD3+CD8+ and the percentage of B-lymphocytes, and the increasing of the percentage of activated T-lymphocytes in pregnant women with various pathologies who received immunosuppressive therapy. The percentage of B-lymphocytes in the umbilical cord blood of newborns was reduced. The differences in the percentage of T-lymphocytes were absent.
Conclusion. The state of health in children who had born in women with immunosuppressive therapy was a satisfactory state in the early neonatal period and the changes in immune indicators were significantly less, compared with those in mothers.
Keywords
Today, pregnancy is no longer considered as an absolute contraindication in women with malignant tumors, autoimmune diseases, and organ transplant recipients. Yet, the need for continuing immunosuppressive drugs in these patients represents a major issue because of the potential teratogenic or toxic effects on the fetus [1]. Therefore, administration of immunosuppressive medications carries an increased risk of pregnancy complications and adverse fetal and maternal outcomes that needs to be considered in decision-making. In this category of patients, careful planning of pregnancy and the choice of drugs to treat the underlying disease is critical to preventing maternal complications and minimizing fetal risks.
The possible teratogenic or toxic effect of the chemotherapeutic agent used during pregnancy is dependent on the total dose and gestational age. Chemotherapy during the first trimester increases the risk of spontaneous abortion, intrauterine death, and congenital disabilities [2–5]. The number of chemotherapy drugs used during pregnancy is limited because many of them can passively cross the placental barrier. Nevertheless, their fetal blood concentrations are significantly lower compared to the mother, which is attributed to changes in pharmacokinetics due to the formation of a special hormonal background, hemodynamic changes, and the presence of placental proteins that regulate the content of the drug in the fetal circulation [6]. After the completion of organogenesis in trimester II and III, the risk of developing fetal defects minimal, but some organs and systems remain vulnerable to chemotherapy toxicities. These may include the fetal immune system, which is affected by prolonged exposure to immunosuppressants.
The effects of immunosuppressive drugs on the fetal and newborn immune systems have not been sufficiently explored [7, 8]. Earlier, we conducted studies of immune status of infants exposed in utero to immunosuppressive agents due to being born to transplant recipients, autoimmune diseases, malignant neoplasms [9–11]. It was shown that compared with their mothers, the newborns had better health status during the early neonatal period and less pronounced changes in the immune system. It is of considerable interest to conduct a comparative analysis of changes in maternal and fetal immune systems exposed to immunosuppressants due immunosuppressive therapy of underlying maternal diseases during pregnancy.
This study was aimed to conduct a comparative analysis of immune function in neonates born to mothers undergoing immunosuppressive chemotherapy during pregnancy.
Material and methods
This prospective study comprised 106 children born and managed at V.I. Kulakov NMRC for OG&P of Minzdrav of Russia of Russia from 2013 to 2017. An immunological studies were conducted in newborns (n = 82) and their mothers (n= 82).
Group 1 consisted of children (n = 18) born to mothers with breast cancer (n = 10) or Hodgkin’s and non-Hodgkin’s lymphomas (n = 8); Group 2 included children (n = 16) born to mothers who are organ transplant recipients (14 kidneys, 2 liver); Group 3 included children (n = 34) born to mothers with systemic lupus erythematosus (SLE), group 4 (control) - children (n = 20) born to healthy women with normal pregnancy with no history of immunosuppressive therapy.
Blood samples were collected from a maternal peripheral vein before delivery and from umbilical cord at birth to assess maternal and newborn immune status.
Peripheral blood lymphocyte phenotyping was performed by flow cytometry on a FACSCanto II Flow Cytometer (Becton Dickinson, USA) using monoclonal antibodies to surface markers CD3, CD16, CD95, labeled with FITC, and to CD4, CD8, CD19, CD56, HLA-DR labeled with PE, (BD Biosciences, USA). Positively stained subpopulations were evaluated with corresponding IgG isotypes. A leukocyte gate, allowing other blood cells to be excluded from the analysis, was detected using monoclonal antibodies to CD45 labeled with PerCP. Data were analyzed using the FACSDiva software (Becton Dickinson, USA). The absolute lymphocyte count of the studied subpopulations was calculated based on the complete blood count. The serum concentration of immunoglobulins of classes M, A and G was determined by a turbidimetric immunoassay using commercial kits (Human, Germany).
Statistical analysis was performed using Microsoft Office Excel 2010 and MedCalc12 for Windows 7. The normality of the distribution was tested by the Shapiro-Wilk test. Quantitative variables showing normal distribution were expressed as means and standard deviation and presented as M (SD) and compared between groups with the Student t test; otherwise the Mann – Whitney U-test was used. In this case, the median (Me) and quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. Kruskal–Wallis test was used for comparing numerical data between groups. Categorical variables were reported as counts and proportions (%) and compared using the χ² test.
Results and discussion
The women included in the study were comparable in age (Table 1). Patients with breast cancer during pregnancy received polychemotherapy according to the following schemes: АС (doxorubicin and cyclophosphamide) and EC (epirubicin and doxorubicin). Pregnant women with lymphomas were administered BEACOPP (Bleomycin, Etoposide, Doxorubicin, Vincristine, Procarbazine, Prednisolone) and ABVD (Doxorubicin, Bleomycin, Vinblastine, Dacarbazine). Group 2 (women with a history of organ transplantation) received two- or three-component therapy during pregnancy: prednisone, cyclosporine, azathioprine, tacrolimus.
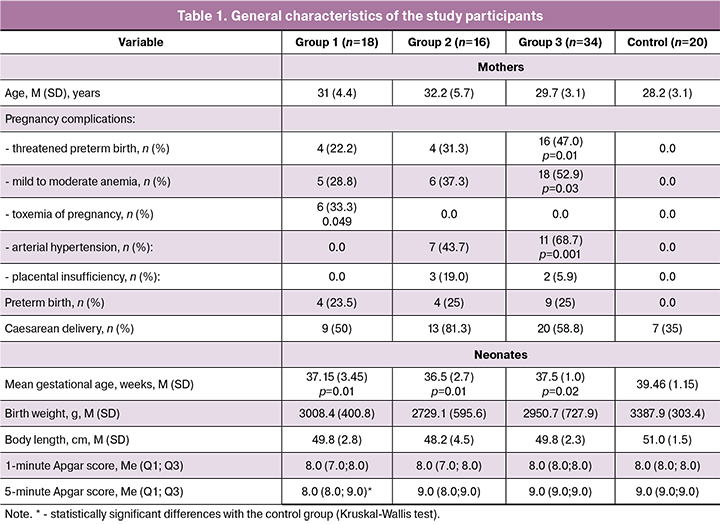
Among patients in group 3, the mean duration of SLE was 9.4 (4.3) years; 29 (85.3%), 4 (11.8%), and 1 (2.9%) of them had disease activity of grade I, II, and III, respectively. During pregnancy, they were administered two-component therapy (glucocorticosteroids and plaquenil), three-component therapy (glucocorticosteroids, plaquenil, and azathioprine) or received glucocorticosteroids without the use of cytostatics.
Table 1 summarizes the most common pregnancy complications: in women with cancer they included threatened preterm birth, mild to moderate anemia, and toxemia of pregnancy; women with transplanted organs had threatened miscarriage, anemia, and arterial hypertension; in women with SLE pregnancy complications included threatened preterm birth, anemia, activation of intravascular coagulation, and placental insufficiency.
Fifty percent of patients with cancer underwent cesarean delivery due to the need for early and more aggressive maternal anticancer therapy. In group 2, 13 patients underwent operative delivery, and only 3 had spontaneous vaginal delivery. More than half of women with SLE also had cesarean delivery. Among women with normal pregnancies (control group), a one third underwent cesarean delivery due to uterine scar from previous surgery, while the others had spontaneous delivery.
Infants born to mothers with cancer had lower birth weight and height in comparison with the control group. They also significantly differed from the control group in mean gestational age and the fifth-minute Apgar score. In this group, 4 (23.5%) babies were born prematurely, of which 1 (6.5%) was born at a gestational age below 32 weeks. Among infants born preterm to mothers with breast cancer, respiratory disorders accounted for the largest proportion of neonatal morbidity including transient tachypnea, mild asphyxiation, and small for gestational age birth. Among infants born to mothers with hemoblastosis, apnea in preterm neonates, grade 1 intraventricular hemorrhage, persistent fetal circulation, and small for gestational age birth were more often detected. Among infants born to mothers with breast cancer both treated and not treated with immunosuppressants, neonatal morbidity was is associated with preterm birth, morphofunctional immaturity, and prematurity, but not with antitumor therapy [12].
Infants born to transplant recipients also had significantly lower birth weight, height, and gestational age compared with the control group. Four (25%) babies were born preterm, of which one (6.25%) was born at a gestational age below 32 weeks. Among infants born to transplant recipients the most common causes of neonatal morbidity included a choroid plexus cyst (n = 3; 18.75%), persistent fetal circulation on the 3rd day of life (n =4; 25.0%), congenital pneumonia (n=1; 8.12%), neonatal jaundice (n = 1; (8.12%), and transient tachypnea (n = 1; 8.12%).
In group 3, the mean gestational age at delivery was significantly lower than in the control group; were no differences in the first- and fifth-minute Apgar scores. Nine (25%) babies were born prematurely, of which one (3%) was born at the gestational age below 32 weeks.
Compared with children in the control group, newborns in the study groups had lower mean values in several parameters, which can be explained by a lower mean gestational age and the presence of preterm neonates in these groups.
Pregnant women with cancer and SLE had lower leukocyte counts than women in the control group, but there were no differences in leukocyte proportions (Table 2).
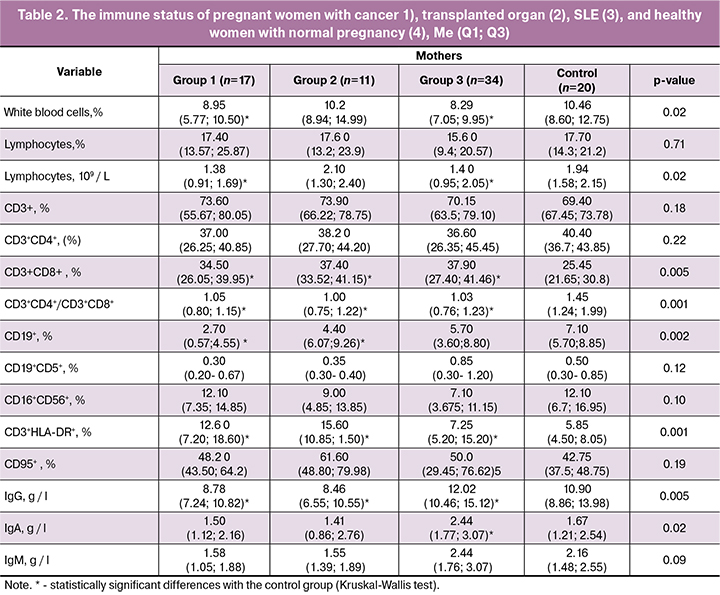
In all three study groups, pregnant women had a decreased CD3+ CD4+/CD3+ CD8+ -T-lymphocytes ratio due to an increase in the proportion of CD3 + CD8+-T cells. Besides, they had an increase in the percentage of activated T-lymphocytes (CD3+ HLA-DR+) in the peripheral blood. Among pregnant women with cancer and transplanted organs, the proportion of B2 lymphocytes (CD19+) was significantly reduced, and they had higher concentrations of IgG than women in the control group. Women with SLE had increased levels of IgG and IgA.
Currently, there is no unambiguous explanation for the cause of these changes in the content of immunocompetent cells. It cannot be ruled out that they may be associated not only with the effects of immunosuppressants. In addition to differences in the type of exposure, treatment regimens, and dosages, many factors can influence immune function, including the nature and activity of the disease. However, the similarities of changes despite differences in the etiology and pathogenesis of diseases in pregnant women imply the possibility of attributing this imbalance to the immunosuppressive effect of the chemotherapy drugs.
Children born to mothers with a transplanted organ had decreased mean total leukocyte counts; children born to mothers with cancer had decreased relative lymphocyte counts. However, all three groups did not differ from the control in absolute content (Table 3). Other authors reported mixed results. In one work, no significant differences were found in leukocyte, granulocyte, and lymphocyte counts between groups of newborns born to mothers receiving immunosuppressive therapy during pregnancy [13]. However, according to Ono et al., at birth, children born to mothers with transplanted kidneys had lower numbers of platelets, white blood cells, neutrophils and eosinophils than children from control group. [14].
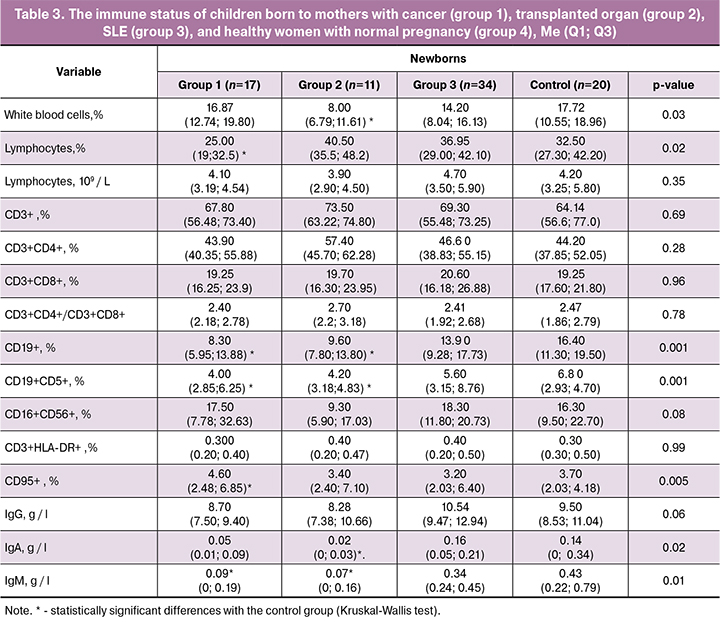
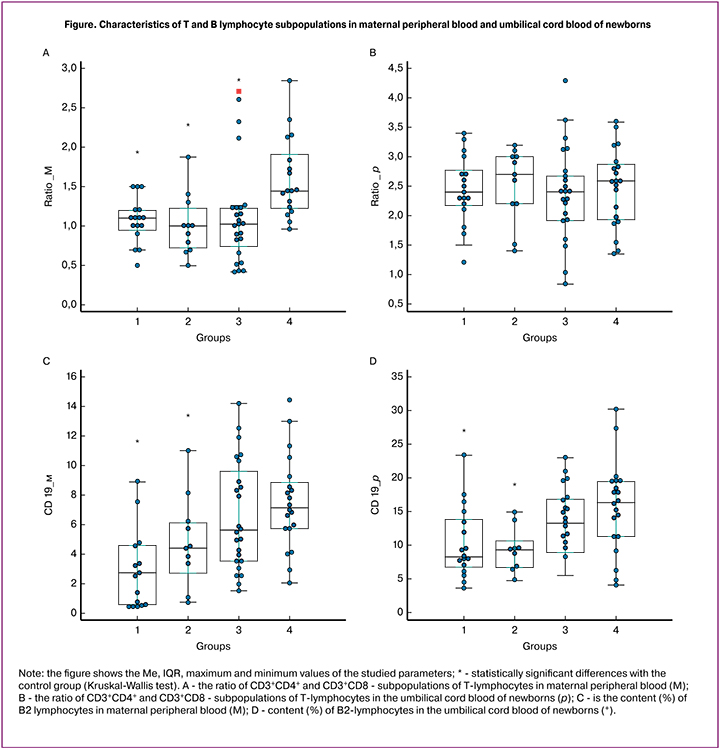
Significant changes in subpopulations of T-lymphocytes in the blood of mothers receiving immunosuppressive therapy during pregnancy were not accompanied by similar changes in the blood collected from the umbilical cord at birth. It should be noted that the ratio of CD3+CD4+ and CD3+CD8+-T-lymphocytes in the umbilical cord blood was significantly higher than in the maternal peripheral blood (Figure A, B) due to a higher proportion of CD3+CD4+ and low of CD3+CD8+-T cells in newborns than in mothers.
Noteworthy is the significant decrease in the percentage of B-lymphocytes (CD19+) in the umbilical cord blood of neonates born to mothers with cancer and transplanted organs, as well as the downward trend in the proportion of these cells in children born to women with SLE (Figure C, D).
The umbilical cord blood levels of class G immunoglobulins in newborns from the study groups did not differ from that in the control group. Statistically significant differences were found in the levels of IgM and IgA in the umbilical cord blood of children born to women with cancer, and IgA in children born to transplant recipients. Lower levels of immunoglobulins were associated with reduced B-lymphocyte counts. However, it should be noted that these groups were small, and newborns of all groups had very low concentrations of IgM and IgA in umbilical cord blood.
Therefore, the content and ratio of T-lymphocyte subpopulations in the umbilical cord blood of newborns in the study groups, who may be affected by prenatal exposure to immunosuppressants, was similar to that observed in their mothers. Based on these findings, it can be concluded that the development of the fetal immune system is not significantly affected by maternal exposure to immunosuppressive therapy. Another pattern was observed concerning fetal B-lymphocytes. B-lymphocyte counts in umbilical cord blood of babies born to mothers with cancer or transplant recipients were significantly decreased. Mothers with SLE and their newborns tended to lower counts of B-lymphocytes. Our data are consistent with studies that also showed a significant decrease in the B cell count or immunoglobulin level in the umbilical cord blood of children born to mothers undergoing immunosuppressive therapy [14–16].
It has been assumed that low B-cell counts in newborns can alter the immune response to the vaccine, and this should be considered when determining the timing of vaccination. It was previously thought that the immune alterations observed in neonates born to mothers receiving immunosuppressive drugs could interfere with vaccine responses. But currently, available evidence suggests that infants born to those mothers can have adequate serological responses without a higher percentage of adverse events [17]. At the same time, it has been suggested that children from mothers receiving immunosuppressive therapy during pregnancy should be immunized as soon as possible against vaccine-preventable diseases, since they are in constant contact with mothers who, being immunocompromised, have an increased risk of infectious diseases.
In our work, changes were found in the content and ratio of immunocompetent cells in pregnant women during immunosuppressive therapy, which in most cases do not result in the suppression of the immune system. No significant changes were found in the immune status of neonates born to these mothers compared with newborns in the control group, which may indicate an insignificant effect on the embryo and fetus of maternal immunosuppressive therapy that is necessary to suppress the activity of the underlying disease and ensure successful completion of pregnancy.
Conclusion
Children born to women undergoing immunosuppressive therapy often have low length and weight growth rates and are small for gestational age. The increased complication rate in the early neonatal period is associated with lower gestational age, and not with fetal exposure to maternal chemotherapy.
Mothers with various diseases undergoing immunosuppressive therapy have an imbalance in cellular immunity, which is manifested by a decrease in the ratio of CD3+CD4+ and CD3+CD8+ subpopulations of T-lymphocytes and B lymphocyte count, and an increase in activated T-lymphocyte counts.
In newborns undergoing intrauterine exposure to maternal immunosuppressant therapy, there were smaller differences in parameters with the control group than that in their mothers. B-lymphocyte counts in the umbilical cord blood were decreased, but no significant changes were found in the content and ratio of T-lymphocyte subpopulations. Considering these findings, it can be concluded that fetal T-cell development is not significantly affected by maternal exposure to immunosuppressive therapy; however, to a certain extent, this therapy affects the development of B-cell immunity.
Significant differences in the ratios of T-lymphocytes of different phenotypes are found between the parameters of the maternal peripheral blood of mothers and the umbilical cord blood of their newborns. The ratio of CD4+ and CD8+ T-lymphocytes in newborns in all groups was significantly higher than in mothers, while the proportion of activated T-lymphocytes (CD3+HLA-DR+) was lower.
Selection of appropriate dosing regimen and the multidisciplinary integrated care aimed at maximizing maternal benefit without apparent fetal harm helps ensure a successful pregnancy outcome without serious negative impacts on the formation and development of the cellular basis of fetal adaptive immunity.
References
- Ponticelli C., Moroni G. Fetal Toxicity of Immunosuppressive Drugs in Pregnancy. Clin Med. 2018; 7(12): 552. doi: 10.3390/jcm7120552
- Brewer M., Kueck A., Runowicz C.D. Chemotherapy in pregnancy. Clin. Obstet. Gynecol. 2011; 54(4): 602-18. doi: 10.1097/GRF.0b013e318236e9f9.
- Ponticelli C., Moroni G. Immunosuppression in pregnant women with systemic lupus erythematosus. Expert Rev. Clin. Immunol. 2015; 11: 549-552. doi: 10.1586/1744666X.2015.1033404
- Martín M.C., Cristiano E., Villanueva M., Bonora M.L., Berguio N., Tocci A., Groisman B. et al. Esophageal atresia and prenatal exposure to mycophenolate. Reprod. Toxicol. 2014; 50: 117-121. doi: 10.1016/j.reprotox.2014.10.015.
- Walton J.R., Prasad M.R. Obstetric and neonatal outcomes of cancer treated during pregnancy. Clin. Obstet. Gynecol. 2011; 54(4): 567-73. doi: 10.1097/GRF.0b013e318236e781
- Esposito S., Tenconi R., Preti V., Groppali E., Principi N. Chemotherapy against cancer during pregnancy. Medicine (Baltimore). 2016; 95(38): e4899. doi: 10.1097/MD.0000000000004899.
- Motta M., Ciardelli L., Marconi M., Tincani A., Gasparoni A., Lojacono A., Chirico G. Immune system development in infants born to mothers with autoimmune disease, exposed in utero to immunosuppressive agents. Am. J. Perinatol. 2007; 24(8): 441-7.
- Dinelli M.S., Ono E., Viana P.O., Spina F.G., Weckx L.Y., Dos Santos A.M.N., Moraes-Pinto M. Response to immunization in children born to renal transplant recipients using immunosuppressive drugs during gestation. Vaccine. 2016; 34(4): 404-407. doi: 10.1016/j.vaccine.2015.12.017.
- Шаталова Е.А., Матвеева Н.К., Ванько Л.В., Кравченко Н.Ф., Жукова А.С., Макиева М.И., Кречетова Л.В., Зубков В.В. Клинико-иммунологическая характеристика новорожденных у матерей с трансплантированными органами. Акушерство и гинекология. 2018; 11: 106-113. [Shatalova E.A., Matveeva N.K., Vanko L.V., Kravtchenko N.F., Zhukova A.C., Makieva M.I., Krechetova L.V., Zubkov V.V. Clinical and immunological characteristics of newborns in mothers with transplanted organs. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2018; 11: 106-113. (in Russ.)]. doi: https://dx.doi.org/10.18565/aig.2018.11.106-113
- Макиева М.И., Матвеева Н.К., Ванько Л.В., Цой Т.А., Жукова А.С., Шаталова Е.А., Полушкина Е.С., Аверьянова М.В., Тимофеева Л.А., Кречетова Л.В., Зубков В.В., Шмаков Р.Г. Клинико-иммунологическая характеристика новорожденных у матерей с онкологическими заболеваниями. Акушерство и гинекология. 2017; 11: 92-99. [Makieva M.I., Matveeva N.K., Vanko L.V., Tsсoy T.A., Zhukova A.C., Shatalova E.A.,Polushkina E.C., Averyanova M.V., Timofeeva L.A., Krechetova L.V., Zubkov V.V., Shmakov R.G. Clinical and immunological characteristics of newborns in mothers with cancer. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2017; 11: 92-99. (in Russ.)]. doi: https://dx.doi.org/10.18565/aig.2017.11.92-99
- Матвеева Н.К., Ванько Л.В., Федорова Е.В., Беляева А.С., Клименченко Н.И., Николаева М.А., Сафронова В.Г., Кошелева Н.М., Кречетова Л.В., Сухих Г.Т. Особенности клеточного звена иммунитета у беременных женщин с системной красной волчанкой и их новорожденных. Акушерство и гинекология. 2014; 12: 48-56. [Matveeva N.K., Vanko L.V., Fedorova E.V., Belyaeva A.C., Klimenchenko A.I., Nikolaeva M.A., Safronova V.G., Kosheleva N.M., Krechetova L.V., Sukhikh G.T. Features of cellular immunity in pregnant women with systemic lupus erythematosus and their newborns. Obstetrics and gynecology. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2014; 12: 48-56. (in Russ.)].
- Волочаева М.В., Шмаков Р.Г., Зубков В.В. Здоровье детей, рожденных женщинами с раком молочной железы, связанным с беременностью. Акушерство и гинекология. 2014; 7: 33-37. [Volochaeva M.V., Shmakov R.G., Zubkov V.V. Health of children born to women with pregnancy-related breast cancer. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2014; 7: 33-37.(in Russ.)].
- Kociszewska-Najman B., Pietrzak B., Cyganek A., Szpotanska-Sikorska M., Schreiber-Zamora J., Jabiry-Zieniewicz Z., Wielgos M. Intrauterine Hypotrophy and Premature Births in Neonates Deliveredby Female Renal and Liver Transplant Recipients. Transplantation Proceedings. 2011; 43: 3048-51. doi: 10.1016/j.transproceed.2011.08.041.
- Ono E., dos Santos A. M., Viana P. O., Dinelli M. I. S., Sass N., De Oliveira L. et al. Immunophenotypic Profile and Increased Risk of Hospital Admission for Infection in Infants Born to Femalе Kidney Transplant Recipients. Am J Transpl. 2015; 15(6): 1654-65.
- Dörner T., Jacobi A.M., Lee J., Lipsky P.E.. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J. of Immunol. Methods. 2011; 363(2): 187-197.
- Drozdowska-Szymczak A., Pietrzak B., Czaplińska N., Schreiber-Zamora J., Jabiry-Zieniewicz Z., Wielgoś M., Immunological Status of Children Born to Female Liver Recipients. Ann Transplant. 2018; 23: 182-189. doi: 10.12659/AOT.907930
- Dinelli M.I.S., Ono E., Viana P. O., Spina F. G., Weckx L.Y., dos Santos A.M.N., de Moraes-Pinto M.I. Response to immunization in children born to renal transplant recipients using immunosuppressive drugs during gestation. Vaccine. 2016; 34: 404-407. https://doi.org/10.1016/j.vaccine.2015.12.017
Received 03.06.2019
Accepted 21.06.2019
About the Authors
Ludmila V. Vanko, Doctor of Medicine, professor, leading researcher of clinical immunology laboratory of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation., telephone +7(495)438 11 83.E-mail: lvanko@mail.ru; 4, Acad. Oparin street, Moscow, Russian Federation 117997.
Victor V. Zubkov, Doctor of Medicine, Director of The Institute for Neonatology and Pediatrics of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation, Professor of the Department of Neonatology Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Healthcare of the Russian Federation.
Phone: +7(495)438 22 66. E-mail: v_zubkov@oparina4.ru; 4, Acad. Oparin street, Moscow, Russian Federation, 117997.
Lubov`V. Krechetova, Doctor of Medicine, head of clinical immunology laboratory National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I.Kulakov of the Ministry of Healthcare of Russian Federation. Phone: +7(495)438-1183. E-mail: k_l_v_@mail.ru. 4, Acad.
Oparin street, Moscow, Russian Federation, 117997.
Mzia I. Makieva, neonatologist, pediatrician, the head of the Department of newborns National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of the Ministry of Healthcare of Russian Federation, phone:+7(495)531 44 44. E-mail: m_makieva@oparina4.ru. 4, Acad.
Oparin street, Moscow, Russian Federation, 117997.
Elena N. Baybarina, MD, Director of Department for Mother and Child Healthcare, Ministry of Health of the Russian Federation, professor.
Phone: +7 (495) 627 24 00 1500BaybarinaEN@rosminzdrav.ru Rakhmanovsky l., 3, Moscow, 127948.
Ekaterina A. Shatalova, neonatologist of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation, phone: +7(495)438 11 83. E-mail: e_shatalova@oparina4.ru; 4, Acad. Oparin street, Moscow, Russian Federation, 117997,
Anastasia S. Zhukova, candidate of biological sciences, research worker of clinical immunology laboratory National Medical Research Center for Obstetrics,
Gynecology and Perinatology named after Academician V.I.Kulakov of the Ministry of Healthcare of Russian Federation, phone: +7(495)438 11 83.
E-mail: a_belyaeva@oparina4.ru; 4, Acad. Oparin street, Moscow, Russian Federation, 117997.
Nataliya I. Klimenchenko, candidate of medicine sciences, senior researcher of Department for obstetric and extra genital pathology National Medical Research Center
for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of the Ministry of Healthcare of Russian Federation, phone: +7 +7(495)438 06 74.
E-mail: natalite@list.ru; 4, Acad. Oparin street, Moscow, Russian Federation, 117997,
Roman G. Shmakov, Doctor of Medicine, Director of The Institute for Obstetrics of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of the Ministry of Healthcare of Russian Federation, phone: +7(495)438 72 00. E-mail: r_shmakov@oparina4.ru; 4, Acad.
Oparin street, Moscow, Russian Federation, 117997
For citations: Vanko L.V., Zubkov V.V., Krechetova L.V., Makieva M.I., Baybarina E.N., Shatalova E.A., Zhukova A.С., Klimenchenko N.I., Shmakov R.G. The influence of immunosuppressive therapy during pregnancy on the immune system of the mother and the newborn.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2019; 11:113-21.(In Russian).
https://dx.doi.org/10.18565/aig.2019.11.113-121



