Russian eligibility criteria for menopausal hormone therapy in patients with cardiovascular and metabolic diseases. Consensus document of the Russian Society of Cardiology, Russian Society of Obstetricians and Gynecologists, Russian Association of Endocrinologists,Eurasian Association of Therapists, Russian Phlebological Association
Shlyakhto E.V., Sukhikh G.T., Serov V.N., Dedov I.I., Arutyunov G.P., Suchkov I.A.
Co-chairs: Е.V. Shlyakhto, G.T. Sukhikh, V.N. Serov, I.I. Dedov, G.P. Arutyunov, I.A. Suchkov
Executive secretary of the working group: Y.A. Orlova
Working group: E.N. Andreeva, S.V. Yureneva, I.S. Yavelov, M.I. Yarmolinskaya, S.V. Villevalde, O.R. Grigoryan, E.N. Dudinskaya, E.A. Ilyukhin, N.A. Koziolova, I.V. Sergienko, A.A. Smetnik, N.I. Tapilskaya
Experts: N.V. Artymuk, A.G. Arutyunov, V.E. Balan, I.I. Baranov, S.A. Bobrov, R.I. Gabidullina, N.Yu. Grigor'eva, I.V. Gubareva, O.V. Dzhenina, Yu.E. Dobrokhotova, S.O. Dubrovina, E.V. En'kova, E.I. Ermakova, S.K. Zyryanov, N.Yu. Katkova, L.Yu. Karakhalis, T.V. Kirsanova, T.Yu. Kuznetsova, T.A. Makarenko, L.I. Mal'tseva, S.V. Mal'chikova, S.V. Nedogoda, S.Yu. Nikulina, T.A. Oboskalova, M.M. Petrova, A.G. Plisyuk, V.I. Podzolkov, N.M. Podzolkova, A.E. Protasova, I.V. Savel'eva, E.A. Sandakova, I.V. Sakhautdinova, M.S. Selikhova, T.M. Sokolova, L.S. Sotnikova, N.V. Spiridonova, E.I. Tarlovskaya, I.V. Fomin, M.B. Khamoshina, A.I. Chesnikova, G.A. Chumakova, I.I. Shaposhnik
Menopausal symptoms can significantly disrupt the lives of women who are at the peak of their careers and family life. Currently, menopausal hormone therapy (MHT) is the most effective approach to alleviate these symptoms. It is important to note that the presence of cardiovascular and metabolic diseases should not automatically rule out MHT in managing menopausal symptoms and enhancing overall quality of life. However, a common obstacle in the use of MHT is the fear of some physicians that it may do more harm than good to their patients. Caution should be exercised, especially in women with coexisting medical conditions. Furthermore, it is important to acknowledge the limited availability of high-quality research on the safety of MHT in the context of major chronic noncommunicable diseases and common comorbidities. This consensus document reviews all current data from clinical trials of various designs and provides a set of criteria for determining the suitability of MHT for women with cardiovascular and metabolic comorbidities. The aim of this document is to equip physicians from diverse specialties who care for menopausal women with an accessible algorithm that can help them avoid potentially risky situations and guide their decisions regarding prescribing menopausal hormone therapy in real-world practice.
For citation: Shlyakhto E.V., Sukhikh G.T., Serov V.N., Dedov I.I., Arutyunov G.P., Suchkov I.A. Russian eligibility criteria for menopausal hormone therapy in patients with cardiovascular and metabolic diseases. Consensus document of the Russian Society of Cardiology, Russian Society of Obstetricians and Gynecologists, Russian Association of Endocrinologists, Eurasian Association of Therapists, Russian Phlebological Association.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (11): 211-232 (in Russian)
https://dx.doi.org/10.18565/aig.2021.267
Keywords
Introduction
By the Decree of the Government of the Russian Federation dated December 29, 2022 No. 4356-r, the National strategy of actions in the interest of women 2023-2030 was approved. One of the important objectives of state policy is to preserve the health of women of all ages, improve their quality of life, and increase the period of active aging [1]. To implement this strategy in health care, an interdisciplinary approach is essential. Internists, together with obstetricians and gynecologists, need to identify women who have entered menopausal transition in order to provide them with the necessary assistance in a timely manner.
Menopausal symptoms can disrupt the lives of women at the peak of their careers and family life: 75% of women 45–55 years old complain of hot flashes; in 28.5% of cases these are hot flashes of moderate or severe severity; The duration of symptoms can be 3–15 years [2]. Currently, menopausal hormone therapy (MHT) is the most effective treatment for these manifestations is menopausal hormone therapy (MHT) [3, 4].
The presence of cardiovascular and metabolic diseases in itself does not exclude the possibility of prescribing MHT to relieve menopausal symptoms and improve the quality of life. However, a common obstacle in the use of MHT is the fear of some physicians that it may do more harm than good to their patients.
Caution is especially important in women with underlying health conditions. Moreover, high-quality research regarding the safety of MHT for major chronic non-communicable diseases and common comorbid conditions is lacking.
Thus, the purpose of the conciliation document is:
To analyze all currently available data from clinical trials of various designs and create a set of criteria for the eligibility of MHT in women with underlying cardiovascular and metabolic diseases.
Based on the presented document, doctors of various specialties who advise menopausal women will receive an accessible algorithm that allows them to avoid potentially dangerous situations and reasonably prescribe MHT in real practice.
Section 1. Basic definitions, symptoms and classification of menopause
The menstrual cycle is one of the most important indicators of a woman's health, and its regularity can vary depending on the stage of reproductive ageing.
The Stages of Reproductive Aging Workshop (STRAW) [5] distinguishes three stages of reproductive aging: the reproductive stage, menopausal transition, and postmenopause. The STRAW+10 classification of the stages of aging in the female reproductive system is shown in Figure 1.1.
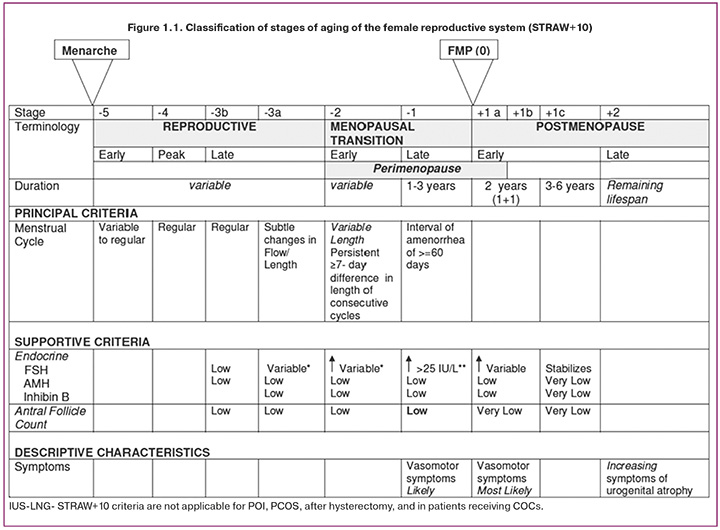
Menopausal transition is characterized by disruption of the regularity of menstrual cycles, which is a reflection of variability in hormonal secretion and ovulatory function.
Menopause is a permanent cessation of menstruation, which is the last independent menstruation caused by an age-related decrease in hormonal activity and the “switching off” of the reproductive function of the ovaries. The date of menopause is assessed retrospectively: after 12 months of absence of menstruation [6, 7].
Perimenopause – includes the period of menopausal transition + 1 year after the last menstruation.
Perimenopause begins with disruption of the regularity of the menstrual cycle (“menopausal transition phase”) and lasts up to one year after the complete cessation of menstruation. This phase of reproductive aging can occur over a wide age range (from 42 to 58 years) and last up to 4–8 years [8].
Postmenopause is defined as the period of life after the last menstrual cycle.
Menopausal syndrome is a complex of vegetative- vascular, mental and metabolic-endocrine disorders that occur in women against the background of fading (or sudden loss) of hormonal function of the ovaries and general aging of the body [9].
The average age at menopause worldwide is 48.8 years (95% CI 48.3–49.2), with significant fluctuations in this indicator depending on the geographical region of residence of women [10], in the Russian Federation it ranges from 49 to 51 year [9]. The prevalence of menopausal symptoms varies depending on the circumstances.
Vasomotor symptoms occur more often in the late period of the menopausal transition and are especially pronounced in perimenopause and the first years of postmenopause [11, 12]. Vasomotor symptoms affect up to 80% of perimenopausal women [13]. Sleep disturbances occur in 39–47% of perimenopausal women and 35–60% of postmenopausal women [14]. Osteoporosis is detected in 34% of women aged 50 years and older in the Russian Federation, and the incidence of osteopenia is 43% [15].
Vasomotor symptoms and other manifestations of menopausal syndrome not only worsen the quality of life of women and limit their functional capabilities, but are also associated with an increase in the risk of developing coronary heart disease (CHD) by 1.34 times, and the risk of any cardiovascular diseases (CVD) by 1.34 times. 1.48 times [16].
Up to 15% of perimenopausal women and up to 80% of postmenopausal women experience symptoms of genitourinary syndrome of menopause (GSM) or vulvovaginal atrophy (VVA) [17]. 41% of women aged 50–79 years had at least one of the symptoms of VVA. The prevalence of urinary disorders (sudden and irresistible urge to urinate, which cannot be delayed, and urinary incontinence) in women depends on the duration of postmenopause and increases from 15.5% with postmenopause up to 5 years and to 41.4% with a postmenopausal duration of more than 20 years [17].
Classification of menopause
Based on the time of onset, they were distinguished as follows:
1. premature menopause or premature ovarian insufficiency (up to 40 years);
2. early (40–44 years);
3. timely (45–55 years);
4. late (over 55 years old).
According to the onset of menopause, natural and iatrogenic(includingsurgical) menopausearedistinguished.
Section 2. Indications and contraindications for MHT
Indications and contraindications for the use of MHT are determined by current Clinical Guidelines and instructions for specific drugs.
Indications for MHT [4]
- Treatment of moderate to severe vasomotor symptoms significantly reduces quality of life.
- Treatment of GSM symptoms, sexual dysfunction.
- Prevention of postmenopausal osteoporosis.
- Replenishment of estrogen deficiency in premature ovarian failure (POI) and early menopause; after bilateral oophorectomy.
Contraindications to MHT administration [4]
- Bleeding from the genital tract of unknown origin.
- Breast cancer (diagnosed, suspected or history).
- Diagnosed or suspected estrogen-dependent malignant neoplasms (endometrium, ovaries, uterus).
- Acute and chronic liver diseases currently or in history (before normalization of liver function tests), including malignant liver tumors.
- Thrombosis(arterialandvenous)andthromboembolism currently or in history (including deep vein thrombosis and pulmonary embolism).
- Myocardial infarction.
- Ischemic or hemorrhagic cerebrovascular disorders.
- Presence of uterine fibroids with submucosal location of the nodule.
- Presence of endometrial polyp.
- Allergy to MHT components.
- Porphyria cutanea (for estrogen component).
- Progestogen-dependent neoplasms (e.g., meningio- ma) (for gestagens).
Section 3. Types of MHT and basic principles of its purpose
Systemic MHT
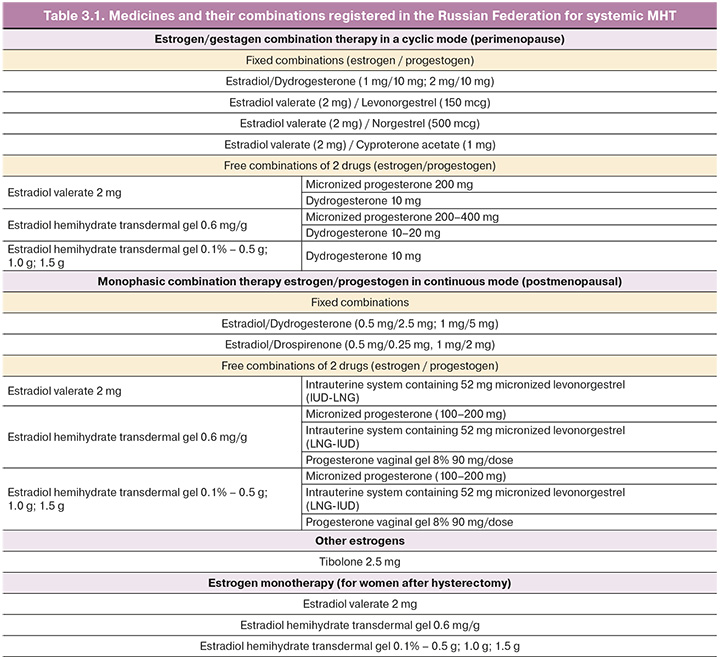
Systemic MHT is the most effective treatment for vasomotor symptoms and other menopausal manifestations including GSM. Most MHT drugs are approved for the prevention of postmenopausal osteoporosis, with the exception of the ultra-low-dose forms.
Table 3.1 presents the drugs for systemic MHT registered in the Russian Federation.
Local MHT
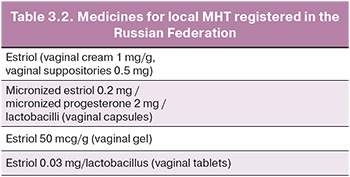
Local estrogen therapy (estriol) is used in peri- and postmenopausal women who complain only of GSM symptoms such as vaginal dryness, dyspareunia, or sexual discomfort associated with this condition.
Long-term observations (6–24 months) show no effect of local estrogens on the endometrium; therefore, additional use of progestogens is not required. Topical estrogens do not increase the risk of venous thromboembolic events (VTE), breast cancer (BC), CVD, endometrial hyperplasia, or endometrial cancer in observational studies [18]. Table 3.2 presents the drugs for local MHT registered in the Russian Federation.
Basic principles of MHT administration
1. Initiation of systemic MHT should be considered in women aged < 60 years and less than 10 years postmenopausal. The optimal time to start MHT is during perimenopause and early post-menopause. There are no age restrictions when prescribing local estrogen (estriol) therapy for GSM symptoms.
2. The therapeutic goal should be to use the most appropriate minimum effective dose of MHT that is consistent with the treatment goals.
3. MHT is individualized based on the risk factors for breast cancer, cardiovascular disease, osteoporosis, and fractures. The choice of dose and dosage form of the drug, its composition, and mode of use should be determined by considering the patient’s age, stage of reproductive aging, gynecological diseases (POI (primary/secondary), PCOS, presence of an intact uterus/hysterectomy, endometriosis), comorbid conditions, and preferences and needs.
4. Availability of indications for prescribing MHT and absence of contraindications.
5. The use of MHT requires periodic dosage adjustments depending on the stage of reproductive aging, age, effectiveness, and tolerability of the treatment. As the patient's age and postmenopausal duration increase, it is advisable to reduce the MHT dose.
6. Monitoring of treatment and regular (at least once a year) reassessment of benefits/risks. The duration of therapy is determined by the purpose of the therapy and benefit/risk balance.
The safety profile of the constituent components is considered when selecting an MHT. Personalizing the dosage of MHT considering the patient’s risk factors (CVD, risk of breast cancer, osteoporosis, comorbid conditions, etc.) allows the selection of the minimum effective dosage and method of drug delivery [14, 15].
The prescription, correction, or cancellation of MHT, as well as dynamic monitoring of the effectiveness and tolerability of treatment, lies in the area of responsibility of the obstetrician-gynecologist.
Section 4. MHT in patients with obesity and carbohydrate metabolism disorders
Insulin resistance, dyslipidemia, arterial hypertension, and abdominal obesity are the main markers of menopausal metabolic syndrome [19]. Compared to the reproductive period, perimenopausal and early postmenopausal women are at a higher risk of progressing to insulin resistance [20]. With age, the risk of developing metabolic syndrome (MS) increases 5 times in women. The incidence of CVD increases 5 times in women with carbohydrate metabolism disorders [21].
Obesity, especially abdominal obesity, is closely associated with MS, significantly increases cardiometabolic risk, and affects the incidence, prognosis, and life expectancy of patients [22].
Obesity is an independent risk factor for the development of VTE. In the randomized Women's Health Initiative (WHI) trial, obese women (BMI >30 kg/m2) had a 3-fold increased risk of VTE compared with women with normal BMI, even in the placebo group [23].
In the case of obesity, it is undesirable to prescribe drugs containing gestagens with residual androgenic and glucocorticoid activity; preference is given to metabolically neutral progestogens [24]. Following the discovery of an association between mineralocorticoid receptors and adipose tissue differentiation, the potential role of progesterone and progestins with anti- mineralocorticoid properties in controlling body weight and adipose tissue proliferation has been established [25]. According to a comparative study of the administration of combined MHT containing drospirenone or dydrogesterone, patients with menopausal metabolic syndrome showed a significant decrease in weight after 6 months of therapy (from 74.2 to 72.4 kg in the E/DDG group (p=0.03) and from 74.5 to 72.7 kg in the E/DRSP group (p=0.05). There was an improvement in fasting glucose (p<0.05) in both groups, and improvements in HOMA-IR (p=0.03) and MAGE were noted in the E/ DRSP group (p<0.001) [26].
The incidence of type 2 diabetes mellitus (DM) in the female population is as follows: 40–44 years, 1.2%; 45–49 years, 2.4%; 50–54 years, 4.2%; and 55–59 years, 9.4% [27]. Timely initiation of MHT may delay the risk of developing the DM2 type. According to the WHI, therapy with conjugated equine estrogens (CEE) + medroxyprogesterone acetate (MPA) significantly reduced the incidence of DM2 by 19% (RR 0.81; 95% CI 0.70–0.94; P=0.005), which corresponds to a reduction of 16 cases per 10,000 woman-years. In the CEE monotherapy cohort, the number of new DM2 diagnoses was reduced by 14% (RR 0.86; 95% CI 0.76– 0.98), corresponding to a reduction of 21 cases per 10,000 woman-years [28].
According to a meta-analysis of 107 studies, MHT reduces the risk of developing DM2 by 30% (RR 0.7; 95% CI 0.6–0.9), and in the case of existing DM, MHT reduces fasting glucose and HOMA levels. IR also leads to an improvement in the lipid profile and a decrease in blood pressure, along with a decrease in the degree of abdominal obesity. During estrogen monotherapy or combined MHT, there was no increase in the risk of cardiovascular mortality in women with type 2 diabetes [29].
For type DM2, oral MHT is preferred unless there are contraindications. When prescribing combined MHT, it is important to consider the metabolic effects of the progestogen included in the combined MHT, and progestogens with a neutral effect on metabolic processes should be chosen [30].
The beneficial effect of MHT on carbohydrate metabolism ceases when therapy is discontinued.
Thus, MHT may be considered a treatment for menopausal symptoms in patients with DM2 type.
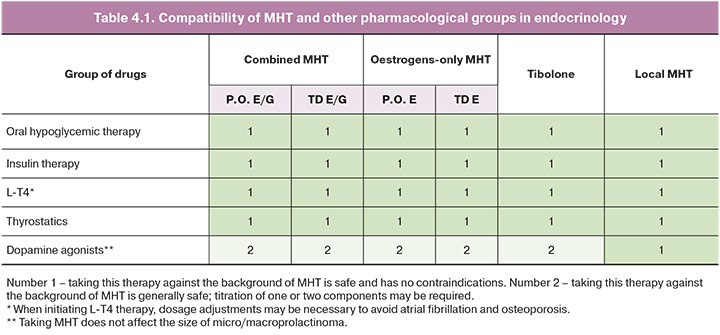
The compatibility of glucose-lowering therapy with MHT, levothyroxine sodium (L-T4) replacement therapy, and thyreostatic and dopaminergic therapy, considering the route of administration, is shown in Table 4.1 [31].
Key Points
- Timely initiation of MHT delays the development of DM2
- Together with the use of MHT in obese women, it is recommended to conduct educational conversations to correct their usual lifestyle.
- Oral MHT is preferred for patients with DM2 type. If there are contraindications to oral administration or an increased risk of thrombosis, transdermal forms of MHT can be used.
- In women with a preserved uterus, progestogens with a neutral effect on metabolic processes should be chosen.
- MHT has a positive effect on glycemic profile in women without DM and in women with type 2 DM.
Section 5. MHT in patients with thrombophilias, venous diseases, venous embolisms
5.1. MHT composition and the risk of venous thromboembolic complications MHT containing oral estrogens is believed to increase the risk of VTE - deep vein thrombosis (DVT) and pulmonary embolism (PE) [32, 33]. However, this effect, noted in randomized controlled trials and meta-analyses based on them, may be largely associated with the administration of rather “thrombogenic” drugs based on CEE and MPA, as well as with the untimely initiation of MHT.
Thus, according to a case-control analysis of the large QResearch and CPRD databases, the administration of MHT CEE in combination with MPA was associated with the highest risk of VTE. There was a significant increase in the risk of VTE for oral estradiol, and this effect was dose dependent. However, for the combination of oral estradiol and dydrogesterone, the risk of VTE did not increase with either cyclic or monophasic combination MHT regimens, regardless of estradiol dose. Administration of transdermal estradiol, either as monotherapy or in combination with MHT, was not associated with an increased risk of VTE. Regardless of BMI, the combination of oral estradiol with dydrogesterone and transdermal estradiol, either alone or in combination with progestin, was not associated with an increased VTE risk. In a cohort of women with a history of VTE episodes and/or receiving anticoagulant therapy, a significant reduction in the risk of VTE was noted when prescribing transdermal estradiol alone, as well as no increase in the risk of VTE with the combined use of transdermal estradiol with a progestogen and oral estradiol with dydrogesterone [34].
According to observational studies, the use of transdermal estradiol in low (<50 μg/day) and higher doses alone, as well as its combination with a gestagen in cyclic or continuous regimens, did not increase the risk of VTE [34–37]. At the same time, on the one hand, there is evidence that the transdermal route of estrogen is associated with a lower risk of VTE than its oral administration, on the other hand, there is evidence that there are no differences [34, 35, 38–40]. No adequate randomized controlled clinical trials have compared these approaches.
A large real-world study EURAS-HRT (more than 30,000 women) confirmed the long-term safety profile of drospirenone-containing MHT drugs in relation to VTE. The risk of VTE with MHT and drospirenone was comparable, and the risk of serious arterial thromboembolic events (mainly acute myocardial infarction and ischemic stroke) was significantly lower than that with other MHT (detailed comparison of the composition and characteristics of other MHT was not performed) [41].
In general, modern low-dose and ultra-low-dose combination oral MHT using estradiol appears to be safe against VTE and has a comparable risk of venous thrombosis as transdermal MHT [34, 40]. However, an assessment of the benefits and risks of prescribing MHT, the choice of drug, its composition, and route of administration should be carried out individually, considering the characteristics of the clinical picture and the presence of risk factors for VTE.
In a case-control analysis of large QResearch and CPRD databases, there was no increased risk of VTE with tibolone [34].
Local estradiol therapy for GSM symptoms does not increase the risk of venous thrombosis and can be used in all categories of patients [31].
LNG-IUS, containing 52 mg of micronized levonorgestrel, can also be used as a component of MHT. According to previous studies, LNG-IUS use did not lead to an increased risk of VTE [41, 42].
When deciding on the possibility and composition of MHT, it should be noted that the risk of VTE cannot be considered in isolation from other thrombotic risks. Thus, even in cases where some increase in the risk of VTE is possible, this effect may be offset by a decrease in the incidence of arterial thrombosis and other cardiovascular events, which ultimately provides a neutral or positive effect on mortality [32, 43, 44].
5.2. MHT in various clinical situations associated with thrombosis
Venous thrombosis
MHT is contraindicated in patients with acute DVT or PE.
Most experts recommend avoiding MHT in patients with a history of VTE [31, 45, 46]. There is evidence that transdermal MHT does not increase the risk of VTE recurrence with anticoagulant treatment, but data on the safety of this approach for VTE are limited [37, 39].
For severe menopausal symptoms, in addition to local estrogen use, the possibility of using the lowest effective dose of transdermal estradiol (<50 μg/day) or ultra-low dose (0.5 mg estradiol) oral combination MHT with appropriate anticoagulant therapy cannot be excluded [36, 37, 45, 46]. It is also possible that modern MHT is safe after planned cessation of anticoagulant use in certain categories of patients with a low risk of recurrent venous thrombosis [37].
Available data do not allow for a clear conclusion about the risk of MHT in acute superficial vein thrombosis (SVT) and a history of SVT [47]. The decision on the possibility of using modern oral and transdermal MHT for SVT should be made individually, considering the characteristics of the clinical situation, the presence of risk factors for VTE, and a history of SVT as a contraindication for use in the instructions for a particular drug.
Studies assessing the risk of DVT and/or PE after SVT have not differentiated between nonvariceal thrombosis and superficial variceal thrombosis (varicothrombophlebitis). Varicothrombophlebitis is primarily caused by varicose veins, which can be eliminated long before MHT is prescribed.
A history of varicothrombophlebitis should be considered a limitation for prescribing MHT, with a history of SVT directly listed as a contraindication in the instructions for a specific drug for MHT.
Varicose veins
The presence of varicose veins is not a contraindication for MHT and should not influence the decision to prescribe MHT. To date, there is no evidence that MHT increases the risk of developing varicose vein thrombosis (varicothrombophlebitis). Ultrasound examination of the veins of the lower extremities is not required before prescribing MHT.
Thrombophilia
There are very little data on the safety of MHT in antiphospholipid syndrome [39]. Due to the high risk of venous and/or arterial thrombosis, oral and transdermal MHT are not recommended in patients with antiphospholipid syndrome. Potentially, this possibility cannot be excluded in women with low disease activity or asymptomatic changes in certain laboratory parameters who do not have additional risk factors for thrombosis [47].
Data regarding the safety of MHT in asymptomatic thrombophilia are limited. Some studies have established an increased risk of developing VTE with oral MHT in the presence of certain types of thrombophilia (protein C deficiency, protein S deficiency, antithrombin deficiency, factor V Leiden, prothrombin gene mutation G20210A, and high levels of coagulation factor VIII) [48, 49]. However, this is not sufficient to clearly prohibit the use of oral MHT against the background of asymptomatic thrombophilia; additional research on this issue is required.
The decision about the possibility and composition of MHT should be made individually, taking into account information about the presence of previously identified asymptomatic thrombophilia, the severity of menopausal symptoms, presence of additional risk factors for VTE, and indication of certain thrombophilia in the list of contraindications in the instructions for a specific drug for MHT [31, 40, 50]. Testing for thrombophilia before starting an MHT is not recommended.
A family history of thrombosis (venous or arterial thrombosis in first-degree relatives under the age of 50 years) indicates an increased risk of VTE but is not a basis for prohibiting MHT [17, 37, 50].
Transdermal MHT does not reportedly increase the risk of VTE in women with asymptomatic thrombophilia, but evidence for its safety in this clinical setting is limited [37, 39, 49].
A limitation of the use of a particular drug is the indication of a family thrombotic history and/or the presence of certain thrombophilia as a contraindication for use in the instructions.
5.3. MHT for surgery and hospitalization for acute non- surgical illness
There is currently no evidence of benefit from discontinuation of MHT before surgery or during hospitalization for acute nonsurgical illness (except for those in which MHT is contraindicated) [51]. When the risk of VTE increases, prophylaxis with anticoagulants offsets the potential prothrombotic effects of hormonal drugs. When stratifying the risk of VTE in such patients, it is recommended that continued MHT be considered as an additional risk factor for VTE.
Section 6. MHT in patients with atherosclerotic cardiovascular diseases
In 1998, the HERS study, the first randomized, placebo-controlled trial of estrogen and progestin hormone therapy (HT) for secondary prevention of CHD among postmenopausal women with established CHD, found no benefit in cardiovascular events or all- cause mortality with HT use. The results of this study argue against initiating HT for secondary prevention of CHD [52].
A more recent meta-analysis of 19 randomized controlled trials involving 40,410 postmenopausal women treated with MHT (most of whom were oral) found no significant increase in all-cause mortality, CVD mortality, or MI with MHT in either the primary or secondary prevention of cardiovascular complications.
Subgroup analysis based on the timing of MHT initiation revealed the following:
- Women who started MHT within 10 years of menopause had lower mortality (RR=0.70; 95% CI=0.52– 0.95) and fewer cardiovascular events (a combination of cardiovascular death and non-fatal MI) (RR=0.52; 95% CI=0.29–0.96) [33];
- Women who started MHT >10 years from the onset of menopause had an increased risk of stroke without any effect on mortality or other CVD outcomes [33].
Currently, the initiation of MHT is not recommended for women with established CHD, including angina [40], and MI is a contraindication to MHT.
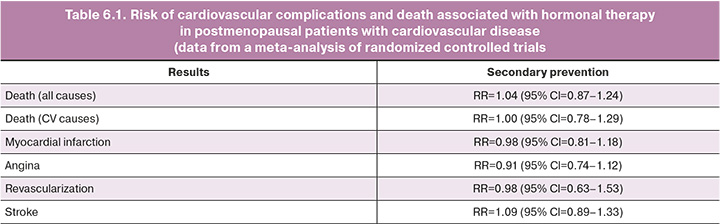
Manifestation of CHD while receiving MHT usually requires discontinuation. Although the authors of the previously mentioned HERS study concluded from its results that given the favorable pattern of ischemic events after several years of MHT, it may be advisable for women with CHD already receiving this treatment to continue it [52]. A meta-analysis that included 5766 patients with existing CVD showed that the absolute risk of death, MI, angina, or revascularization in this category of patients against the background of MHT was low (Table 6.1). Thus, in patients who develop CHD during therapy and are determined to continue MHT, the issue of its cancellation should be decided individually by a cardiologist and gynecologist [33].
Patients with a history of stroke are advised to avoid systemic MHT and consider alternative (nonhormonal) treatment. In the WHI study, an increased risk of ischemic stroke was observed in both the combined MHT group (RR=1.37; 95% CI=1.07–1.76) and the estrogen monotherapy group (RR=1.35; 95% CI=1.07– 1.70), regardless of the patient’s baseline risk [53, 54]. In a meta-analysis of four studies including 719 participants without cardiovascular disease, the risk of stroke was increased (RR=1.32; 95% CI=1.12– 1.56) compared to placebo. A meta-analysis of studies performed in the context of secondary CVD prevention (5172 participants in 5 studies) showed a trend toward an increased risk of stroke (Table 6.1) [33].
Non-atherosclerotic/non-thrombotic CHD is more common in women, but there are currently insufficient data to stratify the risk of MHT use by disease subtype. For women aged 50–59 years with a history of MI without obstructive coronary artery disease, spontaneous coronary artery dissection, coronary microvascular dysfunction, or coronary vasospasm, an individualized approach to MHT administration is required. Systemic MHT should be avoided in spontaneous coronary artery dissection because of a suspected pathophysiological relationship with female sex hormone levels. This recommendation is based on the fact that >90% of patients with spontaneous coronary artery dissection are female.
For symptoms of GSM, women with cardiovascular disease may be treated with local estriol therapy [4, 18, 55]. Please note that the instructions for estrogens for local use contain the same contraindications as estrogens for systemic MHT. This warning is not based on scientific research data but is associated with international requirements for the mandatory indication of uniform contraindications for a drug, regardless of the route of administration [45]. When applied locally, estriol has minimal systemic absorption and is not metabolized into the more active forms of estrogen (estradiol and estrone). Circulating levels of estriol, estradiol, and estrone are maintained within normal postmenopausal values [56, 57]. Several large observational studies have confirmed that there is no increased risk of adverse health outcomes, including CVD, VTE, and cancer, with the use of topical MHT estriol [58, 59].
Key Points
- MHT is not recommended for patients with CHD or a history of acute cerebrovascular accident or transient ischemic attack. Non-hormonal therapy should be used to treat vasomotor symptoms in these patients.
- In patients who develop CHD during MHT and are determined to continue it, the issue of discontinuing MHT should be resolved individually within the framework of a consultation, including a cardiologist and gynecologist.
Section 7. MHT in patients with cardiovascular risk factors
7.1 Dyslipidemias
Clinical studies have shown that compared with placebo or no treatment, MHT can significantly increase high-density lipoprotein cholesterol (HDL-C) levels and reduce total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and lipoprotein (a) levels ((Lp(a)) [60–62]. It should be noted that Lp(a) is an independent risk factor for CVD, and in particular recurrent ischemic stroke [63, 64]. Statin therapy has a weak effect on the level of this proatherogenic lipoprotein, whereas MHT significantly reduces it [65]. Conflicting data exist regarding the effect of MHT on triglyceride (TG) levels. In some studies, there was a significant increase in TG levels [66], whereas in other studies, there was no significant difference in TG levels between the two groups taking placebo and MHT [61, 67–75].
In general, MHT is considered to be a therapy associated with beneficial changes in lipid parameters with both short- and long-term use in postmenopausal women. However, there are features associated with the dose of drugs and method of delivery.
Oral MHT has been shown to increase TG concentrations compared with transdermal MHT [62]. A moderate but significant increase in TG levels during therapy with fenofibrate and/or polyunsaturated fatty acids can have a clinically significant effect on both the progression of atherosclerosis and development of pancreatitis. Thus, for women with hypertriglyceridemia, transdermal or low- dose MHT or tibolone is a safer choice.
At the same time, oral MHT is associated with a positive effect on the level of LDL cholesterol, and the concentration of this pro-atherogenic factor has the greatest influence on the development of atherosclerosis and destabilization of atherosclerotic plaques (AP).
Whether low-dose MHT can have the same effect on lipid profiles as standard doses of MHT remains unclear. One study found that low doses of MHT were associated with higher TC and LDL-С levels and lower TG levels than the standard doses [76]. Other studies have shown a similar benefit for TG in the low-dose estrogen group of MHT but did not find significant differences in TC and LDL-С levels between the two groups (high and low doses).
In addition, it has been found that low doses of estradiol in MHT can reduce HDL-C levels. Epidemiologically, low plasma HDL-C levels have been associated with an increased risk of ischemic CVD [77]. Taken together, the benefits of low-dose MHT and the transdermal route of estradiol on lipid profiles may be limited by TG levels. However, there are conflicting data regarding the effects of tibolone on lipid profiles. A meta-analysis conducted in 2021 showed that tibolone reduces TC, HDL-C, and TG levels. LDL concentrations were significantly reduced if tibolone treatment was continued for ≥26 weeks [78]. No differences were observed between the conventional MHT and tibolone
in terms of their effect on Lp(a) [79].
There is evidence of an increased risk of CHD in women receiving combined estrogen-progestin therapy, but not in women receiving estrogen monotherapy [80]. Unfortunately, no large-scale RCT has assessed the lipid profile according to the type of progestogen used. One observational study found that the addition of progestogens attenuated the beneficial effect of estrogen on lipid profiles [81], and a 2017 meta-analysis found no significant difference in the reduction in Lp(a) concentrations [79].
Although several studies have demonstrated the beneficial effects of MHT on lipid profiles, it must be emphasized that MHT is not recommended for the treatment of dyslipidemia and reduction of cardiovascular disease risk [82].
Key Points
- MHT has a positive effect on lipid profiles in peri- and postmenopausal women.
- MHT is not recommended as a treatment for dyslipidemia because changes in the lipid profile are minimal and not comparable to the effects of lipid- lowering drugs.
- Oral MHT is more effective at lowering LDL-С levels than transdermal MHT.
- For women with hypertriglyceridemia, transdermal or low-dose MHT or tibolone is a safer choice.
7.2 Arterial hypertension
Women-specific risk factors for hypertension and CVD later in life include the timing of menarche, a history of menstrual and reproductive disorders, uterine fibroids, polycystic ovary syndrome, endometriosis, adverse pregnancy outcomes, premature ovarian failure, and menopause. Increased risk during the reproductive period may contribute to a greater increase in CVD risk in peri- and postmenopausal women [83–87].
In hypertension, as in other diseases, there are sex differences that influence epidemiology, pathophysiology, and clinical management.
In 2019, the age-standardized prevalence of hypertension (SBP ≥140 mmHg and/or DBP ≥90 mmHg, or use of antihypertensive therapy) worldwide was 32% in women [88]. However, in Eastern Europe, the prevalence of hypertension in women aged 30–79 years ranges from 34% to 46% [89]. The prevalence of hypertension increases with age [89] but tends to decline more strongly before menopause in women than in men of the same age, with a marked increase in women after menopause [14]. After 65 years, the prevalence of hypertension in women is higher than in men [88–90].
Trajectories of blood pressure throughout life in men and women are explained by differences in the mechanisms of blood pressure regulation and a combination of sex and gender factors [88, 89]. In women before menopause, estrogen helps lower blood pressure in the context of its general vasoprotective effect. Protection is mediated by various mechanisms, including endothelial vasodilation through the enhancement of the nitric oxide pathway, inhibition of the sympathetic nervous system, and renin-angiotensin system activity. Moreover, estrogens reduce endothelin production, oxidative stress, and inflammation [87]. Loss of ovarian function due to natural aging or medical intervention is associated with an increased burden of cardiometabolic risk factors, including increased body weight, plasma glucose and cholesterol levels, and blood pressure, leading to an increased risk of CVD [86, 87, 91, 92]. After menopause, the marked decline in estrogen levels partly explains why BP levels and the risk of hypertension increase [87, 88]. Moreover, due to a sharp decrease in the level of progesterone (a natural antagonist of aldosterone), reactivation of the renin-angiotensin-aldosterone system (RAAS) occurs, with consequences such as fluid retention and increased blood pressure [93].
The pathophysiological characteristics of hypertension specific to women can be distinguished as follows [94]:
- close connection between obesity and hypertension;
- connection of gynecological disorders (anovulation, proliferative gynecological diseases) and unfavorable course of pregnancy (preeclampsia, gestational diabetes mellitus) with cardiometabolic risk and hypertension;
- cardiovasoprotective effect (including vasodilation) of physiological estrogen levels in reproductive age;
- pharmacological use of estrogen in the presence of established endothelial dysfunction can contribute to an increase in blood pressure and the risk of CVD; the administration of exogenous estrogens at dosages used for MHT does not have a negative effect on blood pressure.
- progesterone promotes leptin-mediated endothelial dysfunction in obese women before menopause;
- more pronounced sensitivity to sodium;
- higher incidence of inflammatory diseases associated with hypertension and CVD.
Postmenopausal women experience a more rapid increase in arterial stiffness than men of the same age. Elderly women have higher aortic stiffness than men, which appears to contribute to the development of isolated systolic hypertension, uncontrolled hypertension, heart failure with preserved left ventricular ejection fraction, and aortic stenosis, which is more common in women [95, 96].
Menopause has been found to double the risk of developing hypertension, even after adjusting for age and body mass index [97]. Although MHT contains estrogens, there is no convincing evidence that BP significantly increases in menopausal women with or without hypertension [98]. However, after the initiation of MHT, regular BP measurement should be recommended to confirm continued normal BP or monitor BP levels during antihypertensive therapy [99, 100]. In cases of uncontrolled hypertension, MHT should be discontinued. It is advisable to make the decision to discontinue MHT together with a cardiologist.
Key Points
- MHT may be prescribed if blood pressure is controlled.
- MHT is not indicated for primary or secondary cardiovascular prevention.
7.3. Smoking
Smoking significantly increases the risk of arterial cardiovascular events and is a risk factor for malignancies. Smoking is not a risk factor for VTE in MHT (including combined oral MHT). Although smoking alone is not a reason to stop using MHT, including combination oral medications, caution should be exercised when prescribing oral MHT to smokers, informing them of the health risks associated with smoking, and encouraging
them to stop smoking [ 23, 101, 102].
Key Points
- Women need to be educated about the health risks associated with smoking and encouraged to stop smoking.
- In women who smoke, the decision to use MHT should be made by considering all risk factors.
Section 8. MHT in Special Clinical Situations
8.1. Atherosclerosis of peripheral arteries
Among women aged 45–49 years, the prevalence of peripheral arterial atherosclerosis is 4.89% at the age of 50–55 years (5.73 %), and at the age of 56–60 years (6.73 %). Menopause increases the risk of developing carotid atherosclerosis by 2 times [103]. Premature and early menopause are associated with increased AP volume and prevalence [104].
The use of estrogen monotherapy in postmenopausal women for a year reduces the risk of peripheral arterial atherosclerosis by 52%, as shown in the observational Rotterdam study [105]. In patients with coronary artery disease in the HERS and HERSII RCTs, combined oral MHT did not provide a statistically significant reduction in the number of events associated with peripheral arterial atherosclerosis [53, 106]. One observational study found that MHT, regardless of its choice, reduced the risk of developing peripheral arterial atherosclerosis by 20% [107]. In their descriptive review, Davies et al. As a mechanism for the positive effect of MHT on the course of peripheral atherosclerosis, a decrease in the level of circulating LDL, an increase in the level of HDL-C, and a positive effect on endothelial function were discussed [108].
8.2. Chronic heart failure
In the Russian Federation, according to the EPOHA- CSN population study, the prevalence of CHF in women aged 50 years is 12.2% and in women aged 60 years is 26.2%, mainly with preserved left ventricular ejection fraction (LVEF) [109]. The five-year survival rate of patients with CHF is no more than 50% [110].
Early menopause increases the risk of developing CHF by 33%, as reported in a meta-analysis of three observational studies [111].
An RCT after 10 years of treatment found that women receiving oral estrogen therapy or a combination of MHT given in the first 7 months on average after menopause had a significantly lower risk of death, CHF, and MI, without any increased risk of cancer, VTE, or stroke. [112].
Oral estrogen therapy and combined MHT in patients 50 years of age and older with functional class III– IV CHF and LVEF ≤35% of non-ischemic etiology provided a statistically significant reduction in the risk of all-cause mortality by 40%, as demonstrated in a sub- analysis of the Beta-Blocker Evaluation (BEST) RCT of Survival Trial) [113].
A sub-analysis of the WHI (Women's Health Initiative) RCT showed that oral estrogen monotherapy and combined MHT did not increase the risk of CHF- related hospitalizations, regardless of LVEF and age at MHT initiation [114].
8.3. Atrial fibrillation
Women in all age groups are known to have a lower prevalence of atrial fibrillation (AF) than men, but all- cause mortality is higher in women: AF is independently associated with a 2-fold increased risk of death in women compared with a 1.5-fold increased risk of death in men [115]. In the observational ATRIA study, the annual incidence of thromboembolic events in patients with AF not taking warfarin was 3.5% in women compared with 1.8% in men [116]. Women with additional risk factors for stroke, especially those older (than 65 years), are at greater risk of stroke even if they are on anticoagulation therapy, while the risk of bleeding with anticoagulation was similar in both sexes [117]. Women with AF have more severe symptoms and more severe stroke. In clinical practice, women with AF are less likely to receive specialized care and a more conservative approach is often used [118, 119].
Menopause increases the risk of AF by 82% [120].
Data from the European BiomarCaRE Consortium observational study showed that the prevalence of AF in postmenopausal women (mean age 49.2 years) was 4.4%, which was associated with a 42% increase in the risk of stroke, a 78% increase in the risk of myocardial infarction, and a more than 3.5-fold increase in the incidence of death [121].
A sub-analysis of the WHI RCT and observational studies found that combined MHT, oral estrogen monotherapy, and tibolone increased the risk of AF [120, 122–124].
The contribution of transdermal and local forms of estrogen to the development of AF in women during menopause has not been determined.
8.4. Heart valve pathology
The possibility of prescribing oral MHT to peri- and postmenopausal women with valve pathology is determined by the presence of complications.
- MHT is contraindicated in cases of atrial fibrillation and blood clots in the heart chambers.
- in cases of CHF of non-ischemic etiology and absence of complications, MHT can be prescribed within the framework of an interdisciplinary consultation [125].
Conclusion
- Indications and contraindications for the use of MHT are determined according to the current Clinical Guidelines and instructions for specific drugs.
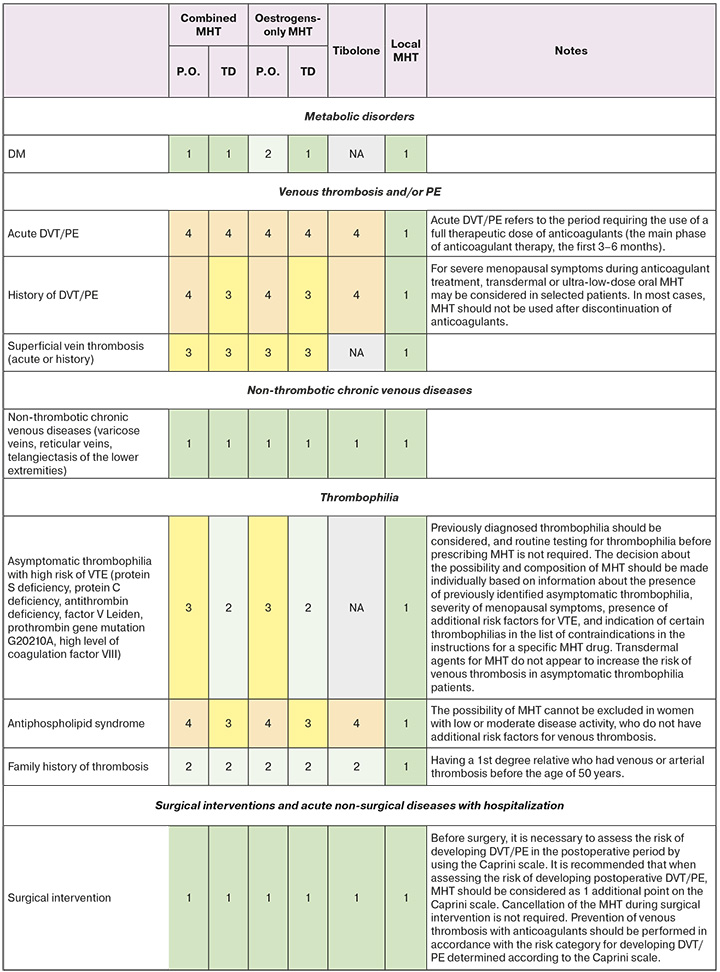
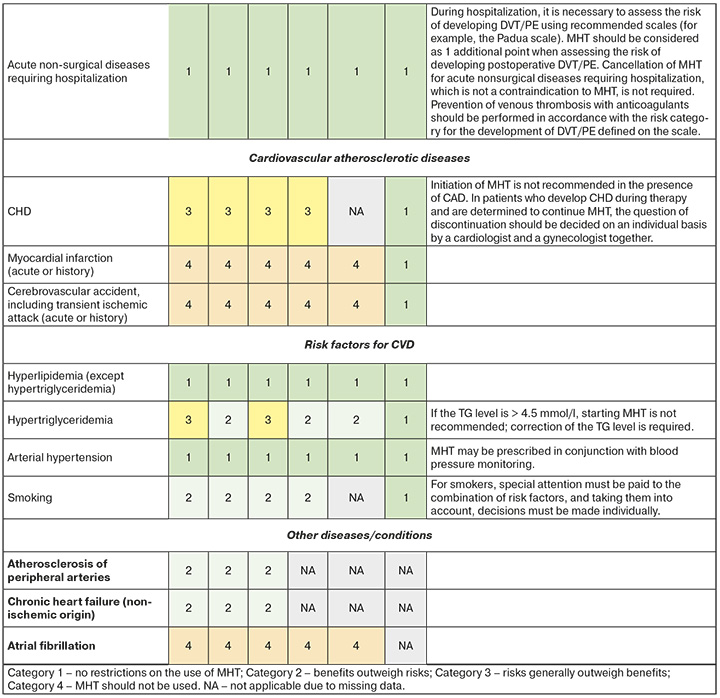
- A set of eligibility criteria for prescribing menopausal hormone therapy to patients with cardiovascular and metabolic diseases is given in Appendix 1.
- To unify the recommendations, the following categories were defined in accordance with WHO international nomenclature [31]:
- CATEGORY 1: No restriction on the use of MHT CATEGORY 2: The benefits outweigh the risks.
- CATEGORY 3: The risks generally outweigh the benefits.
- CATEGORY 4: MHT Should not be used
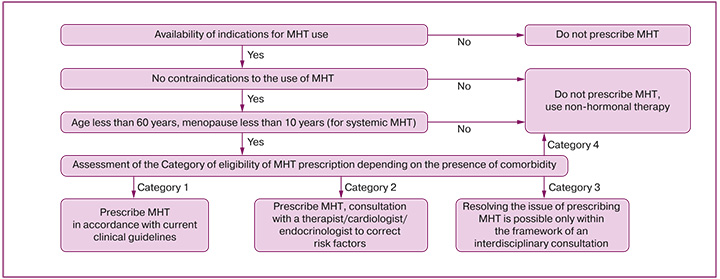
- When a woman complains of hot flashes, sweating, or palpitations, an internist must conduct a survey to identify the relationship between complaints and possible menopausal disorders. The interview should include information about the date of the last spontaneous menstrual period, menstrual irregularity, and current use of hormonal contraception or MHT. If complaints are suspected to be related to menopausal disorders, it is necessary to refer the woman for a consultation with an obstetrician-gynecologist.
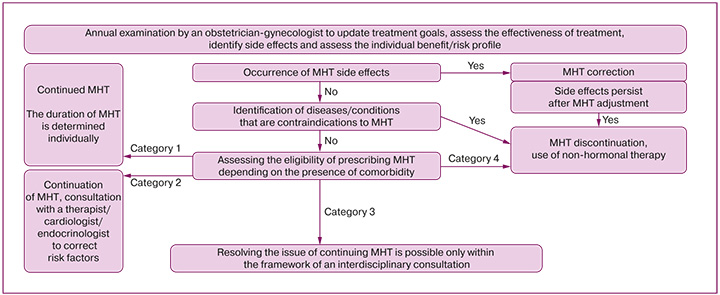
- Prescribing MHT, dose adjustment, changing drugs, stopping MHT, annual dynamic monitoring of the effectiveness and tolerability of treatment, updating therapy goals, and assessing the benefit/risk balance are performed by an obstetrician-gynecologist (Appendices 2 and 3).
- If adverse events of MHT are detected/suspected by a non-gynecologist, the patient should be advised to consult an obstetrician-gynecologist.
- If obstetrician-gynecologists identify or suspect the presence of cardiovascular risk factors and cardiovascular and metabolic diseases, the patient should be advised to consult a general practitioner.
List of Abbreviations
- AH – arterial hypertension BP – blood pressure
- AP – atherosclerotic plaque VVA – vulvovaginal atrophy IUS – intrauterine system
- VTE – venous thromboembolic complications, including deep vein thromboembolism and/or pulmonary embolism
- G – gestagens
- HT – hormonal therapy
- GSM – genitourinary syndrome of menopause DBP – diastolic blood pressure
- DYG – dydrogesterone DRSP – drospirenone
- PAD – peripheral arterial disease E – estradiol
- CHD – coronary heart disease MI – myocardial infarction
- CEE – conjugated equine estrogen MS – menopausal syndrome
- LNG – levonorgestrel Lp(a) – lipoprotein(a) MP – medicinal product
- MHT – menopausal hormone therapy MP – micronized progesterone
- MPA – medroxyprogesterone acetate MS – metabolic syndrome
- NA – not applicable
- NSAIDs – non-steroidal anti-inflammatory drugs ACS – acute coronary syndrome
- ACVA – acute cerebrovascular accident RR – relative risk
- POI – premature ovarian insufficiency
- p.o. – oral administration
- DOACs – direct oral anticoagulants RCT - randomized controlled trial BC – breast cancer
- SBP – systolic blood pressure DM – diabetes mellitus
- PCOS – polycystic ovary syndrome CVD – cardiovascular diseases
- SE – systemic embolism TG - triglycerides
- DVT – deep vein thrombosis
- TD – transdermal administration TIA – transient ischemic attack
- SVT – superficial veins thrombosis (thrombophlebitis), includes thrombosis of varicose and non-varicose superficial veins
- PE – pulmonary embolism
- LVEF – left ventricular ejection fraction AF – atrial fibrillation
- CKD – chronic kidney disease
- HDL-C – high-density lipoprotein cholesterol LDL-C – low-density lipoprotein cholesterol CHF – chronic heart failure
- E – estrogens
No conflicts of interest are declared.
Appendix 1. Eligibility Criteria for MHT
Appendix 2. Decision algorithm for prescribing MHT
Appendix 3. Decision algorithm for discontinuing MHT
References
- Распоряжение Правительства Российской Федерации от 29 декабря 2022 г. N 4356-р «Об утверждении Национальной стратегии действий в интересах женщин на 2023–2030 годы». Доступно на: https://www.consultant.ru/document/cons_doc_LAW_436691. [Decree of the Government of the Russian Federation No. 4356-р dated Dec 29, 2022 “On the approval of the National Strategy for Action in the Interests of Women for 2023–2030”. Available at: https://www.consultant.ru/document/cons_doc_LAW_436691 (in Russian)].
- Улумбекова Г.Э., Худова И.Ю. Оценка демографического, социального и экономического эффекта при приеме менопаузальной гормональной терапии. ОРГЗДРАВ: новости, мнения. Вестник ВШОУЗ. 2020; 6(4(22)): 23-53. [Ulumbekova GE, Khudova IYu. Demographic, social and economic effects of menopause hormonal therapy. Healthcare management: News, Views, Education. Bulletin of VSHOUZ. 2020;6(4(22)):23-53. (in Russian)]. https://doi.org/10.24411/2411-8621-2020-14002
- Lambrinoudaki I, Armeni E, Goulis D, Bretz S, Ceausu I, Durmusoglu F et al. Menopause, wellbeing and health: A care pathway from the European Menopause and Andropause Society. Maturitas. 2022;163:1-14. https://doi.org/10.1016/j.maturitas.2022.04.008
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Менопауза и климактерическое состояние у женщины. М.: 2021. Доступно на: https://cr.minzdrav.gov.ru/recomend/117_2 [Ministry of Health of the Russian Federation. Clinical Guidelines. Menopause and female climacteric states. 2021. Available at: https://cr.minzdrav.gov.ru/recomend/117_2 (in Russian)].
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW et al. Executive summary of the Stages of Reproductive Aging Work- shop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19(4):387-95. https://doi.org/10.1097/ gme.0b013e31824d8f40
- Drewe J, Bucher KA, Zahner C. A systematic review of non-hormonal treatments of vasomotor symptoms in climacteric and cancer patients. SpringerPlus. 2015;4(1):65. https://doi.org/10.1186/s40064-015-0808-y
- Schnatz PF, Romegialli A, Abrantes J, Marakovits K, Cunningham D, O’Sullivan DM. The North American Menopause Society: from abstract to publication. Menopause. 2008;15(5):996-1001 https://doi.org/10.1097/gme.0b013e318166f026
- Paramsothy P, Harlow SD, Nan B, Greendale GA, Santoro N, Craw- ford SL et al. Duration of the menopausal transition is longer in wom- en with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24(2):142-9. https://doi.org/10.1097/ GME.0000000000000736
- Сухих Г.Т., Сметник В.П., Андреева Е.Н., Балан В.Е., Гависова А.А., Григорян О.Р. и др. Менопаузальная гормонотерапия и сохранение здоровья женщин в зрелом возрасте. Клинические рекомендации. 2015. Доступно на: https://www.consultant.ru/document/cons_doc_LAW_320073/ [Sukhih G.T., Smetnik V.P., Andreeva E.N., Balan V.E., Gavisova A.A., Grigoryan O.R. et al. Menopausal hormone therapy and maintaining the health of women in adulthood. Clinical Guidelines. 2015. Available at: https://www.consultant.ru/document/cons_doc_ LAW_320073/ (in Russian)].
- Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeco- nomic position, lifestyle factors and age at natural menopause: a sys- tematic review and meta-analyses of studies across six continents. In- ternational Journal of Epidemiology. 2014;43(5):1542-62. https://doi.org/10.1093/ije/dyu094
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flash- es after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21(9):924-32. https://doi.org/10.1097/ GME.0000000000000196
- Costanian C, Zangiabadi S, Bahous SA, Deonandan R, Tamim H. Re viewing the evidence on vasomotor symptoms: the role of traditional and non-traditional factors. Climacteric. 2020;23(3):213-23. https://doi.org/10.1080/13697137.2019.1711051
- Prior JC. Progesterone for Symptomatic Perimenopause Treatment - Progesterone politics, physiology and potential for perimenopause. Facts, Views & Vision in ObGyn. 2011;3(2):109-20. PMID: 24753856
- Santoro N, Epperson CN, Mathews SB. Menopausal Symptoms and Their Management. Endocrinology and Metabolism Clinics of North America. 2015;44(3):497-515. https://doi.org/10.1016/j. ecl.2015.05.001
- Мельниченко Г.А., Белая Ж.Е., Рожинская Л.Я., Торопцова Н.В., Алексеева Л.И., Бирюкова Е.В. и др. Федеральные клинические рекомендации по диагностике, лечению и профилактике остеопороза. Проблемы эндокринологии. 2017;63(6):392-426. [Mel’nichenko G.A., Belaya J.E., Rozhinskaya L.Ya., Toroptsova N.V., Alekseeva L.I., Biryukova E.V. et al. Russian federal clinical guidelines on the diagnostics, treatment, and prevention of osteoporosis. Problems of Endocrinology. 2018;63(6):392-426. (in [Russian)]. https://doi.org/10.14341/probl2017636392-426
- Muka T, Oliver-Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R et al. Association of Vasomotor and Other Menopausal Symptoms with Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLOS One. 2016;11(6):e0157417. https://doi.org/10.1371/journal.pone.0157417
- Baber RJ, Panay N, Fenton A. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016;19(2):109-50. https://doi.org/10.3109/13697137.2015.1129166
- Hirschberg AL, Bitzer J, Cano A, Ceausu I, Chedraui P, Durmusoglu F et al. Topical estrogens and non-hormonal preparations for postmenopausal vulvovaginal atrophy: An EMAS clinical guide. Maturitas. 2021;148:55-61. https://doi.org/10.1016/j.maturitas.2021.04.005
- Grundy SM. Metabolic Syndrome: A Multiplex Cardiovascular Risk Factor. The Journal of Clinical Endocrinology & Metabolism. 2007;92(2):399-404 https://doi.org/10.1210/jc.2006-0513
- Hu G, The DECODE Study Group. Gender difference in all-cause and cardiovascular mortality related to hyperglycaemia and newly-diagnosed diabetes. Diabetologia. 2003;46(5):608-17. https://doi.org/10.1007/s00125-003-1096-6
- Vishram JKK, Borglykke A, Andreasen AH, Jeppesen J, Ibsen H, Jørgensen T et al. Impact of Age and Gender on the Prevalence and Prognostic Importance of the Metabolic Syndrome and Its Components in Europeans. The MORGAM Prospective Cohort Project. PLoS One. 2014;9(9):e107294. https://doi.org/10.1371/journal. pone.0107294
- Драпкина О.М., Концевая А.В., Калинина А.М., Авдеев С.Н., Агальцов М.В. и др. Профилактика хронических неинфекционных заболеваний в Российской Федерации. Национальное руководство 2022. Кардиоваскулярная терапия и профилактика. 2022;21(4):5-232. [Drapkina O.M., Kontsevaya A.V., Kalinina A.M., Avdeev S.M., Agaltsov M.V., Alexandrova L.M. et al. 2022 Prevention of chronic non-communicable diseases in the Russian Federation. National guidelines. Cardiovascular Therapy and Prevention. 2022;21(4):5-232. (in Russian)]. https://doi.org/10.15829/1728-8800- 2022-3235
- Cushman M. Estrogen Plus Progestin and Risk of Venous Thrombosis. JAMA. 2004;292(13):1573-80. https://doi.org/10.1001/jama.292.13.1573
- Трошина Е.А., Покусаева В.Н., Андреева Е.Н., Григорян О.Р., Мазурина Н.В., Дзгоева Ф.Х. и др. Ожирение у женщин. [Григорян О.Р., Андреева Е.Н. Ожирение и менопауза. С.233-268]. М.: Медицинское информационное агентство; 2017. 272c. [Troshina E.N., Pokusayeva V.N., Andreeva E.N., Grigoryan O.R., Ma-zurina N.V., Dzgoeva F.H. et al. Obesity among women. [Grigoryan O.R., Andreeva E.N. Obesity and menopause. P. 233-268]. M.: Medical Information Agency; 2017. 272 p. (in Russian)]. ISBN 978-5-9986-0296-2
- Caprio M, Antelmi A, Chetrite G, Muscat A, Mammi C, Marzolla V et al. Antiadipogenic Effects of the Mineralocorticoid Receptor Antagonist Drospirenone: Potential Implications for the Treatment of Metabolic Syndrome. Endocrinology. 2011;152(1):113-25. https://doi.org/10.1210/en.2010-0674
- Rizzo MR, Leo S, De Franciscis P, Colacurci N, Paolisso G. Short-term effects of low-dose estrogen/drospirenone vs low-dose estrogen/ dydrogesterone on glycemic fluctuations in postmenopausal women with metabolic syndrome. AGE. 2014;36(1):265-74. https://doi.org/10.1007/ s11357-013-9554-7
- Дедов И.И., Шестакова М.В., Викулова О.К., Железнякова А.В., Исаков М.А. Эпидемиологические характеристики сахарного диабета в Российской Федерации: клинико-статистический анализ по данным регистра сахарного диабета на 01.01.2021. Сахарный диабет. 2021;24(3):204-21. [Dedov I.I., Shestakova M.V., Vikulova O.K., Zheleznyakova A.V., Isakov M.A. Epidemiological characteristics of diabetes mellitus in the Russian Federation: clinical and statistical analysis according to the Federal diabetes register data of 01.01.2021. Diabetes mellitus. 2021;24(3):204-21. (in Russian)]. https://doi.org/10.14341/DM12759
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL et al. Menopausal Hormone Therapy and Health Outcomes During the Intervention and Extended Poststopping Phases of the Women’s Health Initiative Randomized Trials. JAMA. 2013;310(13):1353-68. https://doi.org/10.1001/jama.2013.278040
- Salpeter SR, Walsh JME, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta‐analysis: effect of hormone‐replacement thera py on components of the metabolic syndrome in postmenopausal women. Diabetes, Obesity and Metabolism. 2006;8(5):538-54. https://doi.org/10.1111/j.1463-1326.2005.00545.x
- Grigoryan O.R. Climacteric syndrome in women with diabetes mel litus. Diabetes Mellitus. 2013;16(3):103-8. https://doi.org/10.14341/2072- 0351-824
- Mendoza N, Ramírez I, De La Viuda E, Coronado P, Baquedano L, Llaneza P et al. Eligibility criteria for Menopausal Hormone Therapy (MHT): a position statement from a consortium of scientific societies for the use of MHT in women with medical conditions. MHT Eligibility Criteria Group. Maturitas. 2022;166:65-85. https://doi.org/10.1016/j.maturitas.2022.08.008
- Kim J-E, Chang J-H, Jeong M-J, Choi J, Park J, Baek C et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Scientific Reports. 2020;10(1):20631. https://doi.org/10.1038/s41598-020-77534-9
- Boardman HM, Hartley L, Eisinga A, Main C, Roqué I Figuls M, Bon fill Cosp X et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database of Systematic Reviews. 2015;2015(8):CD002229. https://doi.org/10.1002/14651858. CD002229.pub4
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone re- placement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. https://doi.org/10.1136/bmj.k4810
- Goldštajn MŠ, Mikuš M, Ferrari FA, Bosco M, Uccella S, Noventa M et al. Effects of transdermal versus oral hormone replacement therapy in postmenopause: a systematic review. Archives of Gynecology and Obstetrics. 2022;307(6):1727-45. https://doi.org/10.1007/s00404-022- 06647-5
- Kapoor E, Kling JM, Lobo AS, Faubion SS. Menopausal hormone therapy in women with medical conditions. Best Practice & Research Clinical Endocrinology & Metabolism. 2021;35(6):101578. https://doi.org/10.1016/j.beem.2021.101578
- Morris G, Talaulikar V. Hormone replacement therapy in women with history of thrombosis or a thrombophilia. Post Reproductive Health. 2023;29(1):33-41. https://doi.org/10.1177/20533691221148036
- Blondon M, Timmons AK, Baraff AJ, Floyd JS, Harrington LB, Korpak AM et al. Comparative venous thromboembolic safety of oral and transdermal postmenopausal hormone therapies among women Veterans. Menopause. 2021;28(10):1125-9. https://doi.org/10.1097/ GME.0000000000001823
- Sobel TH, Shen W. Transdermal estrogen therapy in meno- pausal women at increased risk for thrombotic events: a scoping review. Menopause. 2022;29(4):483-90. DOI: 10.1097/ GME.0000000000001938
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29(7):767-94. https://doi.org/10.1097/GME.0000000000002028
- Dinger J, Bardenheuer K, Heinemann K. Drospirenone plus es- tradiol and the risk of serious cardiovascular events in post-menopausal women. Climacteric. 2016;19(4):349-56. https://doi.org/10.1080/13697137.2016.1183624
- Tepper NK, Whiteman MK, Marchbanks PA, James AH, Curtis KM. Progestin-only contraception and thromboembolism: A systematic review. Contraception. 2016;94(6):678-700. https://doi.org/10.1016/j.contraception.2016.04.014
- Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI. Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis. BMJ. 2012;345:e4944. https://doi.org/ 10.1136/bmj.e4944
- Nudy M, Chinchilli VM, Foy AJ. A systematic review and meta-regression analysis to examine the ‘timing hypothesis’ of hormone replacement therapy on mortality, coronary heart disease, and stroke. IJC Heart & Vasculature. 2019;22:123-31. https://doi.org/10.1016/j.ijcha.2019.01.001
- Cho L, Kaunitz AM, Faubion SS, Hayes SN, Lau ES, Pristera N et al. Rethinking Menopausal Hormone Therapy: For Whom, What, When, and How Long? Circulation. 2023;147(7):597-610. https://doi.org/10.1161/ CIRCULATIONAHA.122.061559
- LaVasseur C, Neukam S, Kartika T, Samuelson Bannow B, Shatzel J, DeLoughery TG. Hormonal therapies and venous thrombosis: Con- siderations for prevention and management. Research and Practice in Thrombosis and Haemostasis. 2022;6(6):e12763. https://doi.org/10.1002/ rth2.12763
- Roach REJ, Lijfering WM, Van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR, Cannegieter SC. The risk of venous thrombosis in in- dividuals with a history of superficial vein thrombosis and acquired venous thrombotic risk factors. Blood. 2013;122(26):4264-9. https://doi.org/10.1182/blood-2013-07-518159
- Douketis JD, Julian JA, Crowther MA, Kearon C, Bates SM, Ba- rone M et al. The Effect of Prothrombotic Blood Abnormalities on Risk of Deep Vein Thrombosis in Users of Hormone Replace- ment Therapy: A Prospective Case-Control Study. Clinical and Applied Thrombosis/Hemostasis. 2011;17(6):E106-13. https://doi.org/10.1177/1076029610387587
- Straczek C, Oger E, Yon De Jonage-Canonico MB, Plu-Bureau G, Conard J, Meyer G et al. Prothrombotic Mutations, Hormone Therapy, and Venous Thromboembolism Among Postmenopau- sal Women: Impact of the Route of Estrogen Administration. Circulation. 2005;112(22):3495-500. https://doi.org/10.1161/CIRCULA- TIONAHA.105.565556
- Bezemer ID, Van Der Meer FJM, Eikenboom JCJ, Rosendaal FR, Doggen CJM. The Value of Family History as a Risk Indicator for Venous Thrombosis. Archives of Internal Medicine. 2009;169(6):610-5. https://doi.org/10.1001/archinternmed.2008.589
- Brighouse D. Hormone replacement therapy (HRT) and anaesthesia. British Journal of Anaesthesia. 2001;86(5):709-16. https://doi.org/10.1093/ bja/86.5.709
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B et al. Randomized Trial of Estrogen Plus Progestin for Secondary Prevention of Coronary Heart Disease in Postmenopausal Women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605-13. https://doi.org/10.1001/jama.280.7.605
- Wassertheil-Smoller S, Hendrix S, Limacher M, Heiss G, Kooper- berg C, Baird A et al. Effect of Estrogen Plus Progestin on Stroke in Postmenopausal Women: The Women’s Health Initiative: A Randomized Trial. JAMA. 2003;289(20):2673-84. https://doi.org/10.1001/ja- ma.289.20.2673
- Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE et al. Effects of Conjugated Equine Estrogen on Stroke in the Women’s Health Initiative. Circu- lation. 2006;113(20):2425-34. https://doi.org/10.1161/CIRCULATIONAHA.105.594077
- The 2020 genitourinary syndrome of menopause position state- ment of The North American Menopause Society. Menopause. 2020;27(9):976-92. https://doi.org/10.1097/GME.0000000000001609
- Te West NID, Day RO, Hiley B, White C, Wright M, Moore KH. Es- triol serum levels in new and chronic users of vaginal estriol cream: A prospective observational study. Neurourology and Urodynamics. 2020;39(4):1137-44. https://doi.org/10.1002/nau.24331
- Santen RJ, Mirkin S, Bernick B, Constantine GD. Systemic estradiol levels with low-dose vaginal estrogens. Menopause. 2020;27(3):361- 70. https://doi.org/10.1097/GME.0000000000001463
- Bhupathiraju SN, Grodstein F, Stampfer MJ, Willett WC, Crandall CJ, Shifren JL et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2019;26(6):603-10. https://doi.org/10.1097/GME.0000000000001284
- Crandall CJ, Hovey KM, Andrews CA, Chlebowski RT, Stefanick ML, Lane DS et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Womens Health Initiative Observational Study. Menopause. 2018;25(1):11-20. https://doi.org/10.1097/GME.0000000000000956
- Орлова Я.А., Плисюк А.Г., Долгушин Г.О., Кириллова К.И., Михеев Р.К., Андреева Е.Н. Связь длительной менопаузальной гормональной терапии и показателей сосудистого и репликативного старения у женщин. Профилактическая медицина. 2023;26(7):96-102. [Orlova Ya.A., Plisyuk A.G., Dolgushin G.O., Kirillova K.I., Mikheev R.K., Andreeva E.N. Correlation between prolonged meno- pausal hormonotherapy and indicators of vascular and replicative aging in women. Prevention Medicine. 2023;26(7): 96-102. (in Russian)]. https://doi.org/10.17116/profmed20232607196
- Draper MW, Flowers DE, Huster WJ, Neild JA, Harper KD, Arnaud C. A controlled trial of raloxifene (LY139481) HCl: Impact on bone turnover and serum lipid profile in healthy postmenopausal women. Journal of Bone and Mineral Research. 2009;11(6):835-42. https://doi.org/10.1002/jbmr.5650110615
- Nie G, Yang X, Wang Y, Liang W, Li X, Luo Q et al. The Effects of Menopause Hormone Therapy on Lipid Profile in Postmenopausal Women: A Systematic Review and Meta-Analysis. Frontiers in Pharmacology. 2022;13:850815. https://doi.org/10.3389/fphar.2022.850815
- Ежов М.В., Кухарчук В.В., Сергиенко И.В., Алиева А.С., Анциферов М.Б., Аншелес А.А. и др. Нарушения липидного обмена. Клинические рекомендации 2023. Российский кардиологический журнал. 2023;28(5):250-97. [Ezhov M.V., Kukharchuk V.V., Sergienko I.V., Alieva A.S., An- tsiferov M.B., Ansheles A.A. et al. Disorders of lipid metabo- lism. Clinical Guidelines 2023. Russian Journal of Cardiology. 2023;28(5):250-97. (in Russian)]. https://doi.org/10.15829/1560-4071-2023-5471
- Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF et al. Lipoprotein(a) as a cardiovascular risk factor: current status. European Heart Journal. 2010;31(23):2844-53. https://doi.org/10.1093/eurheartj/ehq386
- Van Dam-Nolen DHK, Van Dijk AC, Crombag GAJC, Lucci C, Kooi ME, Hendrikse J et al. Lipoprotein(a) levels and atherosclerotic plaque characteristics in the carotid artery: The Plaque at RISK (PARISK) study. Atherosclerosis. 2021;329:22-9. https://doi.org/10.1016/j. atherosclerosis.2021.06.004
- Stevenson JC, Chines A, Pan K, Ryan KA, Mirkin S. A Pooled Anal- ysis of the Effects of Conjugated Estrogens/Bazedoxifene on Lipid Parameters in Postmenopausal Women From the Selective Estrogens, Menopause, and Response to Therapy (SMART) Trials. The Journal of Clinical Endocrinology & Metabolism. 2015;100(6):2329-38. https://doi.org/10.1210/jc.2014-2649
- Miller VT, LaRosa J, Barnabei V, Kessler C, Levin G, Smith-Roth A et al. Effects of Estrogen or Estrogen/ Progestin Regimens on Heart Disease Risk Factors in Postmenopausal Women: The Postmeno- pausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1995;273(3):199-208. https://doi.org/10.1001/jama.1995.03520270033028
- Binder EF, Birge SJ, Kohrt WM. Effects of Endurance Exercise and Hormone Replacement Therapy on Serum Lipids in Older Women. Journal of the American Geriatrics Society. 1996;44(3):231-6. https://doi.org/10.1111/j.1532-5415.1996.tb00907.x
- Bunyavejchevin S, Limpaphayom KK. The metabolic and bone density effects of continuous combined 17-beta estradiol and noresthisterone acetate treatments in Thai postmenopausal women: a double- blind placebo-controlled trial. Journal of the Medical Association of Thailand. 2001;84(1):45-53. PMID: 11281499
- Çayan F, Gen R, Akbay E, Dilek U, Dilek S. The Effect of Hor- mone Therapy and Tibolone on Glucose and Lipid Metabolism in Healthy Postmenopausal Women. Turkish Journal of Geriatrics. 2011;14(1):19-25. Available at: https://geriatri.dergisi.org/abstract. php?id=534
- Cheng GJ, Liu JL, Zhang Q, Fan W, Ye HF, Wang ZQ et al. Nylestriol replacement therapy in postmenopausal women. A three-year prospective study. Chinese Medical Journal. 1993;106(12):911-6. PMID: 8198628
- Conard J, Basdevant A, Thomas J-L, Ochsenbein E, Denis C, Guyene TT et al. Cardiovascular risk factors and combined estrogen-progestin replacement therapy: a placebo-controlled study with nomege trol acetate and estradiol. Fertility and Sterility. 1995;64(5):957-62. https://doi.org/10.1016/S0015-0282(16)57909-6
- Conard J, Gompel A, Pelissier C, Mirabel C, Basdevant A. Fibrino- gen and plasminogen modifications during oral estradiol replacement therapy. Fertility and Sterility. 1997;68(3):449-53. https://doi.org/10.1016/S0015-0282(97)00220-3
- Davidson MH, Maki KC, Marx P, Maki AC, Cyrowski MS, Nanavati N et al. Effects of Continuous Estrogen and Estrogen-Progestin Replacement Regimens on Cardiovascular Risk Markers in Postmenopausal Wom- en. Archives of Internal Medicine. 2000;160(21):3315-25. https://doi.org/10.1001/ archinte.160.21.3315
- Duvernoy CS, Rose PA, Kim HM, Kehrer C, Brook RD. Combined Con- tinuous Ethinyl Estradiol/Norethindrone Acetate Does Not Improve Forearm Blood Flow in Postmenopausal Women at Risk for Cardiovascular Events: A Pilot Study. Journal of Women’s Health. 2007;16(7):963-70. https://doi.org/10.1089/jwh.2006.0321
- Casanova G, Dos Reis AM, Spritzer PM. Low-dose oral or non-oral hormone therapy: effects on C-reactive protein and atrial natriuretic peptide in menopause. Climacteric. 2015;18(1):86-93. https://doi.org/10.3109/13697137.2014.940309
- Haase CL, Tybjærg-Hansen A, Ali Qayyum A, Schou J, Nordestgaard BG, Frikke-Schmidt R. LCAT, HDL Cholesterol and Ischemic Cardiovascu- lar Disease: A Mendelian Randomization Study of HDL Cholesterol in 54,500 Individuals. The Journal of Clinical Endocrinology & Metabolism. 2012;97(2):E248-56. https://doi.org/10.1210/jc.2011-1846
- Lv C, Zhang W, Tan X, Shang X, Găman M-A, Salem H et al. The effect of tibolone treatment on lipid profile in women: A systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacolog ical Research. 2021;169:105612. https://doi.org/10.1016/j.phrs.2021.105612
- Anagnostis P, Galanis P, Chatzistergiou V, Stevenson JC, Godsland IF, Lam- brinoudaki I et al. The effect of hormone replacement therapy and tibolone on lipoprotein (a) concentrations in postmenopausal women: A systematic review and meta-analysis. Maturitas. 2017;99:27-36. https://doi.org/10.1016/j.maturitas.2017.02.009
- Falkeborn M, Persson I, Adami H-O, Bergstrom R, Eaker E, Lithell H et al. The risk of acute myocardial infarction after oestrogen and oestrogen- progestogen replacement. British Journal of Obstetrics and Gynaecology. 1992;99(10):821-8. https://doi.org/10.1111/j.1471-0528.1992.tb14414.x
- Shufelt CL, Manson JE. Menopausal Hormone Therapy and Cardiovas- cular Disease: The Role of Formulation, Dose, and Route of Delivery. The Journal of Clinical Endocrinology & Metabolism. 2021;106(5):1245-54. https://doi.org/10.1210/clinem/dgab042
- Anagnostis P, Bitzer J, Cano A, Ceausu I, Chedraui P, Durmusoglu F et al. Menopause symptom management in women with dyslipidemias: An EMAS clinical guide. Maturitas. 2020;135:82-8 https://doi.org/10.1016/j.maturitas.2020.03.007
- Wenger NK, Lloyd-Jones DM, Elkind MSV, Fonarow GC, Warner JJ, Al- ger HM et al. Call to Action for Cardiovascular Disease in Women: Epi- demiology, Awareness, Access, and Delivery of Equitable Health Care: A Presidential Advisory From the American Heart Association. Circulation. 2022;145(23):e1059-71. https://doi.org/10.1161/CIR.0000000000001071
- Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. The Lancet. 2021;397(10292):2385-438. https://doi.org/10.1016/S0140-6736(21)00684-X
- Reckelhoff JF. Gender differences in hypertension: Current Opinion in Nephrology and Hypertension. 2018;27:176-81. https://doi.org/10.1097/ MNH.0000000000000404
- Cho L, Davis M, Elgendy I, Epps K, Lindley KJ, Mehta PK et al. Summary of Updated Recommendations for Primary Prevention of Cardiovascular Disease in Women. Journal of the American College of Cardiology. 2020;75(20):2602-18. https://doi.org/10.1016/j.jacc.2020.03.060
- Gerdts E, Sudano I, Brouwers S, Borghi C, Bruno RM, Ceconi C et al. Sex differences in arterial hypertension. European Heart Journal. 2022;43(46):4777-88. https://doi.org/10.1093/eurheartj/ehac470
- Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 pop ulation-representative studies with 104 million participants. The Lancet. 2021;398(10304):957-80. https://doi.org/10.1016/S0140-6736(21)01330-1
- O’Keeffe LM, Simpkin AJ, Tilling K, Anderson EL, Hughes AD, Lawlor DA et al. Sex-specific trajectories of measures of cardiovascular health during childhood and adolescence: A prospective cohort study. Atherosclerosis. 2018;278:190-6. https://doi.org/10.1016/j.atherosclerosis.2018.09.030
- Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN et al. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiology. 2020;5(3):19-26. https://doi.org/10.1001/jamacardio.2019.5306
- Maas A, Rosano G, Cifkova R, Chieffo A, Van Dijken D, Hamoda H et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. European Heart Jour nal. 2021;42(10):967-84. https://doi.org/10.1093/eurheartj/ehaa1044
- El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation. 2020;142(25):e506-32. https://doi.org/10.1161/CIR.0000000000000912
- Biglia N, Cagnacci A, Gambacciani M, Lello S, Maffei S, Nappi RE. Va- somotor symptoms in menopause: a biomarker of cardiovascular disease risk and other chronic diseases? Climacteric. 2017;20(4):306-12. https://doi.org/10.1080/13697137.2017.1315089
- Chapman N, Ching SM, Konradi AO, Nuyt AM, Khan T, Twumasi-Ankrah B et al. Arterial Hypertension in Women: State of the Art and Knowledge Gaps. Hypertension. 2023;80(6):1140-9. https://doi.org/10.1161/HYPERTEN- SIONAHA.122.20448
- Coutinho T. Arterial Stiffness and Its Clinical Implications in Women. Ca- nadian Journal of Cardiology. 2014;30(7):756-64. DOI: 10.1016/j.cj- ca.2014.03.020
- Picone DS, Kodithuwakku V, Mayer CC, Chapman N, Rehman S, Clim- ie RE. Sex differences in pressure and flow waveform physiology across the life course. Journal of Hypertension. 2022;40(12):2373-84. DOI: 10.1097/HJH.0000000000003283
- Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women-2011 Update: A Guideline From the American Heart Association. Circulation. 2011;123(11):1243-62. https://doi.org/10.1161/ CIR.0b013e31820faaf8
- Issa Z, Seely EW, Rahme M, El-Hajj Fuleihan G. Effects of hormone ther apy on blood pressure. Menopause. 2015;22(4):456-68. https://doi.org/10.1097/ GME.0000000000000322
- Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). Journal of Hypertension. 2023; [Epub ahead of print]. https://doi.org/10.1097/ HJH.0000000000003480
- Кобалава Ж.Д., Конради А.О., Недогода С.В., Шляхто Е.В., Арутюнов Г.П., Баранова Е.И. и др. Артериальная гипертензия у взрослых. Клинические рекомендации 2020. Российский кардиологический журнал. 2020;25(3):149-218. [Kobalava Zh.D., Konradi A.O., Nedogoda S.V., Shlyakhto E.V., Aru- tyunov G.P., Baranova E.I. et al. Arterial hypertension in adults. Clinical guidelines 2020. Russian Journal of Cardiology. 2020;25(3):149-218. (in Russian)]. https://doi.org/10.15829/1560-4071-2020-3-3786
- Curb JD, Prentice RL, Bray PF, Langer RD, Van Horn L, Barnabei VM et al. Venous Thrombosis and Conjugated Equine Estrogen in Women Without a Uterus. Archives of Internal Medicine. 2006;166(7):772-80. https://doi.org/10.1001/archinte.166.7.772
- Blondon M, Wiggins KL, Van Hylckama Vlieg A, McKnight B, Psaty BM, Rice KM et al. Smoking, postmenopausal hormone therapy and the risk of venous thrombosis: a population‐based, case-control study. British Journal of Haematology. 2013;163(3):418-20. https://doi.org/10.1111/bjh.12508
- Li Y, Zhao D, Wang M, Sun J, Liu J, Qi Y et al. Association of menopause with risk of carotid artery atherosclerosis. Maturitas. 2021;143:171-7. https://doi.org/10.1016/j.maturitas.2020.10.007
- Schreinlechner M, Noflatscher M, Reinstadler SJ, Sommer P, Lener D, Reiser E et al. Early onset of menopause is associated with increased peripheral atherosclerotic plaque volume and progression. Atherosclerosis. 2020;297:25-31. https://doi.org/10.1016/j.atherosclerosis.2020.01.023
- Westendorp I, In’T Veld BA, Grobbee DE, Pols H, Meijer WT, Hofman A et al. Hormone Replacement Therapy and Peripheral Arterial Disease: The Rotterdam Study. Archives of Internal Medicine. 2000;160(16):2498-502. https://doi.org/10.1001/archinte.160.16.2498
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M et al. Cardiovascular Disease Outcomes During 6.8 Years of Hormone Therapy: Heart and Estrogen/Progestin Replacement Study Follow-up (HERS II). JAMA. 2002;288(1):49-57. https://doi.org/10.1001/jama.288.1.49
- Rockman CB, Maldonado TS, Jacobowitz GR, Adelman MA, Riles TS. Hormone Replacement Therapy is Associated With a Decreased Prevalence of Peripheral Arterial Disease in Postmenopausal Women. Annals of Vascular Surgery. 2012;26(3):411-8. https://doi.org/10.1016/j.avsg.2011.10.012
- Davies RSM, Vohra RK, Bradbury AW, Adam DJ. The Impact of Hor- mone Replacement Therapy on the Pathophysiology of Peripheral Arterial Disease. European Journal of Vascular and Endovascular Surgery. 2007;34(5):569-75. https://doi.org/10.1016/j.ejvs.2007.06.002
- Поляков Д.C., Фомин И.В., Беленков Ю.Н., Мареев В.Ю., Агеев Ф.Т., Артемьева Е.Г. и др. Хроническая сердечная недостаточность в Российской Федерации: что изменилось за 20 лет наблюдения? Результаты исследования ЭПОХА -ХСН. Кардиология. 2021;61(4):4-14. [Polyakov D.S., Fomin I.V., Belenkov Yu.N., Mareev V.Yu., Ageev F.T., Ar temjeva E.G. et al. Chronic heart failure in the Russian Federation: what has changed over 20 years of follow-up? Results of the EPOCH-CHF study. Kardiologiia. 2021;61(4):4-14. (in Russian)]. https://doi.org/10.18087/ cardio.2021.4.n1628
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38-360. https://doi.org/10.1161/CIR.0000000000000350
- Appiah D, Schreiner PJ, Demerath EW, Loehr LR, Chang PP, Folsom AR. Association of Age at Menopause With Incident Heart Failure: A Prospective Cohort Study and Meta‐Analysis. Journal of the American Heart Association. 2016;5(8):e003769. https://doi.org/10.1161/JAHA.116.003769
- Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L et al. Effect of hormone replacement therapy on cardiovascular events in re- cently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. https://doi.org/10.1136/bmj.e6409
- Lindenfeld J, Ghali JK, Krause-Steinrauf HJ, Khan S, Adams K, Goldman S et al. Hormone replacement therapy is associated with improved survival in women with advanced heart failure. Journal of the American College of Cardiology. 2003;42(7):1238-45. https://doi.org/10.1016/S0735-1097(03)00938- 0
- Liu L, Klein L, Eaton C, Panjrath G, Martin LW, Chae CU et al. Meno- pausal Hormone Therapy and Risks of First Hospitalized Heart Fail- ure and its Subtypes During the Intervention and Extended Post- intervention Follow-up of the Women’s Health Initiative Randomized Trials. Journal of Cardiac Failure. 2020;26(1):2-12. https://doi.org/10.1016/j.cardfail.2019.09.006
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in col- laboration with EACTS. European Heart Journal. 2016;37(38):2893-962. https://doi.org/10.1093/eurheartj/ehw210
- Fang MC, Singer DE, Chang Y, Hylek EM, Henault LE, Jensvold NG et al. Gender Differences in the Risk of Ischemic Stroke and Peripheral Embolism in Atrial Fibrillation: The AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) Study. Circulation. 2005;112(12):1687-91. https://doi.org/10.1161/CIRCULATIONAHA.105.553438
- Dagres N, Nieuwlaat R, Vardas PE, Andresen D, Levy S, Cobbe S et al. Gender-Related Differences in Presentation, Treatment, and Outcome of Patients With Atrial Fibrillation in Europe. Journal of the American College of Cardiology. 2007;49(5):572-7. https://doi.org/10.1016/j.jacc.2006.10.047
- Linde C, Bongiorni MG, Birgersdotter-Green U, Curtis AB, Deisenhofer I, Furokawa T et al. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. EP Europace. 2018;20(10):1565-1565ao. https://doi.org/10.1093/europace/euy067
- Thompson LE, Maddox TM, Lei L, Grunwald GK, Bradley SM, Peterson PN et al. Sex Differences in the Use of Oral Anticoagulants for Atrial Fibrillation: A Report From the National Cardiovascular Data Registry (NCDR®) PINNACLE Registry. Journal of the American Heart Association. 2017;6(7):e005801. https://doi.org/10.1161/JAHA.117.005801
- Lee J, Kim Y, Park H, Kim C, Cho S, Kim J. Clinical Impact of Hormone Replacement Therapy on Atrial Fibrillation in Postmenopausal Women: A Nationwide Cohort Study. Journal of Clinical Medicine. 2021;10(23):5497. https://doi.org/10.3390/jcm10235497
- Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Njolstad I et al. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results From the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation. 2017;136(17):1588-97. https://doi.org/10.1161/CIRCULATIONAHA.117.028981
- Perez MV, Wang PJ, Larson JC, Virnig BA, Cochrane B, Curb JD et al. Effects of Postmenopausal Hormone Therapy on Incident Atrial Fibrillation: The Women’s Health Initiative Randomized Controlled Trials. Circulation: Arrhythmia and Electrophysiology. 2012;5(6):1108-16. https://doi.org/10.1161/CIRCEP.112.972224
- Tsai W-C, Haung Y-B, Kuo H-F, Tang W-H, Hsu P-C, Su H-M et al. Hormone replacement therapy and risk of atrial fibrillation in Taiwanese menopause women: A nationwide cohort study. Scientific Reports. 2016;6(1):24132. https://doi.org/10.1038/srep24132
- Wong JA, Rexrode KM, Sandhu RK, Moorthy MV, Conen D, Albert CM. Menopausal age, postmenopausal hormone therapy and incident atrial fibrillation. Heart. 2017;103(24):1954-61. https://doi.org/10.1136/heartjnl-2016-311002
- Fleury M-A, Clavel M-A. Sex and Race Differences in the Pathophysiology, Diagnosis, Treatment, and Outcomes of Valvular Heart Diseases. Canadian Journal of Cardiology. 2021;37(7):980-91. https://doi.org/10.1016/j.cjca.2021.02.003
About the Authors
Evgeny V. Shlyakhto, MD, PhD, Professor, https://orcid.org/0000-0003-2929-0980; info@scardio.ruGennady T. Sukhikh, Academician of RAS, MD, PhD, Professor, https://orcid.org/0000-0002-7712-1260; secretariat@oparina4.ru
Vladimir N. Serov, Academician of RAS, MD, PhD, Professor, https://orcid.org/0000-0003-2976-7128; v_serov@oparina4.ru
Ivan I. Dedov, MD, PhD, Professor, https://orcid.org/0000-0002-8175-7886; info@rae-org.ru
Grigory P. Arutyunov, MD, PhD, Professor, https://orcid.org/0000-0002-6645-2515; arut@ossn.ru
Igor A. Suchkov, MD, PhD, Professor; suchkov_med@mail.ru
Yana A. Orlova, MD, PhD, https://orcid.org/0000-0002-8160-5612; 5163002@bk.ru
Elena N. Andreeva, MD, PhD, Professor, https://orcid.org/0000-0001-8425-0020, SPIN-код: 1239-2937; endogin@mail.ru
Svetlana V. Yureneva, MD, PhD, Professor, https://orcid.org/0000-0003-2864-066X, SPIN-код: 3623-9149; syureneva@gmail.com
Maria I. Yarmolinskaya, MD, PhD, Professor, https://orcid.org/0000-0002-6551-4147, SPIN-код: 3686-3605; m.yarmolinskaya@gmail.com
Ekaterina N. Dudinskaya, MD, PhD, https://orcid.org/0000-0001-7891-6850, SPIN-код: 6453-5835; katharina.gin@gmail.com
Оlga R. Grigoryan, MD, PhD, Professor, https://orcid.org/0000-0003-4979-7420, SPIN-код: 3060-8242; iceberg1995@mail.ru
Igor S. Yavelov, MD, PhD, Professor, https://orcid.org/0000-0003-2816-1183; yavelov@yahoo.com
Svetlana V. Villevalde, MD, PhD, Professor, https://orcid.org/0000-0001-7652-2962; villevaldes@mail.ru
Evgeniy A. Ilyukhin, PhD; iluhin-e@yandex.ru
Natalya A. Koziolova, MD, PhD, Professor, https://orcid.org/0000-0001-7003-5186; nakoziolova@mail.ru
Igor V. Sergienko, MD, PhD, Professor, https://orcid.org/0000-0003-1534-3965; igorcardio@mail.ru
Antonina A. Smetnik, PhD, https://orcid.org/0000-0002-0627-3902; asmetnik@gmail.com
Natalya I. Tapilskaya, MD, PhD, Professor, https://orcid.org/0000-0001-5309-0087; tapnatalia@yandex.ru
Corresponding author: Yana A. Orlova, 5163002@bk.ru



