Novel coronavirus infection in the first trimester of pregnancy: perinatal and maternal outcomes
Objective: To investigate the characteristic features of the course of pregnancy, labor, and perinatal outcomes in women who had a new coronavirus disease 2019 (COVID-19) in the first trimester of pregnancy. Materials and methods: The first stage of the study consisted of a retrospective analysis of the COVID-19 registry of pregnant and postpartum women from the Ural Federal District (UFD) for 2020-2021. A total of 2347 patients had COVID-19 in the first trimester of pregnancy in the UFD in 2020–2021. The second stage of the study was a single center cross-sectional comparative study in two independent groups. The study group included 131 patients who had COVID-19 in the first trimester of pregnancy; the comparison group comprised 216 patients who gave birth before COVID-19 pandemic (2019). The analysis included the course of pregnancy, labor and delivery, neonatal health status, and histological examination of 10 placentas of women in the study group. Results: Pregnancy was terminated in 19.4% of patients who developed severe COVID-19 in the first trimester. Spontaneous miscarriages were registered in 9.2% of the women with mild and moderate COVID-19. In two cases in patients who had COVID-19 before 6 weeks, fetal malformations were detected that were incompatible with life, which are extremely rare in the population. COVID-19 in the first trimester of pregnancy increased the risk of gestational hypertension (OR=3.3; 95% CI 1.6–6.6; p<0.001) and threatened preterm birth (OR=3.4; 95% CI 1.4–8.0; p=0.004). The mean gestational age at delivery was significantly lower [38.4 (2.0), p<0.001] than in patients who gave birth before the COVID-19 pandemic. The newborns showed a significant decrease in anthropometric parameters and Apgar scores at 1 [7 (6:8), p=0.035] and 5 [8 (7:8), p<0.001] minutes compared to the newborns of the comparison group. At the same time, there were signs of both maternal and fetal blood flow abnormalities in the placenta. Conclusion: Women who had COVID-19 in the first trimester of pregnancy may be at increased risk of adverse perinatal and maternal outcomes.Malgina G.B., Dyakova M.M., Bychkova S.V., Grishkina A.A., Pepelyaeva N.A., Olkov S.S., Melkozerova O.A., Bashmakova N.V., Davydenko N.B.
Keywords
Pregnancy is a very specific and complex period in a woman's life when any health problem can involve the mother's health, the baby's health, or both. Novel coronavirus infection (COVID-19) is considered a global emergency, and both the infection itself and its long-term consequences are dangerous. Particular attention should be paid to the study of the course and outcomes of pregnancy in women who had COVID-19 at different stages of gestation. Research evidence on the course of pregnancy, fetal, and neonatal outcomes of pregnancies complicated by SARS-CoV-2 infection at different stages of gestation continues to accumulate. However, they are often insufficient for a complete comprehensive assessment of mother and fetus, predicting individual risks and long-term consequences. The current literature is especially lacking adequate coverage on the course and outcomes of pregnancy with COVID-19 in the first trimester.
Most publications have shown that COVID-19 is generally asymptomatic and mild in the first trimester of pregnancy; no specific features of the course of infection in first-trimester pregnant women have been identified [1, 2]. However, there have been cases of adverse pregnancy outcomes not long after infection, namely spontaneous abortion, which has not been prevented by therapy aimed at preserving pregnancy [3–6]. Cases of severe intrauterine fetal damage in the form of fetal hydrops, resulting in antenatal fetal death in the first half of pregnancy, have also been described. Long persistence of the virus in fetal tissues, placenta, and fetal membranes was established, while the virus was already eliminated from the mother's body. Experimental studies have shown that ACE2- and TMPRSS2-positive cells are present in the trophectoderm tissues and the future placenta even in early pregnancy, indicating that SARS-CoV-2 may spread through the placenta and cause intrauterine infection of the fetus. Thus, the embryo and fetus become vulnerable to coronavirus infection [7–10].
However, not only vertical transmission of coronavirus infection in the first trimester is associated with the development of pregnancy complications [11]. It was found that in patients with the first trimester coronavirus infection had a hypercoagulable state, which impairs perfusion in the future maternal part of the placenta. At the same time, the authors have not established signs of impaired trophoblast invasion [12]. However, a significantly higher risk of pre-eclampsia was confirmed compared with unexposed pregnant women [13], which, according to the authors, is attributed to maternal factors (overweight, hypertension), which are risk factors for a more severe course of the infectious process [14–16]. Several studies have also reported disorders in maternal carbohydrate metabolism after an infectious disease [17].
In general, analysis of the literature on COVID-19 in the first trimester of pregnancy revealed that there is no consensus on its impact on the further course of pregnancy and perinatal outcomes.
The published studies had a small sample size, which does not allow definitive conclusions to be drawn. Thus, more evidence and study of COVID-19 in the effects of the first trimester on pregnancy, fetoplacental system, fetal status, and perinatal outcomes.
The present study aimed to investigate the characteristic features of the course of pregnancy, labor, and perinatal outcomes in women who had COVID-19 in the first trimester of pregnancy.
Materials and methods
The first stage of the study consisted of a retrospective analysis of the COVID-19 registry of pregnant and postpartum women from the Ural Federal District (UFD) for 2020–2021. A total of 17543 pregnant and puerperal women had COVID-19 in the UFD in 2020–2021, including 2347 first trimester pregnancies.
The second stage of the study was a single center cross-sectional comparative study in two independent groups conducted according to the STROBE reporting guideline for observational studies [18]. Pregnant women with a confirmed diagnosis of mild to moderate COVID-19 (n=823) were treated at the COVID Hospital of the Research Institute of Maternity and Child Care, Ministry of Health of the Russian Federation during the first, second and third waves of the pandemic. Of these patients, 131 had SARS-CoV-2 infection in the first trimester of pregnancy (3 to 12.6 weeks of gestation), with an average gestational age of 7.5 (2.8) weeks. These patients comprised the study group.
The comparison group included 216 patients who gave birth before the COVID-19 pandemic (2019) and did not have ARVI during pregnancy and delivery. This group was recruited by random selection using a random number table according to Sections 6.1, 6.2. GOST "Procedures for Random Sampling and Randomization» [19]. The comparison group was recruited from the database of the Regional Obstetric Monitoring System. The sample size of the comparison group was calculated using the Sealed Envelope Ltd. power calculator [20].
The analysis included the course of pregnancy, the characteristics of delivery, and the clinical state of newborn children from women in these groups.
Inclusion criteria were pregnant women with a confirmed diagnosis of mild to moderate COVID-19 at gestational ages of 3 to 12.6 weeks; signed informed consent to participate in the study. Non-inclusion criteria were gestational age greater than 13 weeks at the time of COVID-19. SARS-CoV-2 infection was confirmed by polymerase chain reaction (PCR), with the detection of the SARS-CoV-2 antigen in nasopharyngeal swab samples (nasopharyngeal/oropharyngeal exudate). Disease severity was determined at admission, according to the criteria outlined in versions 2, 3, 4, 5 of the Methodological recommendations for the organization of medical care for COVID-19 for pregnant, puerperal and parturient women, and newborns [21].
The evaluation of the course of pregnancy and labor included detailed information about the patients, including their age, gestational age at the time of SARS-CoV-2 infection, epidemiological history, comorbidities, complications during pregnancy and labor. The clinical state of the newborns was assessed by the Apgar score at 1 and 5 minutes after they were born, anthropometric data, and degree of prematurity. The women were delivered in the maternity hospitals of Yekaterinburg and Sverdlovsk region.
Macroscopic and microscopic examination of 10 placentas from the women of the study group was performed. Macroscopic examination included measuring the size and thickness of the placenta, determining the nature of attachment, the diameter of the umbilical cord, the presence of pathological tortuosity, and / or false knots. Pathological changes were observed in the membranes and placental tissue. Fragments of the umbilical cord, membranes, sections of the maternal and fetal surface of the umbilical cord and at the edge of the disc were taken from each placenta. The material was then fixed in 10% buffered formalin; paraffin blocks were prepared according to the standard technique. Sections 4 µm thick were stained with hematoxylin and eosin according to the standard technique.
The study was reviewed and approved by the Research Ethics Committee of the Ural Research Institute of Maternity and Child Care (Ref. No. 12, dated 21.09.2021). The main limitation of the second phase of this study is the possibility of systematic selection bias due to the single-center design (in the COVID-19 treatment stage); however, patients subsequently gave birth in various maternity hospitals and perinatal centers in the Sverdlovsk region.
Statistical analysis
Statistical analysis was performed using Microsoft Excel (2010) and SPSS Statistics version 22.0 software (IBM, Microsoft, USA). The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Equality of variance was assessed by f-criterion in the SPSS Statistics version 22.0 package, which is directly included in the procedure for testing the hypothesis of difference in the means. Quantitative variables were expressed as means (M) and standard deviation (SD), categorical variables were presented as number of subjects (n) and percentage (%). Categorical variables were compared by Chi-square (χ2) test for 2×2 contingency tables. If the outcome frequencies were < 10%, a relative risk (RR) with a 95% confidence interval (95% CI) was chosen; otherwise, the odds ratio (OR) with a 95% confidence interval (95% CI) was used. Ordinal variables were presented as the median (Me) with interquartile range (Q1; Q3). Hypothesis testing was performed using the Mann–Whitney U-test.
Results and discussion
The COVID-19 UFD registry for 2020-2021 included 2347 first trimester pregnancies, representing 13.4% of all pregnant and puerperal women who had COVID-19. Twenty-two patients had a pregnancy termination during the disease, which was 0.94%.
In evaluating the clinical manifestations of infection, it should be noted that 926 pregnant women (39.5% of all first-trimester pregnancies) were asymptomatic; 831 (35.4%) had mild COVID-19, 559 (23.8%) had a moderate form, and only 31 patients (1.3% of all first-trimester pregnancies) had severe COVID-19. There were neither maternal near-miss cases nor maternal deaths among first trimester COVID-19 patients.
In patients with asymptomatic, mild, and moderate COVID-19 in the first trimester, the rates of miscarriages during the disease were 0.7; 0.8 and 0.5%, respectively.
However, among patients with severe COVID-19 in the first trimester, 19.4% (6 cases out of 31) experienced pregnancy loss. Therefore, it can be concluded that severe COVID-19 in the first trimester of pregnancy is associated with loss of pregnancy in one of the five pregnancies.
The second stage of the study was a single center cross-sectional comparative study in two independent groups. The study group included 131 patients who had COVID-19 in the first trimester of pregnancy; the comparison group comprised 216 patients who gave birth before COVID-19 pandemic (2019). The mean age of patients in the study group was 30.7 (6.1) years and that of patients in the comparison group was 29.1 (5.7) years.
In terms of parity, patients who had COVID-19 in the first trimester of pregnancy were more likely to be multiparous [86/131 (65.6%) vs. 94/216 (43.1%), p<0.001]. There were significantly fewer primiparous women in the study group: 15/131 (11.5%) in the study group and 63/216 (29.2%) in the comparison group, p<0.001. There were 30/131 (22.9%) multigravida primiparous women with a poor obstetric history (abortions, miscarriages) in the study group and 60/216 (27.5%) in the comparison group, p=0.73 (Table 1).
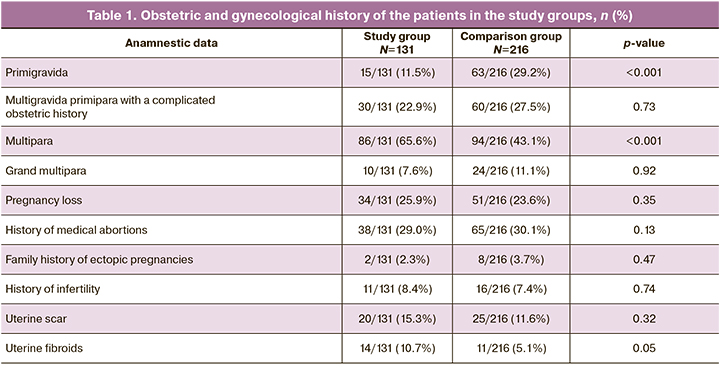
As can be seen from this table, the groups were comparable in terms of diseases and conditions in the obstetric history. There were no significant differences in the rates of recurrent pregnancy loss, infertility, previous surgical deliveries, medical abortions, uterine myoma, and the proportion of multiparous women.
Evaluation of the medical history of the study groups revealed significant differences in history of gastrointestinal diseases; 15.7% of the patients in the study group and 8.4% in the control group had chronic gastroduodenitis, peptic ulcer disease, chronic cholecystitis and chronic pancreatitis. Furthermore, there were significant differences in the frequency of urinary diseases (mainly chronic pyelonephritis). The patients in the study group were 2.4 times more likely to have these diseases than those in the comparison group. Hypothyroidism was also 2.5 times more common in the study group. However, at the beginning of pregnancy, all the above diseases were in the compensatory stage. There were no significant differences between the groups in the rates of obesity, hypertension, diabetes mellitus, and HIV infection, which worsened the prognosis of COVID-19 [14–16].
At admission, 95/131 (72.5%) and 36/131 (27.5%) patients in the first trimester of pregnancy had mild and moderate COVID-19, respectively. The clinical picture of mild COVID-19 was characterized by symptoms of acute rhinopharyngitis, anosmia and hyposmia, and weakness. In moderate COVID-19, in addition to the above symptoms, there were radiologically confirmed pneumonia, fever, C-reactive protein levels over 10 mg/L, and symptoms of respiratory failure [22].
The cases of spontaneous abortion against the backdrop of SARS-CoV-2 infection deserve a separate analysis. In our study, there were 2 cases of miscarriage during COVID-19 disease in first-trimester patients. It should be noted that both miscarriages occurred in the third wave of COVID-19.
Patient B. multipara, 29 years old, was admitted to the COVID hospital for the Research Institute for Maternity and Child Care on 08.17.21 with 5–6 weeks of pregnancy and complicated obstetric and gynecological history (medical abortion). Threatened miscarriage. SARS-CoV-2 infection, confirmed, mild COVID-19. On admission, the patient noted profuse brown vaginal discharge, and constant pulling lower abdominal pain. On ultrasound examination there was no fetal heartbeat, during the examination, abortion in progress was diagnosed. The patient underwent removal of uterine contents using vacuum aspiration under intravenous anesthesia without any complications. On day 3 after surgery, the patient was tested negative for SARS-CoV-2 RNA in the nasopharyngeal smear by PCR.
Patient B. multipara, 42 years old, was hospitalized on 08/22/21 with 11-12 weeks of pregnancy and complicated obstetric and gynecological history (medical abortion). Uterine scar after previous cesarean delivery. Grade I chronic arterial hypertension. Chronic pyelonephritis, remission. Threatened miscarriage. Mild COVID-19, confirmed. On 08/29/1921, on the 7th day after admission, at 12–13 weeks, the patient complained of recurrent cramping lower abdominal pain, abundant bloody vaginal discharge. On ultrasound examination the fetal heartbeat was not detected, during the examination, abortion in progress was diagnosed. The patient underwent removal of uterine contents using vacuum aspiration under intravenous anesthesia. The postoperative period was without complications. On the fourth day after surgery, the result of the COVID-19 PCR test was negative.
In addition to the cases of spontaneous abortion, we had 2 cases of termination of pregnancy termination for medical reasons due to rare fatal fetal malformations.
Patient B., a 26-year-old primipara. She was treated for mild COVID-19 in the COVID hospital of the Research Institute of Maternity and Child Care from July 29, 20 to August 08, 20 at the gestational age of 4–5 weeks. Transvaginal ultrasound examination at 12–13 weeks' gestation revealed nuchal fold thickness greater than the 96th percentile, hypoplasia/absence of visualization of nasal bones and omphalocele, pronounced spinal deformity (Fig. 1), heart rotated, euentration of abdominal wall organs into choroidal cavity, renal parenchyma with increased echogenicity, cranio-caudal dimension 57.6 mm. The umbilical cord was located between the membranes 26 mm, free loops were not visualized, dilated jugular sac.
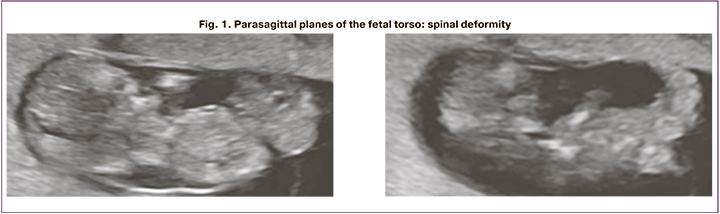
Conclusion: Pregnancy at 12–13 weeks. Congenital malformation in the fetus: body stalk anomaly.
It should be noted that the body stalk anomaly refers to congenital abdominal wall malformations and is characterized by the absence of the umbilical cord and umbilical ring. As a result of incomplete fusion of the amnion and chorion at the periphery due to impaired development of the cephalic, caudal and lateral fetal torso folds, no umbilical cord is formed at 3–4 weeks of embryogenesis. The incidence of this pathology is 1:14000–42000 pregnancies [23–25].
Patient A., a 40-year-old multipara. At 4–5 weeks of gestation, she had moderate COVID-19. A first-trimester ultrasound at 12–13 weeks revealed a defect of the fetal central nervous system: lumbosacral rachischisis, posterior cranial fossa abnormality, and increased thickness of the nuchal fold thickness.
We know from the literature that rachischisis is rachischisis is a neural tube defect belonging to the group of myelodysplasias of the lumbosacral spine. This defect is part of the structure of cloacal exstrophy, the so-called OEIS-complex (O – omphalocele, E – exstrophy of the bladder, I – imperforate anus, S – spinal deformities, including spinal anomalies, spina bifida, and sacral defects). The detection rate for the OEIS complex is 1:200,000–400,000 [23–25].
In general, the incidence of pregnancy loss in the study group was 12 patients out of 131, which was 9.2%. In 2 of these 12 cases, the pregnancy ended at the height of the disease, while in the remaining 10 cases miscarriages occurred after COVID-19 at 6–7 to 15–16 weeks on average on day 14 (0.5) after discharge from the hospital and clinical recovery from COVID-19. Moreover, most of these patients had mild COVID-19.
A total of the 117 of 131 first trimester COVID-19 patients had gestational age greater than 22 weeks. Their pregnancy course and outcomes were evaluated (Table 2).
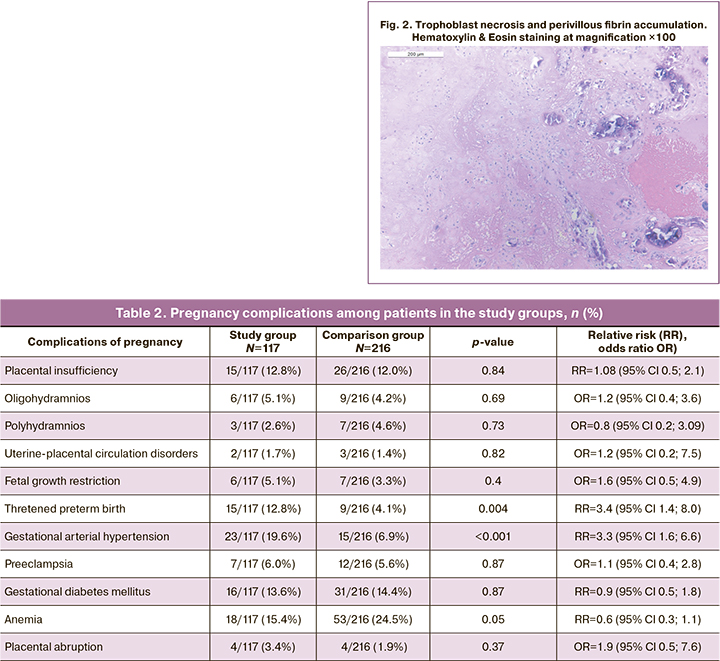
This table shows that there were no significant differences between the study group and the comparison group for most pregnancy complications. However, pregnancy after COVID-19 in the first trimester was significantly more often complicated by threatened preterm labor compared to the group of patients who had pregnancy before the COVID-19 pandemic (2019). Furthermore, hypertensive conditions that complicate pregnancy in the form of gestational arterial hypertension were significantly more frequent in the patients of the study group patients. There was no significant increase in the incidence of preeclampsia. Patients after COVID-19 in the first trimester had no increased risk of gestational diabetes mellitus, placental insufficiency, impaired uteroplacental circulation, or placental abruption.
When analyzing the data in Table 3, the following is noteworthy: in patients who had COVID-19 in the first trimester, the mean duration of delivery was significantly shorter than in those who gave birth before COVID-19 pandemic. While the overall cesarean section rates were almost identical, patients in the control group were more likely to have planned delivery while those in the study group more often had emergency operative delivery.
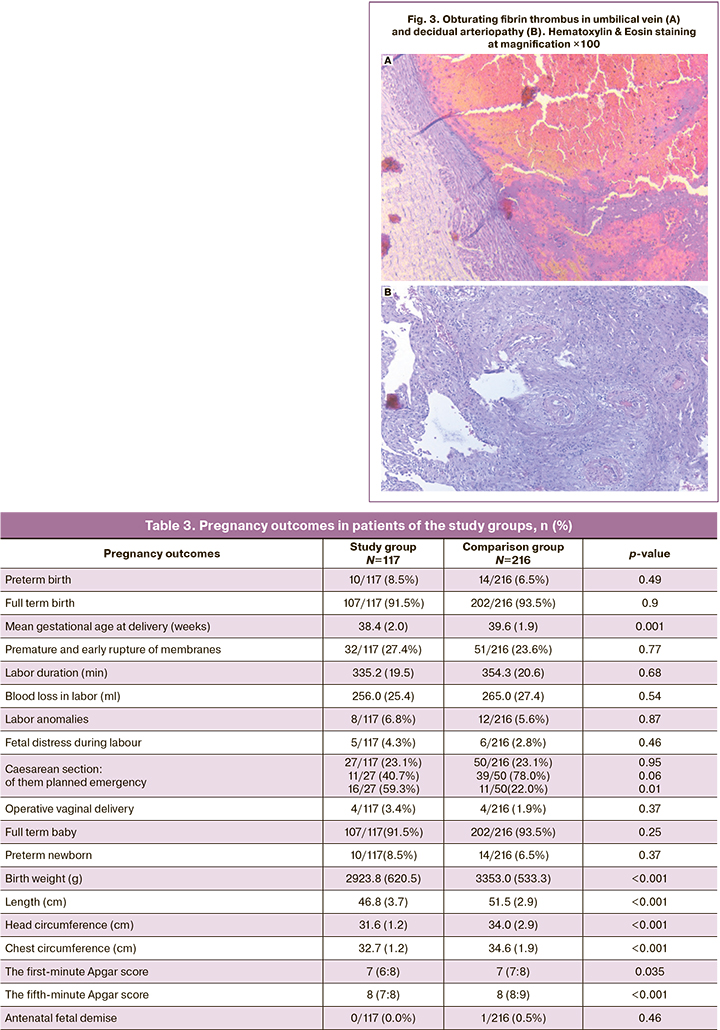
It should be noted that newborns from patients who had COVID-19 in the first trimester of pregnancy had statistically significantly lower anthropometric parameters than those from patients who gave birth before the COVID-19 pandemic. This can be attributed to increase in the number of preterm newborns, as well as to unfavorable conditions of newborn development in the maternal womb, starting from the earliest stages, as well as to a decrease in the mean gestational age at delivery by more than one week. Despite equal medians, differences in newborn Apgar scores at the 1st and 5th minutes after birth were significant by the Mann–Whitney U-test with interquartile intervals.
In light of this, we analyzed the morphology of placentas from mothers who had COVID-19 in the first trimester of pregnancy (Fig. 2, 3). In women with COVID-19 in the first trimester, the placentas showed signs of maternal and fetal blood flow (impaired maternal and fetal perfusion). Almost all placentas showed decidual arteriopathy, atherosis, fibrinoid necrosis of decidual plate vessels, hypertrophy of the walls of placental arterioles. Among the impaired fetal vascular abnormalities, the most frequently registered were intramural fibrin deposits in the vessels, stromal vascular karyorrhexis, and non-obturating thrombi in the vessels. The villous chorion showed a deficit in terminal villi and delayed maturation at the stage of intermediate mature villi. There was focal edema of the villous stroma, chorangiosis. All placentas had increased fibrin content and thrombi in the intervillous space.
Conclusion
From the findings of our study the following conclusions can be drawn: COVID-19 in the first trimester of pregnancy significantly increases the risk of gestational arterial hypertension and the risk of preterm birth; the mean delivery time was significantly shorter than in patients who gave birth before COVID-19 pandemic.
Among women with mild to moderate COVID-19 in the first trimester, 9.2% experienced pregnancy loss. Severe COVID-19 in the first trimester was associated with loss of pregnancy in every fifth pregnancy (19.4%) at the height of the disease. Two patients who had COVID-19 before 6 weeks of gestation developed life-threatening fetal malformations, which are extremely rare in the population.
There was a significant decrease in anthropometric characteristics of newborns by all parameters (birth weight, body length, head and chest circumference) and Apgar scores at the 1st and 5th minutes after birth, despite equal medians. This was associated with earlier delivery and placental abnormalities: women with SARS-CoV-2 infection in the first trimester showed signs of both maternal and fetal blood flow abnormalities (impaired maternal and fetal perfusion) in their placentas.
References
- Cosma S., Carosso A.R., Cusato J., Borella F., Carosso M., Bovetti M. et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a case-control study of 225 pregnant patients. J. Obstet. Gynecol. 2021; 224(4): 391.e1-391.e7. https://dx.doi.org/10.1016/j.ajog.2020.10.005.
- Fallach N., Segal Y., Agassy J., Perez G., Peretz A., Chodick G. et al. Pregnancy outcomes after SARS-CoV-2 infection by trimester: A large, population-based cohort study. PLoS One. 2022; 17(7): e0270893. https://dx.doi.org/10.1371/journal.pone.0270893.
- Balachandren N., Davies M.C., Hall J.A., Stephenson J.M., David A.L., Barrett G. et al. SARS-CoV-2 infection in the first trimester and the risk of early miscarriage: a UK population-based prospective cohort study of 3041 pregnancies conceived during the pandemic. Hum. Reprod. 2022; 37(6): 1126-33.https://dx.doi.org/10.1093/humrep/deac062.
- Kiremitli S., Kiremitli T., Ulug P., Kirkinci A., Kurnuc F.Z., Yilmaz N. et al. Does being infected with SARS-CoV-2 in the first-trimester increase the risk of miscarriage? An Acad. Bras. Cienc. 2022; 94(2): e20211283.https://dx.doi.org/10.1590/0001-3765202220211283.
- González Rodríguez L., Oreja Cuesta A.B., Pardo Pumar M.I., Ferriols-Pérez E., Pedró Carulla R., Bernardo Vega R. et al. SARS-CoV-2 infection in early first-trimester miscarriages: a prospective observational study. Spanish Obstetric Emergency Group. Reprod. Biomed. Online. 2022; 44(1): 127-30.https://dx.doi.org/10.1016/j.rbmo.2021.09.010.
- Kazemi S.N., Hajikhani B., Didar H., Hosseini S.S., Haddadi S., Khalili F. et al. COVID-19 and cause of pregnancy loss during the pandemic: a systematic review. PLoS One. 2021; 16(8): e0255994. https://dx.doi:org/10.1371/journal.pone.0255994.
- Hernández-Díaz S., Smith L.H., Wyszynski D.F., Rasmussen S.A. First trimester COVID-19 and the risk of major congenital malformations-International Registry of Coronavirus Exposure in Pregnancy. Birth Defects Res. 2022; 114(15): 906-14. https://dx.doi.org/10.1002/bdr2.2070.
- Valdespino-Vázquez M.Y., Helguera-Repetto C.A., León-Juárez M., Villavicencio-Carrisoza O., Flores-Pliego A., Moreno-Verduzco E.R. et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. Med. Virol. 2021; 93(7):4480-7. https://dx.doi.org/10.1002/jmv.26965.
- Shende P., Gaikwad P., Gandhewar M., Ukey P., Bhide A., Patel V. Persistence of SARS-CoV-2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Hum. Reprod. 2021 36(4): 899-906. https://dx.doi.org/10.1093/humrep/deaa367.
- Sills E.S., Wood S.H. An experimental model for peri-conceptual COVID-19 pregnancy loss and proposed Interventions to Optimize Outcomes. Int. J. Mol. Cell. Med. 2020 Summer; 9(3): 180-7. https://dx.doi.org/10.22088/IJMCM.BUMS.9.3.180.
- Weatherbee B.A.T., Glover D.M., Zernicka-Goetz M. Expression of SARS-CoV-2 receptor ACE2 and the protease TMPRSS2 suggests susceptibility of the human embryo in the first trimester. Open Biol. 2020; 10(8): 200162.https://dx.doi.org/10.1098/rsob.200162.
- Jaiswal N., Puri M., Agarwal K., Singh S., Yadav R., Tiwary N. COVID-19 as an independent risk factor for subclinical placental dysfunction. J. Obstet. Gynecol. Reprod. Biol. 2021; 259: 7-11. https://dx.doi.org/10.1016/j.ejogrb.2021.01.049.
- Serrano B., Mendoza M., Garcia-Aguilar P., Bonacina E., Garcia-Ruiz I., Garcia-Manau P. Shared risk factors for COVID-19 and preeclampsia in the first trimester: An observational study. Acta Obstet. Gynecol. Scand. 2022; 101(7): 803-8. https://dx.doi.org/10.1111/aogs.14371.
- Allotey J., Fernandez S., Bonet M., Stallings E., Yap M., Kew T. et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020; 370: m3320. https://dx.doi.org/10.1136/bmj.m3320.
- Адамян Л.В., Вечорко В.И., Конышева О.В., Харченко Э.И. Беременность и COVID-19: актуальные вопросы (обзор литературы). Проблемы репродукции. 2021; 27(3): 70‑7. https://dx.doi.org/10.17116/repro20212703170. [Adamyan L.V., Vechorko V.I., Konysheva O.V., Kharchenko E.I. Pregnancy and COVID-19: current issues (literature review). Russian Journal of Human Reproduction. 2021; 27(3):70‑7. (in Russian)]. https://dx.doi.org/10.17116/repro20212703170.
- Белокриницкая Т.Е., Фролова Н.И., Колмакова К.А., Шаметова Е.А.Факторы риска и особенности течения COVID-19 у беременных: сравнительный анализ эпидемических вспышек 2020 и 2021 г. Гинекология. 2021; 23(5): 421-7. https://dx.doi.org/10.26442/20795696.2021.5.201107. [Belokrinitskaya T.E., Frolova N.I., Kolmakova K.A., Shametova E.A. Risk factors and features of COVID-19 course in pregnant women: a comparative analysis of epidemic outbreaks in 2020 and 2021. Ginecology. 2021; 23(5): 421-7. (in Russian)]. https://dx.doi.org/10.26442/20795696.2021.5.201107.
- Cui D., Liu Y., Jiang X., Ding C., Poon L.C., Wang H. et al. Single-cell RNA expression profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet. Gynecol. 2021; 57(2): 248-56. https://dx.doi.org/10.1002/uog.22186.
- von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008; 61(4): 344-9.
- ГОСТ Р ИСО 24153-2012. Статистические методы. Процедуры рандомизации и отбора случайной выборки. 2014. Доступно по:https://docplan.ru/Index2/1/4293787/4293787702.htm [GOST R ISO 24153-2012. Statistical methods. Randomization and random sampling procedures. 2014. (in Russian)].
- Sealed Envelope Ltd. 2012. Power calculator for binary outcome superiority trial. Available al: https://www.sealedenvelope.com/power/binary-superiority
- Министерство здравоохранения Российской Федерации. Временные методические рекомендации. Организация оказания медицинской помощи беременным, роженицам, родильницам и новорожденным при новой коронавирусной инфекции COVID-19. Версия 5. 28.12.2021. 135c. Доступно по: https://static0.minzdrav.gov.ru/system/attachments/attaches/000/059/052/original/BMP_preg_5.pdf [Ministry of Health of the Russian Federation. Temporary guidelines. Organization of medical care for pregnant women, women in labor, women in labor and newborns with a new coronavirus infection COVID-19. Version 5. 28.12.2022. 135p.(in Russian)]. Available at: https://static0.minzdrav.gov.ru/system/attachments/attaches/000/059/052/original/BMP_preg_5.pdf
- Мальгина Г.Б., Дьякова М.М., Бычкова С.В., Пепеляева Н.А., Ольков С.С., Мелкозерова О.А., Башмакова Н.В., Давыденко Н.Б. Особенности клинических проявлений легких и среднетяжелых форм новой коронавирусной инфекции у беременных в динамике эпидемического процесса. Акушерство и гинекология. 2022; 3: 23-31. https://dx.doi.org/10.18565/aig.2022.3.23-31. [Malgina G.B., Dyakova M.M., Bychkova S.V., Pepelyaeva N.A., Olkov S.S., Melkozerova O.A., Bashmakova N.V., Davydenko N.B. Clinical manifestations of mild and moderate novel coronavirus disease in pregnant women in epidemic dynamics. Akusherstvo i Ginekologiya/Obstetrics and Ginecology. 2022; 3: 23-31. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.3.23-31.
- Алтынник Н.А., Кубрина М.В. Пренатальная ультразвуковая диагностика редких летальных комплексов на примере четырех случаев и обзор литературы. Пренатальная диагностика. 2019; 18(1): 27-34.https://dx.doi.org/10.21516/2413-1458-2019-18-1-27-34. [Àltynnik N.A., Kubrina M.V. Prenatal ultrasound diagnosis of rare lethal syndromes on the example of four cases and review of literature. Prenatal Diagnosis. 2019; 18(1): 27-34. (in Russian)]. https://dx.doi.org/10.21516/2413-1458-2019-18-1-27-34.
- Patil N.G., Mudanur S.R., Nemagouda A.S., Kori S., Lahoriet L.Y. OEIS complex: a rare fetal anomaly. Int. J. Reprod. Contracept. Obstet. Gynecol. 2014; 3(4): 1100-3.
- Chikkannaiah P., Dhumale H., Kangle R., Shekar R. Limb body wall complex: a rare anomaly. J. Lab. Physicians. 2013; 5(1): 65-7. https://dx.doi.org/10.4103/0974-2727.115930.
Received 07.09.2022
Accepted 30.11.2022
About the Authors
Galina B. Malgina, Dr. Med. Sci., Director, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, galinamalgina@mail.ru,https://orcid.org/0000-0002-5500-6296, 620028, Russia, Ekaterinburg, Repin str., 1.
Maria M. Dyakova, doctor, Junior Researcher, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, +7(950)550-06-52, mariadakova40@mail.ru, https://orcid.org/0000-0001-7911-6783, 620028, Russia, Ekaterinburg, Repin str., 1.
Svetlana V. Bychkova, PhD, Leading Researcher, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, +7(343)37-155-20, simomm@mail.ru, https://orcid.org/0000-0002-8892-7785, 620028, Russia, Ekaterinburg, Repin str., 1.
Anastasia A. Grishkina, PhD, Pathologist, Department of Immunology, Clinical Microbiology, Pathomorphology and Cytodiagnosis, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, xumukyc.ru@mail.ru, https://orcid.org/0000-0001-7433-2217, 620028, Russia, Ekaterinburg, Repina str., 1.
Natalia A. Pepelyaeva, PhD, Head of the Department, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, pepelyaevana@niiomm.ru,
https://orcid.org/0000-0003-3278-2249, 620028, Russia, Ekaterinburg, Repin str. 1.
Sergey S. Olkov, PhD, Deputy Head of the Pediatrics Clinic, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, olkovss@niiomm.ru,
https://orcid.org/0000-0002-6142-370, 620028, Russia, Ekaterinburg, Repin str., 1.
Oksana A. Melkozerova, Dr. Med. Sci., Deputy Director for Science, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, abolmed@mail.ru, https://orcid.org/0000-0002-4090-0578, 620028, Russia, Ekaterinburg, Repin str., 1.
Nadezhda V. Bashmakova, Dr. Med. Sci., Professor, Chief Researcher, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia,
bashmakovanv@niiomm.ru, https://orcid.org/0000-0001-5746-316X, 620028, Russia, Ekaterinburg, Repin str., 1.
Natalia B. Davydenko, PhD, Head of the Department, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, orgomm@mail.ru,
https://orcid.org/0000-0002-1617-5521, 620028, Russia, Ekaterinburg, Repin str., 1.
Corresponding authors: Maria M. Dyakova, mariadakova40@gmail.com; Svetlana V. Bychkova, simomm@mail.ru
Authors' contributions: Malgina G.B. – conception and design of the study, manuscript drafting and editing; Dyakova M.M., Grishkina A.A. – data collection and analysis, manuscript drafting; Bychkova S.V. – literature search of Russian and international studies in Russian and international databases, review of the relevant literature, manuscript drafting; Pepelyaeva N.A, Olkov S.S. – material collection; Melkozerova O.A., Bashmakova N.V. – manuscript editing; Davydenko N.B. – literary search of Russian and international studies in Russian and international databases, review of the relevant literature.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Ural Research Institute of Maternity and Child Care (Ref. No. 12 dated 21.09.2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Malgina G.B., Dyakova M.M., Bychkova S.V., Grishkina A.A., Pepelyaeva N.A., Olkov S.S., Melkozerova O.A., Bashmakova N.V., Davydenko N.B. Novel coronavirus infection in the first trimester of pregnancy: perinatal and maternal outcomes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 12: 90-99 (in Russian)
https://dx.doi.org/10.18565/aig.2022.212



