Impact of menopausal hormone therapy on immune system parameters
The decline in sex hormone levels with age is known to affect the functioning of the immune system in both men and women. Immunological aging is a consequence of age-related changes in the function of immune cells and the composition of their subpopulations. Taking menopausal hormone therapy has been shown to neutralize these changes. The route of estrogen administration in postmenopausal women may play a crucial role in these processes. Objective: To investigate the effect of combined menopausal hormone therapy (MHT), including transdermal estradiol gel combined with micronized intravaginal progesterone, on blood immune parameters. Materials and methods: The subpopulation composition of leukocytes was assessed in 23 peri- and postmenopausal women aged 44–57 years who were referred to the Gynecological Endocrinology Department of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. The treatment duration was 3 months. Clinical and laboratory examinations were performed before therapy and three months after MHT. Results: After 3 months of MHT, the patients were found to have a significant reduction in the severity of vasomotor (by 4 times), physical (by 3 times), and psychoemotional and sexual symptoms (by 2 times) (p<0.05). The use of MHT significantly reduced the incidence of sleep disturbances by a factor of 1.6, from 82.6 to 52.2% (p=0.020), muscle and joint pain by a factor of 1.4, from 73.9 to 52.2% (p=0.026), hot flashes by a factor of 2.7, from 82.6 to 30.4% (p<0.001), and night sweats by a factor of 1.8, from 73.9 to 39.1% (p=0.012). The effect of MHT on immune system parameters was manifested by a significant increase in the percentage of T-helper cells (CD45+CD3+CD4+) and changes in the expression of CD163, CD206, and CX3CR1 markers in subpopulations of classical, intermediate, and non-classical blood monocytes. Conclusion: Our findings suggest that combined MHT including transdermal estradiol in combination with intravaginal micronized progesterone influences patients' blood immune parameters. It affects both lymphocytes and monocytes, with changes in the monocyte phenotype in different subpopulations that are likely to contribute to the immunopotentiating properties of MHT. Authors' contributions: Yureneva S.V., Vishnyakova P.A. – conception and design of the study; Averyanova M.V., Vishnyakova P.A., Yakushevskaya O.V., Kiseleva V.V., Iskusnykh M.E. – data collection and analysis; Averyanova M.V., Vishnyakova P.A., Kiseleva V.V. – statistical analysis and manuscript drafting; Yureneva S.V., Vishnyakova P.A., Elchaninov A.V., Fatkhudinov T.Kh. – manuscript editing. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: This study was conducted under Government contract No. 121032500100‑3. The work was supported by the Russian Science Foundation [grant number 22‑75‑00048]. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No: 11 of 11.11.2021). Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Averyanova M.V., Yureneva S.V., Kiseleva V.V., Yakushevskaya O.V., Iskusnykh M.E., Elchaninov A.V., Fatkhudinov T.Kh., Vishnyakova P.A. Impact of menopausal hormone therapy on immune system parameters. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (4): 68-77 (in Russian) https://dx.doi.org/10.18565/aig.2023.59Averyanova M.V., Yureneva S.V., Kiseleva V.V., Yakushevskaya O.V., Iskusnykh M.E., Elchaninov A.V., Fatkhudinov T.Kh., Vishnyakova P.A.
Keywords
The increasing life expectancy of modern women underlines the importance of maintaining their health, quality of life, social activities, and performance. Menopause is a normal part of a woman's life cycle and consists of a number of changes in the body, including those in the immune system.
Female sex hormones, including estrogens and progesterone, act as regulators of innate and adaptive immunity; therefore, women have a high reactivity of both humoral and cell-mediated immune responses. Several studies have concluded that women are more resistant to viral diseases, particularly SARS-CoV-2 infection [1].
Analysis of COVID-19 data from Italy, Spain, Germany, Switzerland, Belgium, and Norway showed that among all age groups over 20 years of age, males had a higher mortality rate than females [2]. The fact that men of all ages and women above 50 years of age have the highest risk of developing serious complications from COVID-19 raises the question of the role of sex steroids in morbidity [3, 4]. This suggests that certain protective mechanisms in the female body may potentiate a therapeutic effect, resulting in reduced morbidity and mortality from COVID-19 [5].
Immunological aging is associated with chronic inflammatory processes, increased susceptibility to infectious diseases, and predisposition to cancer and autoimmune diseases in the elderly [6]. Immunological aging is a consequence of age-related changes in the function of immune cells and their subpopulation composition [6]. Distinctive features of immunological aging include an immune profile characterized by a reduced CD4+:CD8+ T-cell ratio, increased numbers of differentiated memory and effector T-cells, depletion of naïve T-cells, decreased numbers of B-cells, and increased levels of proinflammatory cytokines.
Taking menopausal hormone therapy (MHT) has been shown to largely neutralize these changes. For example, several studies have shown that MHT leads to an increase in circulating B-cells, especially in the B2 B-cell population and, to a lesser extent, T-cells [7, 8]. As it is well known that sex steroids modulate the immune response, it can be assumed that MHT may slow down immune aging. However, the exact molecular pathways linking MHT and immune aging have not been elucidated [9].
The purpose of MHT in peri- and post-menopausal women is to partially compensate for sex hormone deficiencies using optimal doses of hormonal drugs, which can improve the general condition, relieve menopausal symptoms, and prevent metabolic disorders [10].
Estrogens in MHT are administered both orally and transdermally. Owing to the absence of primary hepatic metabolism, transdermal forms of estrogen are known to have a more favorable safety profile than equivalent doses of oral estrogen, and there is no increased risk of venous thromboembolism or gallstone disease, and there is a reduced risk of cardiovascular events (heart attack and stroke) [10].
The route of estrogen administration in postmenopausal women may have implications in systemic inflammation. For example, studies have shown that transdermal estrogens attenuate the hypothalamic-pituitary-adrenal system response to low-dose endotoxins in vivo, which was accompanied by a parallel decrease in endotoxin-induced stimulation of inflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1ra. This is probably due to the primary passage through the liver by the oral route of estrogen delivery, which contributes to increased synthesis of C-reactive protein and inflammatory markers [11, 12].
The current literature lacks studies investigating the mechanisms underlying the effects of MHT on the immune system. Thus, further experimental and clinical studies are needed to elucidate the changes in the concentrations of other mediators of the molecular pathways linking MHT and the immune system. In the present study, we investigated the effects of MHT containing transdermal estradiol gel in combination with intravaginal micronized progesterone on immune system parameters. Given the important role of the immune system in the immune response of the elderly and the development of cancer and autoimmune diseases with age, this is a timely topic.
Materials and methods
This single-center, open-label, single-group experimental clinical study was conducted from 2021 to 2022 and included 23 women aged 44–57 years who were managed in the Gynecological Endocrinology Department of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. The patients were in peri- and post-menopause (stages -1, +1 of STRAW +10, Stages of Reproductive Aging Workshop, Clinical and Hormonal Characterization of Stages of Reproductive Aging, 2011 [13])
Obese patients (body mass index (BMI)≥30 kg/m²), those with systemic autoimmune diseases, cancer, and acute respiratory or exacerbation of chronic diseases during the last 3 months were not included in the study.
Patients were administered combined MHT, including 0.06% transdermal estradiol hemihydrate gel – 2.5 g, containing 1.5 mg of estradiol, daily + vaginal micronized progesterone in cyclic or continuous mode (200 mg from the 14th day of the cycle for 14 days monthly/100 mg, daily). The duration of treatment was 3 months. Clinical and laboratory examinations were performed before the start of therapy and 3 months after MHT.
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P (Ref. No: 11 of 11.11.2021). All patients signed an informed consent form to participate in this study.
The severity of menopausal syndrome was determined using the Greene Climacteric Scale (GCS), including psychoemotional (items 1–11), physical (items 12–18), vasomotor (items 19, 20) symptoms, and sexual disorders (item 21). Based on the total Green's scale score, menopausal symptoms were rated as mild (1–11 points), moderate (12–19 points), or severe (≥20 points) [13].
Peripheral blood mononuclear cell isolation. For the isolation of peripheral blood mononuclear cells, whole blood (2 ml) was drawn into separate EDTA contained tubes, 8 ml of erythrocyte lysis buffer was added, and the tube was placed at +4॰C for 10 min. The tube was centrifuged at 300 g for 5 min at +4॰C, the supernatant was removed, 10 ml of red blood cell lysis buffer was added to the precipitate and centrifuged again, and the supernatant was removed. The precipitate was resuspended in 1000 µl of phosphate-salt buffer with BSA (bovine serum albumin) and stored at +4॰C until measurement.
Flow cytofluorimetry. Cells were resuspended in 100 μl autoMACS solution (1×105 cells in 100 μl) with BSA and stained with the following antibodies: CD11b (130-110-554), CD14 (130-110-518), CD206 (130-104-129), CD3 (130-113-138), CD4 (130-113-789), CD40 (130-110-950), CD56 (130-114-549), CD8 (130-125-858), CD80 (130-117-719), CD86 (130-116-164), CX3CR1 (130-096-435), HLA-DR (130-111-789), СD19 (130-113-165), CD45 (130-097-527), CD16 (130-045-701), and CD163 (130-097-630), all-Miltenyi Biotec. The analysis was performed on a FACScan flow cytometer (Becton Dickinson, USA) using the CellQuest and FlowJo software.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8 and Statistica 10.0. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. Normally distributed continuous variables were compared between the two groups using Student’s t-test or Wilcoxon test for data that were not normally distributed. The McNemar test was used to test for differences between the paired groups. Correlation analysis was performed using nonparametric Spearman’s correlation coefficient. The significance threshold was set at p<0.05.
Results
The clinical and anamnestic data of postmenopausal patients are presented in Table 1.
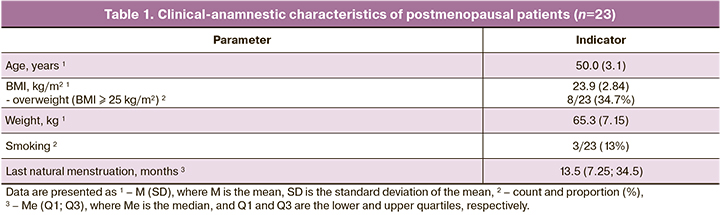
The mean age of the women was 50.0 (3.1) years, and their last natural menstruation was 6–56 months ago, with a median of 13.5 (7.25; 34.5) months. Using the Green Climacteric Scale, patients were diagnosed with mild, moderate and severe menopausal syndrome in 6/23 (26.1%), 7/23 (30.4%), and 10/23 (43.5%) cases, respectively. The baseline median Green's Climacteric Scale score was 19.0 (12.0; 27.0), corresponding to moderate menopausal syndrome. After 3 months of MHT, this score decreased significantly to 8.5 (5.0; 12.0), that is, by a factor of 2.2, corresponding to mild menopausal syndrome (p=0.001). Patients experienced a 4-fold decrease in the severity of vasomotor symptoms, 3-fold decrease in physical symptoms, and 2-fold decrease in psycho-emotional and sexual symptoms (p<0.05).
After three months of therapy, the frequency of menopausal symptoms was significantly reduced. Hot flashes reduced by 2.7 times, from 82.6 to 30.4% (p<0.001), night sweats by 1.8 times, from 73.9 to 39.1% (p=0.012), sleep disturbances by 1.6 times, from 82.6 to 52.2% (p=0.020), muscle and joint pains by 1.4 times, from 73.9 to 52.2% (p=0.026).
Correlation analysis was performed to examine the relationship between immune status indicators and clinical and anamnestic characteristics (age, BMI, length of time elapsed since the last menstruation, and severity of menopausal syndrome by Green's Climacteric Scale score). Correlation analysis showed a significant positive correlation between CD3+CD8+ cytotoxic T lymphocytes (r=0.524, 95% CI (0.086–0.745), p≤0.001) and CD3-CD56+CD16+ natural killer cells (NK-cells) (r=0.448, 95% CI (0.010–0.710), p=0.001) and duration of postmenopause; negative correlation between CD3+CD4+ T-helper cell count and duration of postmenopause (r=-0.397, 95% CI (-0.683–0.041), p=0.005); and a significant positive correlation between CD3-CD19+HLA-DR+ B-lymphocyte count and the severity of menopausal syndrome by Green's scale and duration of postmenopause (r = 0.419, 95% CI (0.008–0.709), p=0.046). The data are presented in Table 2. Among the other immunological parameters, nine showed significant correlations of different directions with age, BMI, severity of menopausal syndrome, and length of time since the last menstruation. The strongest direct correlation was found between CD14++CD16-CD86+, CD14++CD16-CD163+ and age (r=0.547, 95% CI (0.174–0.783), p=0.007; r=0.508, 95% CI (0.121–0.761), p=0.013, respectively).
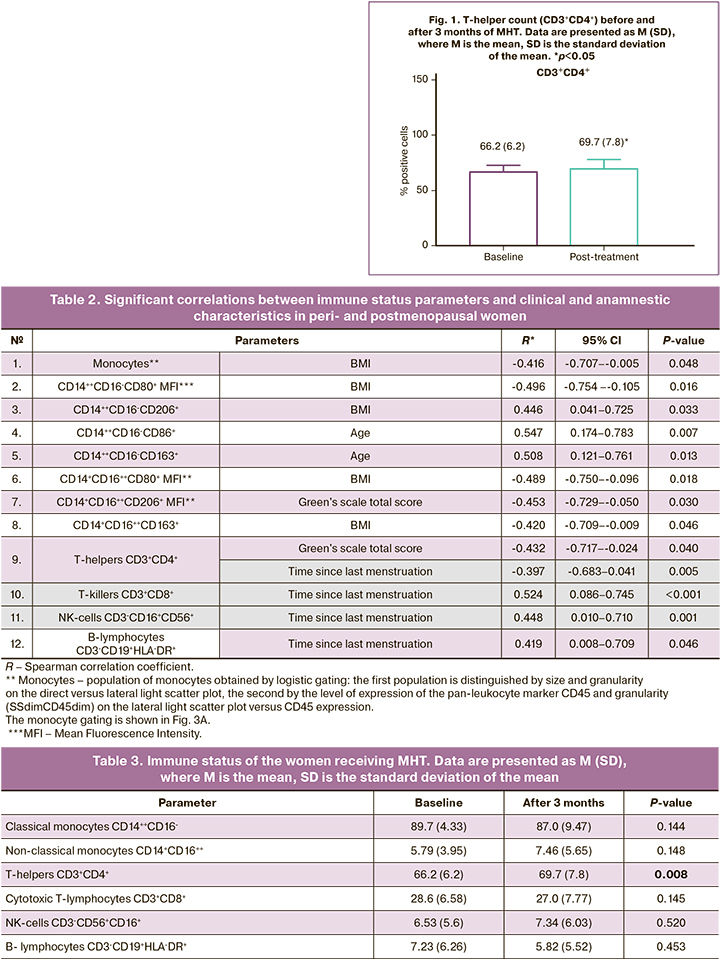
The immune system parameters examined in 23 women receiving MHT by flow cytofluorimetry included the number of classical (CD14++CD16-) and non-classical (CD14+CD16++) monocytes, total monocyte population (CD11b, CX3CR1, HLA-DR, CD45), and expression levels of proinflammatory (CD86, CD80, and CD40) and anti-inflammatory markers (CD163 and CD206). Peripheral blood lymphocyte subpopulations were also analyzed, including T-and B-lymphocytes and NK cells. Among T-lymphocytes the number of T-helpers (CD3+CD4+) and T-killers (CD3+CD8+) was determined. A statistically significant increase in the number of T-helper cells was observed after MHT containing transdermal estradiol gel combined with intravaginal micronized progesterone. The results are presented in Table 3 and Figure 1.
Changes in the number of monocyte subpopulations during MHT
The monocyte population, determined by logistic gating of the population identified by direct versus side light scatter plot by size and granularity, and the population identified by pan-leukocyte marker CD45 expression level and granularity (SSdimCD45dim) on the side light scatter plot versus CD45 expression, determined the expression levels of CD11b and CD40. No statistically significant differences were observed before and after the therapy. Among the classical (CD14++CD16-) monocytes, the staging of which is shown in Figure 3, therapy resulted in a statistically significant increase in CD14++CD16-CX3CR1+ cells (Wilcoxon nonparametric test was used for comparison, p=0.0011), as shown in Figure 2.
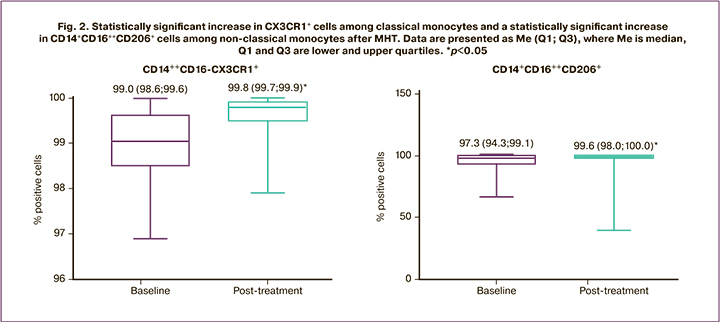
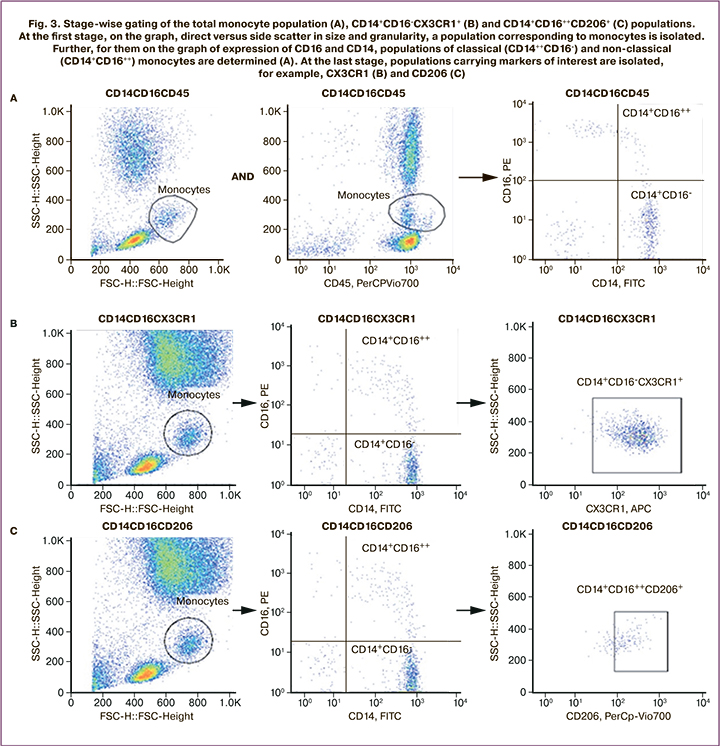
Analysis of the expression levels of proinflammatory (CD86, CD80, and CD40) and anti-inflammatory (CD163 and CD206) markers among classical (CD14++CD16-) and non-classical (CD14+CD16++) monocytes showed a statistically significant increase in the number of CD14++CD16++CD206+ cells (nonparametric Wilcoxon test for comparison, p=0.012). The results are shown in Figure 2.
Discussion
Decreased ovarian function in aging women is associated with the development of various degenerative diseases such as osteoporosis and atherosclerosis, as well as changes in certain parameters of the immune system. MHT is widely used to control or alleviate menopausal syndrome and prevent these disorders in postmenopausal women [14]. The effects of MHT on the immune system have been extensively studied. Before prescribing MHT to patients, we performed a correlation analysis of immune status parameters and clinical and anamnestic characteristics, which revealed significant correlations between monocyte subpopulation levels and age, and BMI. It should be noted that similar studies on similar samples of peri- and postmenopausal patients are difficult to find in the literature. Most of the literature suggests a positive correlation between BMI and monocyte count and classic monocyte percentage and a negative correlation with non-classical monocyte levels [15–17]. Data on the correlation of monocyte counts with age vary; Snodgrass R.G. et al. observed a decrease in their numbers with age [18], whereas Pirabe A. et al. showed no age-associated differences in monocyte levels and phenotypic composition [19].
Estrogens are known to exert a dose-dependent modulating effect on all levels of innate and acquired immunity through specific nuclear and non-nuclear cell receptors [20]. Estrogen receptor alpha is expressed on T- and B-lymphocytes, NK cells, and macrophages, while monocytes, B cells, and macrophages express estrogen receptor beta. Estrogen deficiency in postmenopausal women can alter both cellular and humoral immune responses [21]. Reduced antiviral responses and antibody production have been reported in women with estrogen deficiency [22]. The mechanisms of immune responses against the background of estrogen deficiency are complex and not yet clear. T-cells play a central role in immunomodulation. T helper (Th1) cells, through IL-2, TNF-α, and interferon gamma, contribute to the cellular immune response to fight viruses, other intracellular microorganisms, and malignant cells and can stimulate delayed-type hypersensitivity reactions. Th2 cells, through IL-4, IL-5, IL-6, IL-10, and IL-13, induce a humoral immune response against extracellular microorganisms by activating antibody production [23]. Thus, by inducing exquisitely balanced Th1 and Th2 type immune responses, protection against infectious agents or malignant cells is developed [24]. Several researchers have demonstrated a progressive decrease in the number of naïve T cells [25, 26] and reduced memory T-cell and NK-cell function in postmenopausal women [27]. The present study showed that three months of MHT with 1.5 mg daily 0.06% estradiol hemihydrate transdermal gel + vaginal micronized progesterone in cyclic or continuous mode was associated with a statistically significant increase in T-helper numbers. This can be seen as a positive effect of MHT, acting against the decrease in the CD4/CD8+ cell ratio observed in aging women compared to that in women of reproductive age. Researchers have demonstrated the benefits of MHT in increasing lymphocyte production and serum macrophage colony-stimulating factor levels [7, 27]. Xia X. et al. showed that oral and transdermal MHT improved the balance of Th1/Th2 cytokines by acting on T lymphocytes, predominantly through estrogen receptors [28]. Medeiros S.F. et al. observed an increase in the CD4+/CD8+ ratio with combined MHT in postmenopausal women [29]. At the same time, several studies have found no effect of MHT on lymphocytes [30–32].
Monocytes are circulating precursors of macrophages and dendritic cells [33] that play an important role in both inflammation and homeostasis. In both cases, monocytes arise continuously from hematopoietic precursors in the bone marrow and migrate to the tissues via the bloodstream. Monocytes are subdivided into three subsets: classical, intermediate, and non-classical, based on their expression on the cell surface of CD14 and CD16 in humans and Ly6C, CX3CR1, and CCR2 in mice. In tissues, monocytes further differentiate into monocyte-derived macrophages and dendritic cells to mediate innate and adaptive immune responses and to maintain tissue homeostasis [34].
In the present study, among the total monocyte population, MHT resulted in a statistically significant increase in CD14++CD16-CX3CR1+ cells among classical monocyte subpopulations. CX3CR1 is a fractalkin receptor (CX3CL1) expressed during inflammatory activation of endothelial cells. Analysis of the expression levels of proinflammatory (CD86, CD80, and CD40) and anti-inflammatory (CD163 and CD206) markers among classical (CD14+CD16-) and non-classical (CD14+CD16++) monocytes showed a statistically significant increase in CD14+CD16++CD206+ among the latter. CD206 is a mannose receptor that is important for the detection of infectious agents by cells of the monocyte-macrophage system. This result indicates that MHT potentiates both classical and non-classical monocytes after 3 months of administration.
The Ben-Hur H. study found that monocyte counts increased in postmenopausal women and decreased with estrogen therapy [35]. Similar data on CD206+ and CD86+ cell counts among monocyte subpopulations after MHT are not available in the literature; therefore, we analyzed changes in other immune parameters, such as cytokine secretion. A study by Yasui T. et al. showed that IL-6 levels tended to be elevated in the group of women using oral estrogens as part of MHT and remained unchanged throughout the study when taking transdermal forms of estrogen [36]. IL-6 is synthesized by Kupffer cells in the liver [37]. Therefore, an increase in IL-6 levels following oral estrogen administration may be related to hepatic metabolism. In contrast, IL-6 production by mononuclear cells and serum IL-6 levels have been shown to decrease following estrogen transdermal therapy [38]. IL-6 is involved not only in inflammation but also in the regulation of endocrine and metabolic functions, and it is produced and released by the adrenal glands when stimulated by corticotropin [39], as well as by monocytes, T-lymphocytes, endothelial cells, and adipocytes. Berg et al. reported that IL-6 secretion was lower in mononuclear cells during estrogen monotherapy, but not during combination of estrogen and progesterone in postmenopausal women [40].
Yasui T. et al found significant differences in concentrations of IL-8 and macrophage inflammatory protein (MIP-1b) (proinflammatory cytokines) between groups of patients receiving oral and transdermal estrogens as part of MHT. Both oral and transdermal therapies significantly reduced serum IL-8 levels after 12 months of MHT. Serum concentrations of IL-8, monocyte chemoattractant protein-1 and MIP-1b were reduced by transdermal estrogen delivery, but oral therapy resulted in higher IL-8 concentrations [36].
Conclusion
Our findings suggest that MHT, including transdermal estradiol in combination with intravaginal micronized progesterone, is associated with changes in the patients' blood immune parameters. It affects both lymphocytes and monocytes, with changes in the monocyte phenotype in different subpopulations that are likely to contribute to the immunopotentiating properties of MHT.
References
1. Ghare Naz M.S., Banaei M., Dashti S., Tehrani F.R. An overview of sex hormones in relation to SARS‑CoV‑2 infection. Future Virol. 2021; Jul: 10.2217/ fvl‑2021‑0058. https://dx.doi.org/10.2217/fvl‑2021‑0058.
2. Marina S., Piemonti L. Gender and age effects on the rates of infection and deaths in individuals with confirmed SARS‑CoV‑2 infection in Six European Countries. SSRN Electron J. 2020; 16. https://dx.doi.org/10.2139/ssrn.3576790.
3. Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID‑19 outcomes. Nat. Rev. Immunol. 2020; 20(7): 442‑7. https://dx.doi.org/10.1038/ s41577‑020‑0348‑8.
4. Кречетова Л.В., Инвияева Е.В., Садыков В.Ф., Вторушина В.В., Иванец Т.Ю., Силачев Д.Н., Пырегов А.В., Долгушина Н.В., Сухих Г.Т. Состояние иммунной системы у пациентов с различной степенью тяжести COVID‑19. Акушерство и гинекология. 2021; 8: 75‑85. [Krechetova L.V., Inviyaeva E.V., Sadykov V.F., Vtorushina V.V., Ivanets T.Yu., Silachev D.N., Pyregov A.V., Dolgushina N.V., Sukhikh G.T. Immune status of COVID‑19 patients with different disease severity. Obstetrics and Gynecology. 2021; (8): 75‑85. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.8.75‑85.
5. Averyanova M., Vishnyakova P., Yureneva S., Yakushevskaya O., Fatkhudinov T., Elchaninov A., Sukhikh G. Sex hormones and immune system: menopausal hormone therapy in the context of COVID‑19 pandemic. Front. Immunol. 2022; 13: 928171. https://dx.doi.org/10.3389/fimmu.2022.928171.
6. Feehan J., Tripodi N., Apostolopoulos V. The twilight of the immune system: The impact of immunosenescence in aging. Maturitas. 2021; 147: 7‑13. https://dx.doi.org/10.1016/j.maturitas.2021.02.006.
7. Kamada M., Irahara M., Maegawa M., Ohmoto Y., Murata K., Yasui T. et al. Transient increase in the levels of T‑helper 1 cytokines in postmenopausal women and the effects of hormone replacement therapy. Gynecol. Obstet. Invest. 2001; 52(2): 82‑8. https://dx.doi.org/10.1159/000052948.
8. Porter V.R., Greendale G.A., Schocken M., Zhu X., Effros R.B. Immune effects of hormone replacement therapy in post‑menopausal women. Exp. Gerontol. 2001; 36(2): 311‑26. https://dx.doi.org/10.1016/s0531‑5565(00)00195‑9.
9. Vrachnis N., Zygouris D., Vrachnis D., Antonakopoulos N., Fotiou A., Panagopoulos P. et al. Effects of hormone therapy and favonoids capable on reversal of menopausal immune senescence. Nutrients. 2021; 13(7). https://dx.doi.org/10.3390/nu13072363.
10. Юренева С.В., Сыркашева А.Г., Перминова С.Г., Смольникова В.Ю., Митюрина Е.В., Рогачевский О.В. Ведение женщин в переходном периоде и ранней постменопаузе. Учебное пособие. 2022. [Yureneva S.V., Syrkasheva A.G., Perminova S.G., Smolnikova V.Yu., Mityurina E.V., Rogachevsky O.V. Management of women in the transition period and early postmenopause. Tutorial. 2022. (in Russian)].
11. Lovre D., Peacock E., Katalenich B., Moreau C., Xu B., Tate C. et al. Conjugated estrogens and bazedoxifene improve β cell function in obese menopausal women. J. Endocr. Soc. 2019; 3(8): 1583‑94. https://dx.doi.org/10.1210/ js.2019‑00074.
12. Mauvais-Jarvis F., Manson J.E., Stevenson J.C., Fonseca V.A. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr. Rev. 2017; 38(3): 173‑88. https://dx.doi.org/10.1210/er.2016‑1146.
13. Российское общество акушеров‑гинекологов. Клинические рекомендации. Менопауза и климактерическое состояние у женщины. 2021. [Russian Society of Obstetricians and Gynecologists. Clinical Guidelines. Menopause and the menopausal state in women. 2021. (in Russian)].
14. Hammond C.B. Menopause and hormone replacement therapy: an overview. Obstet. Gynecol. 1996; 87(Suppl. 2):2S‑15S. https://dx.doi.org/10.1016/ 0029‑7844(95)00429‑7.
15. Figueroa-Vega N., Marín-Aragón C.I., López-Aguilar I., Ibarra-Reynoso L., Pérez-Luque E., Malacara J.M. Analysis of the percentages of monocyte subsets and ILC2s, their relationships with metabolic variables and response to hypocaloric restriction in obesity. PLoS One. 2020; 15(2): e0228637. https://dx.doi.org/10.1371/journal.pone.0228637.
16. Friedrich K., Sommer M., Strobel S., Thrum S., Blüher M., Wagner U., Rossol M. Perturbation of the monocyte compartment in human obesity. Front. Immunol. 2019; 10: 1874. https://dx.doi.org/10.3389/fimmu.2019.01874.
17. Rogacev K.S., Ulrich C., Blömer L., Hornof F., Oster K., Ziegelin M. et al. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur. Heart J. 2010; 31(3): 369‑76. https://dx.doi.org/10.1093/eurheartj/ ehp308.
18. Snodgrass R.G., Jiang X., Stephensen C.B. Monocyte subsets display age‑dependent alterations at fasting and undergo non‑age‑dependent changes following consumption of a meal. Immun. Ageing. 2022; 19(1): 41. https://dx.doi.org/10.1186/s12979‑022‑00297‑6.
19. Pirabe A., Heber S., Schrottmaier W.C., Schmuckenschlager A., Treiber S., Pereyra D. et al. Age related differences in monocyte subsets and cytokine pattern during acute COVID‑19‑A prospective observational longitudinal study. Cells. 2021; 10(12): 3373. https://dx.doi.org/10.3390/cells10123373.
20. Cheskis B.J., Greger J.G., Nagpal S., Freedman L.P. Signaling by estrogens. J. Cell. Physiol. 2007; 213(3): 610‑7. https://dx.doi.org/10.1002/jcp.21253.
21. Gameiro C., Romao F. Changes in the immune system during menopause and aging. Front. Biosci (Elite Ed). 2010; 2(4): 1299‑303. https://dx.doi.org/10.2741/e190.
22. Pennell L.M., Galligan C.L., Fish E.N. Sex affects immunity. J. Autoimmun. 2012; 38(2‑3): J282‑91. https://dx.doi.org/10.1016/j.jaut.2011.11.013.
23. Martocchia A., Stefanelli M., Cola S., Falaschi P. Sex steroids in autoimmune diseases. Curr. Top. Med. Chem. 2011; 11(13): 1668‑83. https://dx.doi.org/ 10.2174/156802611796117595.
24. Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996; 383(6603): 787‑93. https://dx.doi.org/10.1038/383787a0.
25. Cossarizza A., Ortolani C., Paganelli R., Barbieri D., Monti D., Sansoni P. et al. CD45 isoforms expression on CD4+ and CD8+ T cells throughout life, from newborns to centenarians: implications for T cell memory. Mech. Ageing Dev. 1996; 86(3): 173‑95. https://dx.doi.org/10.1016/0047‑6374(95)01691‑0.
26. Stulnig T., Maczek C., Böck G., Majdic O., Wick G. Reference intervals for human peripheral blood lymphocyte subpopulations from “healthy” young and aged subjects. Int. Arch. Allergy Immunol. 1995; 108(3): 205‑10. https://dx.doi.org/10.1159/000237155.
27. Kamada M., Irahara M., Maegawa M., Yasui T., Takeji T., Yamada M. et al. Effect of hormone replacement therapy on post‑menopausal changes of lymphocytes and T cell subsets. J. Endocrinol. Invest. 2000; 23(6): 376‑82. https://dx.doi.org/10.1007/BF03343741.
28. Xia X., Zhang S., Yu Y., Zhao N., Liu R., Liu K., Chen X. Effects of estrogen replacement therapy on estrogen receptor expression and immunoregulatory cytokine secretion in surgically induced menopausal women. J. Reprod. Immunol. 2009; 81(1): 89‑96. https://dx.doi.org/10.1016/j.jri.2009.02.008.
29. de Medeiros S.F., Maitelli A. Cellular and humoral immune responses after short‑term oral hormone therapy in postmenopausal women. Climacteric. 2011; 14(6): 677‑82. https://dx.doi.org/10.3109/13697137.2011.570387.
30. Itaborahy R.M.R., de Medeiros S.F. Influence of estrogen therapy on immune markers in postmenopausal women. Climacteric. 2016; 19(5): 496‑500. https://dx.doi.org/10.1080/13697137.2016.1212828.
31. Straub R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007; 28(5): 521‑74. https://dx.doi.org/10.1210/er.2007‑0001.
32. Yang J.H., Chen C.D., Wu M.Y., Chao K.H., Yang Y.S., Ho H.N. Hormone replacement therapy reverses the decrease in natural killer cytotoxicity but does not reverse the decreases in the T‑cell subpopulation or interferon‑gamma production in postmenopausal women. Fertil. Steril. 2000; 74(2): 261‑7. https://dx.doi.org/10.1016/s0015‑0282(00)00622‑1.
33. Tacke F. [Monocyte subpopulations in inflammation processes: principles and perspectives. Dtsch. Med. Wochenschr. 2009; 134(33): 1645‑8. (in German)]. https://dx.doi.org/10.1055/s‑0029‑1233994.
34. Kawamura S., Ohteki T. Monopoiesis in humans and mice. Int. Immunol. 2018; 30(11): 503‑9. https://dx.doi.org/10.1093/intimm/dxy063.
35. Ben-Hur H., Mor G., Insler V., Blickstein I., Amir-Zaltsman Y., Sharp A. et al. Menopause is associated with a significant increase in blood monocyte number and a relative decrease in the expression of estrogen receptors in human peripheral monocytes. Am. J. Reprod. Immunol. 1995; 34(6): 363‑9. https://dx.doi.org/10.1111/j.1600‑0897.1995.tb00965.x.
36. Yasui T., Saijo A., Uemura H., Matsuzaki T., Tsuchiya N., Yuzurihara M. et al. Effects of oral and transdermal estrogen therapies on circulating cytokines and chemokines in postmenopausal women with hysterectomy. Eur. J. Endocrinol. 2009; 161(2): 267‑73. https://dx.doi.org/10.1530/EJE‑09‑0063.
37. Ucan H.B., Kaplan M., Salman B., Yilmaz U., Mentes B.-B., Aybay C. Effect of oophorectomy and exogenous estrogen replacement on liver injury in experimental obstructive jaundice. World J. Gastroenterol. 2008; 14(18): 2818‑24. https://dx.doi.org/10.3748/wjg.14.2818.
38. Berg G., Ekerfelt C., Hammar M., Lindgren R., Matthiesen L., Ernerudh J. Cytokine changes in postmenopausal women treated with estrogens: a placebo‑controlled study. Am. J. Reprod. Immunol. 2002; 48(2): 63‑9. https://dx.doi.org/10.1034/j.1600‑0897.2002.01061.x.
39. Judd A.M., Call G.B., Barney M., McIlmoil C.J., Balls A.G., Adams A., Oliveira G.K. Possible function of IL‑6 and TNF as intraadrenal factors in the regulation of adrenal steroid secretion. Ann. N. Y. Acad. Sci. 2000; 917: 628‑37. https://dx.doi.org/10.1111/j.1749‑6632.2000.tb05428.x.
40. Saucedo R., Rico G., Basurto L., Ochoa R., Zárate A. Transdermal estradiol in menopausal women depresses interleukin‑6 without affecting other markers of immune response. Gynecol. Obstet. Invest. 2002; 53(2): 114‑7. https://dx.doi.org/10.1159/000053005.
Received 07.03.2023
Accepted 30.03.2023
About the Authors
Marina V. Averyanova, postgraduate student at the Department of Gynecological Endocrinology, obstetrician-gynecologist, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(968)979-23-97, marisha199022@mail.ru, https://orcid.org/0000-0002-2995-5228, 117997, Russia, Moscow, Oparin str., 4.Svetlana V. Yureneva, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Department of Vocational Education, Leading Researcher, Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, syureneva@gmail.com, https://orcid.org/0000-0003-2864-066Х, 117997, Russia, Moscow, Oparin str., 4.
Victoria V. Kiseleva, Junior Researcher, Laboratory of Regenerative Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, victoria.kurnosova.1991@gmail.com, 117997, Russia, Moscow, Oparin str., 4.
Oksana V. Yakushevskaya, PhD, obstetrician-gynecologist, oncologist, Researcher at the Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)531-44-44, aluckyone777@gmail.com, https://orcid.org/0000-0002-7430-1207, 117997, Russia, Moscow, Oparin str., 4.
Marina E. Iskusnykh, Laboratory Assistant, Research Institute of Molecular and Cellular Medicine, RUDN, iskusnyh.m@yandex.ru, 117997, Russia, Moscow, Miklukho-Maklaya str., 8.
Andrey V. Elchaninov, Dr. Med. Sci., Head of the Laboratory of Regenerative Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, elchandrey@yandex.ru, 117997, Russia, Moscow, Oparin str., 4.
Timur Kh. Fatkhudinov, Dr. Med. Sci., Deputy Director, Avtsyn Research Institute of Human Morphology; Head of the Department of Histology, Cytology and Embryology, Director of the Research Institute of Molecular and Cellular Medicine, RUDN, tfat@yandex.ru, 117997, Russia, Moscow , Miklukho-Maklaya str., 8.
Polina A. Vishnyakova, PhD, Senior Researcher, Laboratory of Regenerative Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, p_vishnyakova@oparina4.ru, https://orcid.org/0000-0001-8650-8240, 117997, Russia, Moscow, Oparin str., 4.



