The impact of the immune status on COVID-19 severity
Aim. To investigate the impact of patient immune status on the severity of COVID-19.Dolgushina N.V., Krechetova L.V., Ivanets T.Yu., Vtorushina V.V., Inviyaeva E.V., Sukhikh G.T.
Materials and methods. The prospective study included 63 employees of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia with confirmed COVID-19. The patients were stratified into three groups based on the disease severity, including asymptomatic (group 1, n=17), mild (group 2, n=29), and moderate (group 3, n=17) form of COVID-19. On days 3–7 from the onset of the disease, peripheral venous blood samples were collected from the study subjects and tested for serum levels of anti-SARS-CoV-2 IgG antibodies and immune profile by ELISA. After day 20+, testing for serum levels of anti-SARS-CoV-2 IgG antibodies was repeated using ELISA.
Results. Patients who had a higher BMI, blood group A(II), lower leukocyte and lymphocyte counts, higher relative monocyte count, changes in the immune profile in the form of a lower number of CD3+, CD3+CD8+, СD19+, CD19+CD5+, and phagocytic activity of neutrophils, developed more severe forms of COVID-19. They had severe clinical manifestations of the disease, and 100% of them developed antiviral immunity.
Conclusion. This study identified several clinical, laboratory, and immune profile features that may be considered as predictive factors of severe COVID-19 and can be used in clinical practice to predict the clinical course of the disease.
Keywords
The novel coronavirus epidemic first broke out in China at the end of 2019. On 02/11/2020, the World Health Organization announced COVID-19 (Coronavirus disease 2019) as the name of this new disease, and the International Committee on Taxonomy of Viruses has named its causative agent SARS-CoV-2.
Currently, the evidence regarding epidemiology, clinical features, prevention, and management of COVID-19 is limited and inconclusive.
The clinical spectrum of COVID-19 could range from asymptomatic to severe bilateral pneumonia, multiple organ failure, and sepsis [1]. At the same time, there has been an ongoing search for causes and predictors affecting the severity of COVID-19 [2–4].
The immune response to viral infection plays a significant role in the development of severe forms of the disease [5, 6]. There is preliminary evidence of a direct relationship between COVID-19 severity and the intensity of humoral immune response, development of the «cytokine storm», and systemic inflammatory response [6].
Considering this, investigating predictors, causes, and concomitant factors affecting COVID-19 severity could make an essential contribution to understanding the pathogenesis of the severe forms of the disease and help develop therapeutics for treatment and prevention of these complications.
The present study aimed to investigate the impact of patient immune status on the severity of COVID-19.
Materials and methods
The prospective study included 63 employees of the V.I. Kulakov NMRC for OG&P of Ministry of Health of Russia infected with COVID-19. The inclusion criteria were a confirmed diagnosis of COVID-19, age 18+ years, signed informed consent for participation in the study, and the possibility of blood sampling on days 3–7 and 20+ after the onset of the disease (the development of clinical symptoms or a positive SARS-CoV-2 test). The exclusion criteria were HIV infection and other congenital and acquired immunodeficiencies, any chronic infectious, oncological, autoimmune and rheumatic diseases, pregnancy and lactation for women, taking immunomodulatory drugs for at least three months before the onset of the disease and during the illness.
Depending on the severity of the disease, the employees were stratified into three groups: group 1–17 people with an asymptomatic form of the disease, group 2–29 people with a mild form of the disease, group 3–17 people with a moderate form of COVID-19.
The patients were stratified into three groups based on the disease severity, including asymptomatic (group 1, n=17), mild (group 2, n=29), and moderate (group 3, n=17) form of COVID-19.
The criteria for the asymptomatic disease were positive SARS-CoV-2 RNA test by RT-PCR on an oropharyngeal swab in the absence of any clinical manifestations of the disease. The criteria for a mild COVID-19 were the detection of SARS-CoV-2 RNA by RT-PCR on an oropharyngeal swab in combination with the clinical manifestations, including subfebrile fever (<38°C) and no criteria for a severe and moderate infection. The criteria for the moderate disease were the detection of SARS-CoV-2 RNA by RT-PCR on an oropharyngeal swab in combination with any of the following clinical manifestations: fever (≥38°C), shortness of breath on exertion, and typical chest computed tomography (CT) findings with minimal or moderate lung involvement.
The virus was identified using the Reagent kit for detecting RNA of SARS-CoV coronaviruses and similar SARS-CoV by real-time reverse transcription and polymerase chain reaction (SARS-CoV-2/SARS-CoV) (DNA-Technology, Russia). Three regions of the genome were selected as targets, including coronavirus ЅARЅ-CoV-2 specific regions of the N and E genes, as well as the conserved region of the E gene, typical for a group of coronaviruses similar to ЅARЅ-CoV(including ЅARЅ-CoV and ЅARЅ-CoV-2). Amplification was performed on a DT-964 device (NPO DNA-Technology LLC, Russia). The results were processed automatically using the device software.
On days 3–7 from the onset of the disease, peripheral venous blood samples were collected from the study subjects and tested for serum levels of anti-SARS-CoV-2 IgG antibodies and immune profile, including total lymphocyte count with an analysis of the composition of lymphocyte subpopulation: CD3+, CD3+CD4+, CD3+CD8+, CD19+, CD3-CD56+CD16+, CD3+CD56+CD16+, CD19+CD5+, Treg, with the calculation of the ratio of T-lymphocytes with cytotoxic and helper function (CD8+/CD4+), assessment of peripheral blood activated lymphocyte count (CD3+HLA-DR+, CD3+CD25+, CD25+), as well as the phagocytic activity of neutrophils (PAN) with the calculation of the stimulation index (SI). On day 20+ from the onset of the disease, testing for serum levels of anti-SARS-CoV-2 IgG antibodies was repeated.
Serum levels of anti-SARS-CoV-2 IgG antibodies were measured with reagent kits for enzyme-linked immunosorbent assay (ELISA) «SARS-CoV-2-IgG-ELISA» produced by the National Research Center for Hematology of Minzdrav of Russia. The results were recorded by Tecan's Infinite® F50 microplate reader (Tekan, Austria). According to the manufacturer's instructions, the test is intended for the qualitative and semi-quantitative determination of antibodies. The results were interpreted by the calculation of the positivity index (PI) by the formula: PI = OD of the sample/Cut-off, where OD is the optical density of the sample. With PI> 1.1 and PI <0.9, the sample was considered positive and negative, respectively. PI value ranging from 0.9 to 1.1 was deemed to be ambiguous. For samples in which results were ambiguous, a second sample was obtained and retested.
Phenotyping of peripheral blood lymphocytes was performed by flow cytometry using monoclonal antibodies (mAbs) labeled with FITC or PE against antigens CD3(FITC), CD4(PE), CD5(PE), CD8(PE), CD16(PE), CD19(FITC), CD56(PE), CD25(FITC), and HLA-DR(FITC) (Becton Dickinson and eBioscience, USA). The distribution of the main immunocompetent T cell subpopulations (CD3+, CD4+, CD8+), B cells (CD19+), B1 cells (CD19+ CD5+), NK cells (CD56+ CD16+) was assessed. The lymphocyte gate, which allows the exclusion of other blood cells from the analysis, was detected using anti-CD45 mAbs labeled with peridinin chlorophyll protein (PerCP) (Dako, Denmark). Corresponding FITC or PE-labeled isotypic IgGs were used to assess positively stained subpopulations.
Whole blood Tregs with the intracellular expression of FOXP3 were defined as a subpopulation with the СD4+CD25highCD127low phenotype using a combination of mAbs to CD4 antigens labeled with PerCP (eBioscience, USA), CD25 labeled with FITC (Becton Dickinson, USA) and PE-labeled CD127 (eBioscience, USA). The proportion of Tregs among CD4+ cells was estimated. Monoclonal antibodies were added directly to whole blood and then treated with FACS Lysing Solution (Becton Dickinson, USA).
PAN was assessed using the FagoFlow method (ExBio, Czech Republic). The test is based on the assessment of the oxidative burst in granulocytes after stimulation with E. coli. The ratio of the mean fluorescence intensity (SIF) of activated granulocytes of stimulated samples and negative controls reflects the intensity of the oxidative burst of granulocytes after stimulation with E. coli; it is referred to as the stimulation index (SI).
Lymphocyte phenotyping and PAN assessment were performed on a Gallios Flow Cytometer (Beckman Coulter, USA) using the Kaluza software.
Statistical analysis
Statistical analysis was performed using Statistica 10 software package (USA). The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as (M ± SD). Categorical variables were compared by the χ2-test. Continuous variables were compared between two groups using the two-sample t-test and among three groups using an analysis of variance (ANOVA). Therelationshipoftheidentifiedpredictorswith different forms of COVID-19 was assessed by multivariate analysis (logistic regression) using the forward stepwise predictor selection procedure. Differences between the groups were considered statistically significant at p<0.05.
The Research Ethics Committee of the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology approved this study.
Results
The gender and mean age of the patients did not differ statistically significantly in the study groups (p˃0.05), although patients with more severe COVID-19 tended to be older and male. Body mass index (BMI) was higher in the group of patients with more severe disease (p=0.0028). A(II) blood group was the most common (n=36; 57.1%). Also, there were more patients with A(II) blood group among patients with clinical manifestations of infection, and patients with 0(I) blood group were statistically significantly more likely to be asymptomatic (p=0.0370). The comorbidity rates were generally low and did not differ significantly between the study groups (Table 1).
The duration of clinical manifestations of the disease, on average, was (11.5±7.4) days (from 3 to 30 days). In groups 2 and 3 it was (9.8±6.7) and (14.5 ± 7.9) days, respectively (p=0.0397). Among 46 symptomatic individuals, the most common clinical manifestations (in descending order of frequency) were loss of smell, fever, headache, fatigue, myalgia, and cough, which were observed in 36 (78.3%), 34 (73.9%), 31 (67.4%), 30 (65.2%), 21 (45.6%), and 19 (41.3%) patients, respectively. Pneumonia confirmed by chest computed tomography (CT-1 or CT-2) was diagnosed in 12 patients. All symptomatic employees were managed in outpatient settings. The most frequently administered therapy included broad-spectrum antibiotics (32/46; 69.5%) and low molecular weight heparins (LMWH) in prophylactic dosages (17/46; 36.9%).
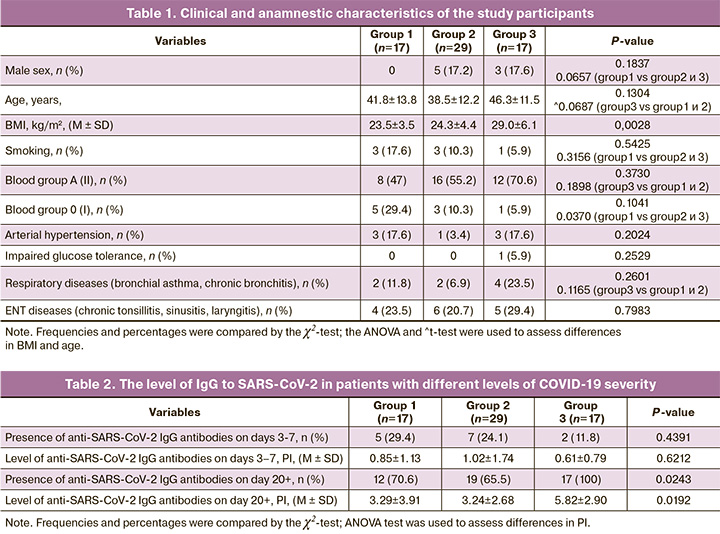
On days 3–7 from the onset of the disease, seroconversion (a change from a seronegative to a seropositive condition) was observed in only 14 people (22.2%), while on day 20+ from the onset of the disease, antibodies were detected in 48 people (76.2%). At the same time, antibodies were detected in 100% of individuals with moderate cases of COVID-19, in contrast to groups 1 and 2 (70.6% and 65.5%); antibody levels were significantly higher in group 3 (p=0.0192) (Table 2, Fig. 1).
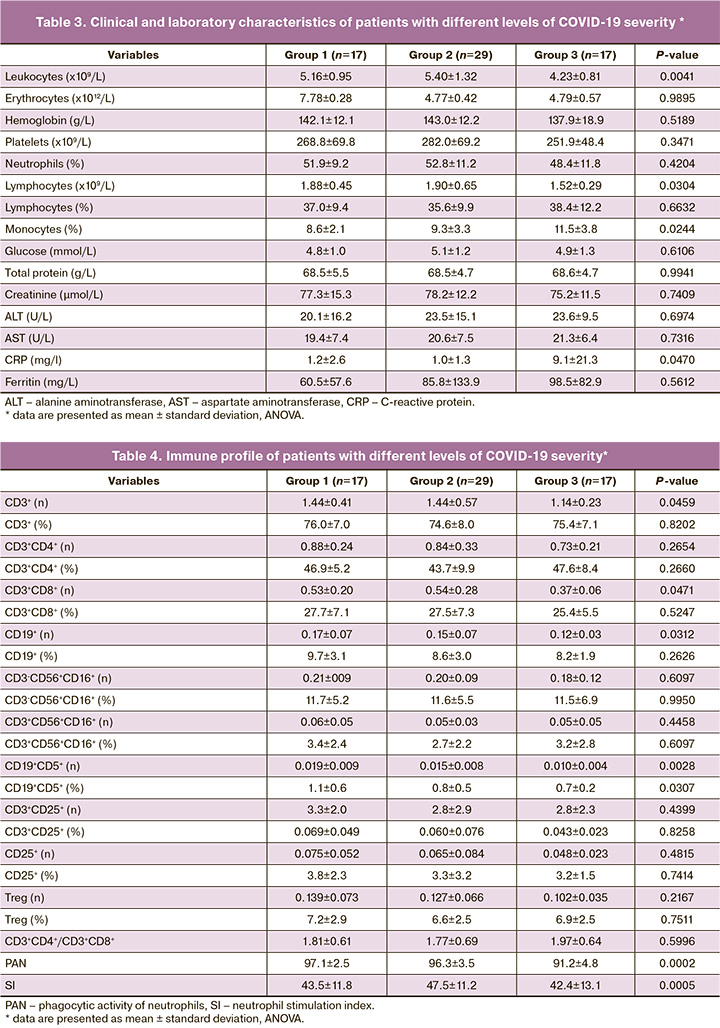
 On days 3–5 of the disease, employees with moderate cases of COVID-19 (group 3) were found to have lower counts of leukocytes and lymphocytes and higher relative levels of monocytes and C-reactive protein (CRP) (p <0.05). The findings of blood biochemistry tests did not differ significantly between the groups (Table 3, Fig. 2).
On days 3–5 of the disease, employees with moderate cases of COVID-19 (group 3) were found to have lower counts of leukocytes and lymphocytes and higher relative levels of monocytes and C-reactive protein (CRP) (p <0.05). The findings of blood biochemistry tests did not differ significantly between the groups (Table 3, Fig. 2).
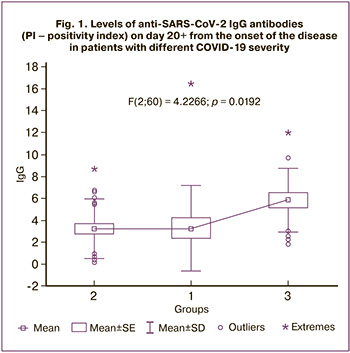
On days 3–5 of the disease, employees with moderate cases of COVID-19 (group 3) were found to have a lower absolute number of CD3+, CD3+CD8+, СD19+, CD19+CD5+, PAN, and SI (Table 4, Fig. 3).
Note: The axes show the ratios of the mean values of significantly different parameters of the immune profile of employees with moderate COVID-19 to the mean values of the immune profile parameters of asymptomatic individuals: gray line – the line of equality of values with the parameters of the immune profile of asymptomatic individuals; the dotted line characterizes the level of parameters in employees with a mild course of the disease, the solid line – in employees with a moderate course of COVID-19
The likelihood of developing a more severe form of COVID-19, depending on the studied clinical and laboratory data, was calculated using multivariate analysis. The prediction model included factors that showed statistical significance when building a multivariate model: BMI, absolute leukocyte count, absolute lymphocyte count, and PAN:

where P (COVID) is the likelihood of developing a more severe form of COVID-19, Exp is the exponent, BMI is the body mass index, Leuk is the absolute leukocyte count serum (x109/L), Lymph is the absolute lymphocyte count (x109/l), PAN – the phagocytic activity of neutrophils.
Therefore, a higher BMI, lower absolute leukocyte and lymphocyte counts, and low PAN levels predispose to the development of a more severe form of COVID-19.
Discussion
The findings of the present study suggest that the development of more severe forms of SARS-CoV-2 infection was associated with a higher BMI (p = 0.0028) and blood group (p = 0.0370). Employees with A (II) blood group more likely to develop more severe disease, while employees with 0 (I) blood group more often were asymptomatic. Our data are consistent with those of Zhao J. et al. and other researchers, who analyzed a large cohort of patients with COVID-19 (n=2173) and also found an increased incidence in people with AV blood phenotype and a low incidence in people with 0 (I) blood group [3, 8]. This can be explained by the influence of AB0 antigens on the immune system and the influence of protective antibodies and the complement system on the spread of pathogens [9]. It was suggested that some viruses including SARS [11] could bind to AB0 antigens and thus spread in the human body [10]. Guillon P. et al., who built a mathematical model of the virus transmission dynamics, suggested that in people with 0 (I) blood group, anti-histo-blood group antibodies have a protective antiviral function [12].
With regard to BMI, there has already been evidence that overweight and obese individuals are 2.3 times more likely to develop severe infections, as evidenced by a meta-analysis [13]. This association has been attributed to the frequent combination of obesity with somatic, endocrine, and other diseases, metabolic and immune disorders. We did not get a statistically significant difference in the number of males and older persons in the group with a more severe form of the disease, although there was such a trend. Male sex and older age, along with obesity, are proven risk factors for the development of severe COVID-19 [1, 2]. The absence of a significant difference in sex and age in our study is due to the small number of men and the relatively young age of the employees included in the study. The same applies to the incidence of comorbidities among employees, which did not differ significantly between groups due to their low prevalence in the study cohort.
Twenty days after the onset of the disease, IgG antibodies were detected in most employees, and the development and intensity of antiviral immunity were associated with COVID-19 severity. Seroconversion was observed in 100% of moderate cases and only in 2/3 of employees with mild and asymptomatic infection (p=0.0243). These observations are consistent with the literature, according to which the majority of patients experience seroconversion during the first three weeks of the disease, IgM and IgG seroconversion co-occurs, and reaches a peak at 2–3 weeks of the disease [14]. It was also shown that in mild cases, the level of antibodies was lower than in patients with more severe forms of the disease [15]. We did not find literature data on antiviral immune response in asymptomatic individuals. At the same time, numerous studies reported antiviral immune response only in patients with clinical manifestations of COVID-19. Thus, our research is one of the first to demonstrate seroconversion in the asymptomatic COVID-19 and show that the intensity of specific antiviral immune response in the absence of symptoms does not differ from that in the mild form of the disease. Our findings suggest that patients with more severe forms of infection, at the onset of the disease, have a lower leukocyte and lymphocyte counts and higher relative levels of monocytes and CRP (p<0.05). Our data do not agree with the data of some researchers, who reported higher leukocyte counts and a lower level of monocytes in more severe forms of infection [16, 17]. At the same time, according to other studies, the development of COVID-19 is associated with lower leukocyte and lymphocyte counts compared with SARS-CoV-2 negative patients [18, 19]. This difference in data is likely due to different study designs. Logic dictates that in advanced forms of the disease, the blood cell composition shifts in a pro-inflammatory direction (leukocytosis, neutrophilia, relative lymphopenia, left shift to band neutrophils), which is shown in cross-sectional studies. A low level of immune cells before the onset of COVID-19 (longitudinal studies) is associated with the development of more severe forms of the disease. At the same time, the higher relative level of monocytes and CRP revealed in our study in the group with a more severe form of infection may reflect the body's protective response to viral invasion due to an already developing disease (3–7 days from the onset of the disease).
Analysis of the immune profile on days 3–5 of the disease confirmed that people with the subsequent development of a more severe form of the disease are characterized by a lower immune cell count (CD3+, CD3+CD8+, СD19+, CD19+CD5+). Similar data were obtained Xu B. et al., who found that people with severe COVID-19 showed a significant decrease in all fractions of T and B lymphocytes [20]. Studies by Chihuan Q. et al. and Jin-Wen S. et al. also confirm the presence of lymphopenia in patients with severe COVID-19 due to a deficiency of all major subpopulations of immune cells. The authors attribute these observations to dysregulation of the immune response, which is associated with the unregulated activation of CD3+CD8+cells [21, 22]. Our study showed a tendency to a similar immune response in moderate COVID-19 cases.
It is interesting to note a significant decrease in phagocytic activity of neutrophils in individuals who subsequently developed severe COVID-19 and not only reduced production of reactive oxygen species by neutrophils (oxidative burst) but also reduced intensity of their production, reflected in the stimulation index (SI). Phagocytic activity of neutrophils demonstrates the ability of these blood macrophages (one of the main cells capable of phagocytosis) to ingest and destroy pathogens like bacteria, viruses, and damaged cells. A decrease in phagocytic activity of neutrophils can be observed with chronic infectious diseases, immunodeficiencies, neoplasms, the use of immunosuppressants (excluded in our study), as well as with congenital defects of the phagocytic system, malabsorption, malnutrition, etc. PubMed currently includes only a limited number of publications investigating the relationship between PAN and COVID-19 [23]. A decrease in the innate immunity activity in the form of impaired neutrophilic phagocytosis of pathogens can negatively affect the development of any infectious and inflammatory process, including those caused by SARS-CoV-2.
At the final stage, we proposed a predictive model, which included factors that showed statistical significance in constructing a multivariate model, namely BMI, absolute leukocyte count, absolute lymphocyte count, and PAN. This model can be used to predict the development of a more severe COVID-19.
Conclusion
Our study identified several clinical, laboratory, and immune profile features that may be considered as predictive factors of severe COVID-19 and can be used in clinical practice to predict the clinical course of the disease.
References
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395(10223): 507-13. https://dx.doi.org/10.1016/S0140-6736(20)30211-7.
- Age, sex, existing conditions of COVID-19. Cases and deaths. Current statistics. Available at: https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/
- Zhao J., Yang Y., Huang H., Li D., Gu D., Lu X. et al. Relationship between the AB0 blood group and the COVID-19 susceptibility. Clin. Infect. Dis. 2020; ciaa1150. https://dx.doi.org/10.1093/cid/ciaa1150.
- Patanavanich R., Glantz S.A. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob. Res. 2020; 22(9): 1653-6. https://dx.doi.org/10.1093/ntr/ntaa082.
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. et al. Coronavirus infections and immune responses. J. Med. Virol. 2020; 92(4): 424-32. https://dx.doi.org/ 10.1002/jmv.25685.
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017; 39(5): 529-39. https://dx.doi.org/10.1007/s00281-017-0629-x.
- Временные методические рекомендации «Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19)». Версия 7 (утв. Министерством здравоохранения РФ 3 июня 2020 г.). [Temporary guidelines "Prevention, diagnosis and treatment of new coronavirus infection (COVID-19)". Version 7 (approved by the Ministry of health of the Russian Federation on June 3, 2020) (in Russian)]. https://base.garant.ru/74212510/.
- Wu Y., Feng Z., Li P., Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin. Chim. Acta. 2020; 509: 220-3. https://dx.doi.org/10.1016/j.cca.2020.06.026.
- Cooling L. Blood groups in infection and host susceptibility. Clin. Microbiol. Rev. 2015; 28(3): 801-70. https://dx.doi.org/10.1128/CMR.00109-14.
- Lee B., Dickson D.M., DeCamp A.C., Ross Colgate E., Diehl S.A., Uddin M.I. et al. Histo-blood group antigen phenotype determines susceptibility to genotype-specific rotavirus infections and impacts measures of rotavirus vaccine efficacy. J. Infect. Dis. 2018; 217(9): 1399-407. https://dx.doi.org/ 10.1093/infdis/jiy054.
- Cheng Y., Cheng G., Chui C.H., Lau F.Y., Chan P.K.S., Ng M.H. et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005; 293(12): 1450-1. https://dx.doi.org/10.1001/jama.293.12.1450-c.
- Guillon P., Clément M., Sébille V., Rivain J.-G., Chou C.-F., Ruvoën-Clouet N. et al. Inhibition of the interaction between the SARS-CoV spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008; 18(12): 1085-93. https://dx.doi.org/10.1093/glycob/cwn093.
- Yang J., Hu J., Zhu C. Obesity aggravates COVID-19: a systematic review and meta-analysis. J. Med. Virol. 2020; 10.1002/jmv.26237. https://dx.doi.org/ 10.1002/jmv.26237.
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y-K. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020; 26(6): 845-8. https://dx.doi.org/10.1038/s41591-020-0897-1.
- Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S. et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020; (6): CD013652. https://dx.doi.org/10.1002/14651858.CD013652.
- Zhang H., Cao X., Kong M., Mao X., Huang L., He P. et al. Clinical and hematological characteristics of 88 patients with COVID-19. Int. J. Lab. Hematol. 2020; 10.1111/ijlh.13291. https://dx.doi.org/10.1111/ijlh.13291.
- Elshazli R.M., Toraih E.A., Elgaml A., El-Mowafy M., El-Mesery M., Amin M.N. et al. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: a meta-analysis of 6320 patients. PLoS One. 2020; 15(8): e0238160. https://dx.doi.org/ 10.1371/journal.pone.0238160.
- Ferrari D., Motta A., Strollo M., Banfi G., Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clin. Chem. Lab. Med. 2020; 58(7): 1095-9. https://dx.doi.org/10.1515/cclm-2020-0398.
- Cheng Z., Lu Y., Cao Q., Qin L., Pan Z., Yan F. et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am. J. Roentgenol. 2020; 215(1): 121-6. https://dx.doi.org/10.2214/AJR.20.22959.
- Xu B., Fan C.-Y., Wang A.-L., Zou Y.-L., Yu Y.-H., He C. et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J. Infect. 2020; 81(1): e51-e60. https://dx.doi.org/ 10.1016/j.jinf.2020.04.012.
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020; 71(15): 762-8. https://dx.doi.org/10.1093/cid/ciaa248.
- Song J.W., Zhang C., Fan X., Meng F.P., Xu Z., Xia P. et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020; 11(1): 1-10. https://dx.doi.org/10.1038/s41467-020-17240-2.
- Merad M., Martin J.C. Author Correction: Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020; 20(7): 448. https://dx.doi.org/10.1038/s41577-020-0353-y.
Received 28.08.2020
Accepted 02.09.2020
About the Authors
Nataliya V. Dolgushina, Dr. Med. Sci., Deputy Director – Head of the Department of Research Administration, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.Tel.: +7(495)438-49-77. E-mail: n_dolgushina@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Liubov V. Krechetova, Dr. Med. Sci. , Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(495)438-11-83.
E-mail: l_krechetova@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Tatiana Yu. Ivanets, Dr. Med. Sci., Head of the Clinical Diagnostic Laboratory, V.I. Kulakov NMRC for OG & P of Minzdrav of Russia. Tel.: +7(910)404-26-69.
Е-mail: t_ivanets@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Valentina V. Vtorushina, M.D., Ph.D., Allergist-Immunologist at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel: +7(916)980-78-95. E-mail: vtorushina@inbox.ru. 4 Oparin str., 117997, Moscow, Russia.
Eugenia V. Inviyaeva, Ph.D. (bio.sci.), Senior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel: +7(495)438-11-83. E-mail e_inviyaeva@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Vladimir A. Klimov, M.D., Ph.D., Head of the Department of Medical Care Organization, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
4 Oparin str., 117997, Moscow, Russia.
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of the RAS, Director of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel: +7(495)438-18-00.
E-mail: g_sukhikh@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
For citation: Dolgushina N.V., Krechetova L.V, Ivanets T.Yu., Vtorushina V.V., Inviyaeva E.V., Klimov V.A., Sukhikh G.T. The impact of the immune status on COVID-19 severity.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 9: 129-137 (in Russian)
https://dx.doi.org/10.18565/aig.2020.9.129-137



