Antibodies to angiotensin-converting enzyme 2 in infertile patients with a history of COVID-19 and in fertile women
Angiotensin-converting enzyme 2 (ACE2) is a key component of the renin-angiotensin system (RAS) that affects fertility in women. Antibodies against ACE2 have predictive value for COVID-19 and may contribute to RAS dysregulation and reproductive failure.Menzhinskaya I.V., Ermakova D.M., Syrkasheva A.G., Drapkina Yu.S., Dolgushina N.V.
Objective: To investigate the prevalence and levels of anti-ACE2 autoantibodies in infertile patients with a history of COVID-19 and in fertile women.
Materials and methods: Serum anti-ACE2 autoantibodies (M, G) were determined by ELISA in infertile patients with a history of COVID-19 (group 1, n=121), without a history of COVID-19 (group 2, n=79), and in fertile women (group 3, n=80). The association between antibodies against ACE2, SARS-CoV-2, thyroid antigens, and hormones was investigated.
Results: Patients in groups 1 and 2 had higher rates of inflammatory gynecologic diseases, pelvic surgery, spontaneous miscarriages, and thyroid pathology than those in group 3. Anti-ACE2 antibodies were detected more frequently (40.5% and 38.8 %) and had higher levels in infertile patients than in fertile women (20%). Women with a history of COVID-19 were more likely to have anti-ACE2 IgG. Antibodies against ACE2 were significantly correlated with those against FSH.
Conclusion: Patients with infertility, irrespective of a history of COVID-19, have a higher prevalence and higher anti-ACE2 antibody levels than fertile women. Anti-ACE2 antibodies are associated with primary and secondary infertility, and may be involved in the pathophysiology of infertility.
Authors' contributions: Menzhinskaya I.V., Dolgushina N.V. – conception and design of the study; Ermakova D.M.,
Syrkasheva A.G., Drapkina Yu.S. – material collection and analysis; Menzhinskaya I.V. – laboratory part of the study, statistical analysis; Menzhinskaya I.V., Ermakova D.M. – manuscript drafting; Menzhinskaya I.V., Dolgushina N.V. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was supported by the contribution of the Future Charity Fund as part of the Stop Coronavirus Together campaign.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Menzhinskaya I.V., Ermakova D.M., Syrkasheva A.G.,
Drapkina Yu.S., Dolgushina N.V. Antibodies to angiotensin-converting enzyme 2
in infertile patients with a history of COVID-19 and in fertile women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (2): 71-78 (in Russian)
https://dx.doi.org/10.18565/aig.2022.284
Keywords
Angiotensin-converting enzyme 2 (ACE2) is a key component of the renin-angiotensin system (RAS) that regulates blood pressure, fluid and electrolyte balance, and systemic vascular resistance in the body [1]. ACE2 converts pro-inflammatory components of the RAS axis, such as angiotensin I (Ang I) and especially angiotensin II (Ang II), into anti-inflammatory RAS axis components such as Ang1-9 and Ang1-7 [2, 3]. Ang II acts on angiotensin type 1 (AT1) receptors and activates the NADPH oxidase complex to produce superoxide and stimulate cellular pro-oxidant and pro-inflammatory responses, whereas Ang1-7 acts on Mas receptors to stimulate cellular antioxidant and anti-inflammatory responses.
Scientific interest in ACE2 has increased, particularly during novel coronavirus infection COVID-19, because ACE2 serves as the major receptor required for the entry of SARS-CoV-2 into human cells, and increased ACE2 can contribute to cellular infection [4]. Dysregulation of the tissue RAS has been suggested as a major mechanism for the development of severe forms of COVID-19 [5]. Binding of SARS-CoV-2 to ACE2 decreases ACE2 content on the cell surface, shifting the RAS balance towards the pro-inflammatory axis (Ang II/AT1 axis), which leads to inflammation and fibrosis and increases disease severity [3, 6]. Autoantibodies to ACE2 may contribute to the pro-inflammatory effects of RAS and are considered useful for assessing the risk of severe COVID-19 [7–9].
However, it is important to note that ACE2 is expressed not only in type II pneumocytes, but also in endothelial cells, myocardium, intestinal mucosa, endocrine glands (thyroid, adrenal glands), adipose tissue, tissues of male and female reproductive organs (ovaries, uterus), and placental and umbilical cord tissues [10–12]. The high expression of placental ACE2 suggests the possibility of intrauterine fetal infection with SARS-CoV-2 [13].
ACE2 is a key enzyme that regulates the conversion of Ang II and Ang1-7, which have important effects on female reproductive processes [12]. Ang II stimulates steroid hormone synthesis, promotes follicle development and oocyte maturation, influences ovulation, initiates menstruation through spiral vasoconstriction, and supports corpus luteum function. The balance between Ang II and Ang1-7 is important for regulating endometrial regeneration and myometrial contractile activity. In addition, Ang II increases the proliferation of uterine epithelial and stromal cells and enhances endometrial fibrosis. Ang 1-7 stimulates the synthesis of estradiol and progesterone and promotes ovulation and meiosis in oocytes. Ang1-7 is associated with human oocyte maturation [14]. Altered ACE2 activity can lead to impaired folliculogenesis, ovulation, and corpus luteum function, and contribute to dysfunctional uterine bleeding associated with endometrial hyperplastic processes [12]. Ovarian RAS dysregulation may be involved in the pathophysiology of polycystic ovarian syndrome and ovarian hyperstimulation syndrome [15].
Based on the above, it can be speculated that women with infertility, especially those with a history of COVID-19, may produce autoantibodies against ACE2, contributing to changes in ACE2 functional activity, RAS dysregulation, and the development of reproductive dysfunction.
Therefore, the present study aimed to investigate the prevalence and levels of anti-ACE2 autoantibodies in infertile patients with a history of COVID-19 and fertile women.
Materials and methods
This cross-sectional study included 200 women who were examined for infertility at the V.I. Kulakov NMRC for OG&P. Group 1 included patients who had mild-to-moderate COVID-19 in the period from September 2020 to December 2021, 153 (44–243) days before the study (n=121). Group 2 included patients with no history of COVID-19 (n=79). The severity of COVID-19 was established in accordance with the provisional guidelines of the Russian Ministry of Health "Prevention, diagnosis, and treatment of new coronavirus infection (COVID-19)". Inclusion criteria for groups 1 and 2 were signed consent to participate in the study, 18-45 years, various forms of primary and secondary infertility; for group 1, a history of COVID-19 disease confirmed by polymerase chain reaction (PCR) and a positive test for IgG antibodies against SARS-CoV-2; and for group 2, negative test for IgG antibodies against SARS-CoV-2 and no history of COVID-19.
Group 3 (comparison group) consisted of relatively healthy, fertile women of reproductive age without a history of COVID-19 (n=80). The inclusion criteria were as follows: age 18–45 years, preserved menstrual function, negative PCR test for SARS-CoV-2 RNA, negative tests for IgM and IgG antibodies to SARS-CoV-2 and no history of COVID-19.
The exclusion criteria for all groups were pregnancy and lactation, acute inflammatory and infectious diseases, severe somatic and autoimmune diseases, cancer, use of hormonal and immunomodulatory therapies, and vaccination against COVID-19.
The identification of SARS-CoV-2 in an oropharyngeal swab was performed using the "Reagent kit for the detection of SARS-CoV-2 and similar SARS-CoV RNA by reverse transcription and real-time PCR (SARS-CoV-2/SARS-CoV)" (LLC NPO DNA-Technology, Russia). To determine class M and G antibodies against SARS-CoV-2, we used the immunochromatographic test Xematest anti-SARS-CoV-2 (XEMA LLC, Russia). A semiquantitative assessment of the level of class G antibodies to SARS-CoV-2 was performed using the "Reagent kit for the detection of class G antibodies to SARS-CoV-2 spike protein by enzyme immunoassay" ("DS-EIA-ANTI-SARS-CoV-2-G(S)") manufactured by Diagnostic Systems NPO (Russia).
The determination of autoantibodies (M, G) to ACE2 was carried out by modification of indirect ELISA using recombinant human ACE2 provided by XEMA LLC (Russia), monoclonal antibody conjugates against human IgM and IgG with horseradish peroxidase and other reagents, and buffer solutions for ELISA produced by XEMA LLC (Russia). ACE2 was immobilized on MaxiSorp polystyrene microplates (Thermo Scientific Nunc, Denmark) at the concentration of 2.5 µg/ml, serum samples were examined at the dilution 1:100, horseradish peroxidase conjugates against human IgM and IgG were used in working dilutions. Optical density (OD) was measured at 450 nm using a MULTISKAN EX photometer (Thermo Electron (Shanghai) Instrument Co., China). The result was considered positive if the mean OD of the test sample exceeded the mean OD of the negative control sera by more than two standard deviations (2δ).
Progesterone (PG) and follicle stimulating hormone (FSH) antibodies were detected by the modified ELISA described above using Progesterone conjugated with BSA (Progesterone 3-(O-carboxymethyl) oxime: BSA) and a highly purified preparation of FSH (Sigma-Aldrich, USA), conjugates against human IgM and IgG with horseradish peroxidase and reagents for ELISA produced by XEMA LLC (Russia) [16, 17].
Antibodies against thyroid antigens, thyroglobulin (TG), and thyroid peroxidase (TPO) were detected using immunoassay kits (ORGENTEC Diagnostika GmbH, Germany).
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Statistical analysis
Statistical analysis was performed using Microsoft Excel spreadsheets and MedCalc v. 12 (MedCalc Software Ltd., Belgium), Statistica v. 10 (StatSoft Inc., USA). The normality of the distribution was tested using the Shapiro–Wilk and Kolmogorov–Smirnov tests. Variables that did not meet normality assumptions were reported as median (Me) and interquartile range (Q1; Q3). Differences between the two groups with non-normally distributed data were analyzed using the Mann–Whitney U-test. The Kruskal–Wallis test was used to compare numerical data among the three groups, followed by the Bonferroni multiple comparison test. Categorical variables were described as counts with percentages, and comparisons between different groups were performed using the χ2 test. Spearman’s correlation coefficient was calculated to determine the correlation between the variables. The association between anti-ACE2 antibodies and infertility was assessed using relative risk (RR) with a 95% confidence interval. Differences were considered statistically significant at P<0.05.
Results
The patients in the study groups were comparable in terms of age, the number of women of early (35 years) and late (>35 years) reproductive age (ERA and LRA), and the rates of sexually transmitted infections (STD) and somatic diseases, including cardiovascular disease, chronic gastrointestinal diseases (GIT), urinary tract diseases, and ENT diseases (Table 1). However, groups 1 and 2 differed from fertile women by significantly fewer pregnancies and term births, and higher rates of spontaneous miscarriages. groups 1 and 2 also differed from the control group in having a higher incidence of gynecological conditions, such as endometriosis and endometrial polyps, pelvic surgery (tubectomy and myomectomy), and endocrine conditions, especially thyroid disease. In a pairwise comparison with group 3, group 1 patients were diagnosed more frequently with chronic salpingo-oophoritis (P=0.03) and uterine myoma (P=0.01), whereas group 2 patients had chronic endometritis (P=0.02) and adenomyosis (P=0.005). In contrast, allergic diseases were observed more frequently in group 3 in fertile women than in groups 1 (P=0.04) and 2 (P=0.001).
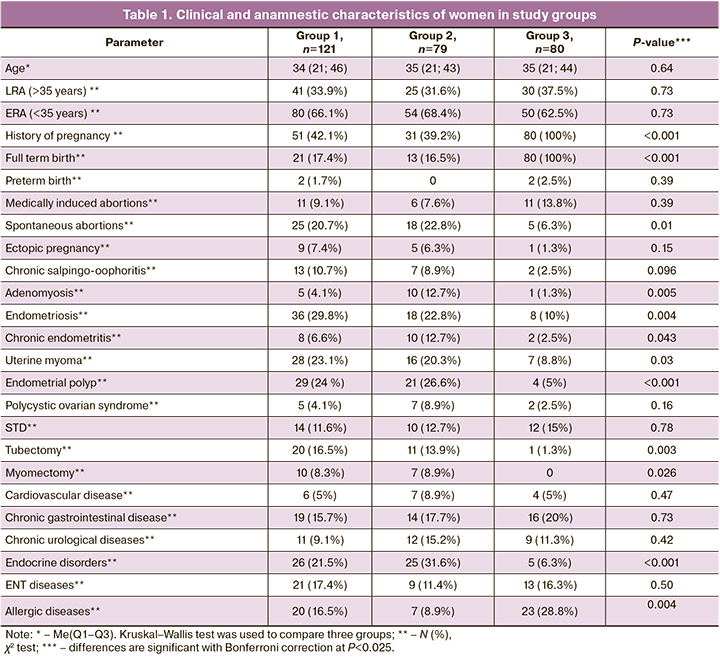
It should be noted that groups 1 and 2 did not differ significantly in the rates of gynecological and somatic diseases, except for adenomyosis, which was more frequently observed in group 2 (P=0.024). The most frequently diagnosed comorbidities in these groups were endometriosis, uterine myoma, endometrial polyps, thyroid pathology, and spontaneous miscarriages. The rates of primary infertility in groups 1 and 2 were 71/121 (58.7%) and 45/79 (57%), respectively, and the rates of secondary infertility were 50/121 (41.3%) and 34/79 (43%), respectively. Patients in groups 1 and 2 did not differ in the duration of infertility [4 (3–6.5) years and 5 (3–6) years (P=0.63)] or the number of previous IVF attempts, 1 (1-8) and 1 (1–5), respectively (P=0.37).
Group 1 patients with mild to moderate COVID-19 showed higher levels of specific antiviral IgG antibodies against SARS-CoV-2 (PI 6.08 (2.80–10.79)) than group 2 patients without COVID-19 (PI 0.13 (0.12–0.96) and group 3 women (PI 0.12 (0.11–0.9) (P<0.001).
Allergic diseases, chronic GIT and ENT diseases, STD, chronic urological diseases, and medical abortions were the most common in the medical history of women in group 3.
Anti-ACE2 antibodies of classes M and G were detected more frequently in infertile women, both in those with COVID-19 (47/121 [38.8%]) and in those without COVID-19 (32/79 [40.5%]) than in fertile women (16/80 [20%]) (P=0,008). Notably, the antibody detection rate in infertile patients in groups 1 and 2 did not differ (P=0.81) and was independent of the history of COVID-19. However, anti-ACE2 antibodies were 1.9 and 2 times more likely to be detected in infertile patients in groups 1 and 2 than in fertile women, with OR of 1.9 [1.2–3.2] (P=0.008) and 2.0 [1.2–3.4] (P=0.007), respectively. The antibody detection rates in patients with primary and secondary infertility in groups 1 [30/71 (42.3%) and 17/50 (34%], respectively) and 2 [21/45 (46.7%) and 11/34 (32.4%], respectively) did not differ significantly (P=0.2; P=0.36).
Patients with primary and secondary infertility who tested positive for anti-ACE2 antibodies most commonly had a history of endometriosis [14/51 (27.5%) and 6/28 (21.4%] ), uterine myoma [12/51 (23.5%) and 7/28 (25%] ), endometrial polyp [12/51 (23.5%) and 9/28 (32.1%] ), tubectomy [9/51 (17.6%) and 5/28 (17.9%] ), endocrine [9/51 (17.6%) and 12/28 (42.9%] ), and allergic diseases [8/51 (15.7%) and 4/28 (14.3%] ). Thyroid diseases were diagnosed more frequently in seropositive patients with secondary infertility (P=0.016).
Women in the comparison group who were positive for anti-ACE2 antibodies had a high incidence of allergic diseases (7/16 [43.8%]), ENT diseases (3/16 [18.8%]), GIT diseases (3/16 [18.8%]), and STD (3/16 [18.8%]).
The detection rates of anti-ACE2 antibody classes M and G did not differ significantly among the three groups, but there was a tendency for a higher detection rate of antibodies in patients with infertility (Table 2). In a pairwise comparison of the groups, the detection rates of these antibodies (M, G) also did not differ between groups 1 and 2 (P-values, 0.36 and 0.40, respectively). The detection rates of IgM antibodies in infertile patients in groups 1 and 2 and IgG antibodies in group 1 were significantly higher than those in fertile women (P-values, 0.04; 0.01, and 0.03, respectively). However, anti-ACE2 IgG antibodies were 5.6 times more likely to be detected in post-COVID-19 infertile patients than in fertile women (OR 5.6 [2.1–15.2]; P<0.001).
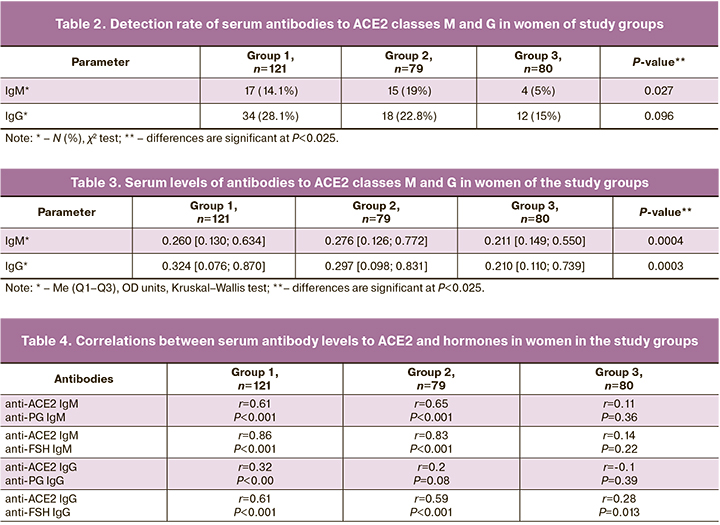
Comparison of serum levels of antibodies to ACE2 in women in the study groups revealed significant differences in median levels of anti-ACE2 autoantibodies (both IgM and IgG) between the three groups (Table 3). In a pairwise comparison of the groups, significantly higher levels of anti-ACE2 class M and G autoantibodies were observed in patients with infertility than in fertile women (P=0.001 or P<0.001). The mean autoantibody levels to ACE2 (M, G) in groups 1 and 2 were not significantly different (P=0.38; P=0.22), that is, they were independent of the patient’s history of COVID-19.
When comparing the detection rate and median level of anti-ACE2 antibodies of classes M and G in ERA and LRA infertile patients, no significant differences were found between groups 1 and 2, except for the level of IgM antibodies to ACE2 in ERA patients with a history of COVID-19 (0.273 [0.130; 0.634] OD units), which was significantly higher than that in similar LRA patients (0.224 [0.132; 0.551] OD units) (P=0.015).
In addition to antibodies to ACE2, antibodies to hormones (FSH and PG) and thyroid antigens (TPO and TG) were detected in patients with infertility: in group 1, 32/121(26.4%), 28/121(23.1%), 8/121(6.6%), and 8/121(6.6%) women, respectively; in group 2, 21/79(26.6%), 19/79(24.1%), 3/79(3.8%), and 3/79(3.8%) women, respectively. A direct correlation between the levels of M- and G-class antibodies to ACE2 and hormones, especially those expressed with antibodies to FSH, was found when comparing the profile of autoantibodies in patients with infertility in groups 1 and 2 (Table 4). At the same time, in groups 1 and 2, patients positive for anti-ACE2 antibodies were significantly more likely to have antibodies (M, G) to hormones [to PG in 17/47 (36.2%) and 11/32 (34, (34.4%), to FSH in 28/47 (59.6%) and 17/32 (53.1%)] than those who were negative for anti-ACE2 antibodies [PG in 12/74 (16.2%) and 4/47 (8.5%), to FSH in 9/74 (12.2%) and 3/32 (6.4%)] (P<0,05). Similar correlations were weak or absent in fertile women. No correlations were found between IgG antibodies to ACE2 and IgG antibodies to SARS-Cov-2 in group 1 or between IgG antibodies to ACE2 and IgG antibodies to thyroid antigens in all study groups.
Discussion
According to research evidence, ACE2 levels in plasma or urine are associated with various diseases, such as renal, cardiovascular and metabolic disorders [18]. Plasma ACE2 levels often correlate with arterial hypertension, heart failure, microalbuminuria, and nephropathy in diabetic patients [19, 20]. At the same time, despite higher plasma levels of ACE2, these patients had reduced ACE2 enzyme activity and significant levels of circulating autoantibodies against ACE2 [21]. It has been suggested that angiotensin peptide concentrations, particularly Ang1-7, Ang II/Ang1-7 ratio, and levels of anti-ACE2 autoantibodies can be used as diagnostic and prognostic biomarkers in various inflammatory diseases [22].
Recently, a significant increase in anti-ACE2 autoantibody levels has been observed in patients with COVID-19 compared to uninfected controls, particularly in patients with moderate to severe infection [8]. However, the association between antibodies and disease severity is more pronounced in patients with diabetes. SARS-Cov-2 infection is believed to cause the RAS to shift to pro-inflammatory effects, since virus binding to ACE2 reduces the levels of transmembrane ACE2 and converts it to a soluble circulating form. Increased levels of circulating ACE2 contribute to the formation of specific autoantibodies. In turn, the presence of high levels of anti-ACE2 autoantibodies leads to a further decrease in transmembrane ACE2 activity in the lungs and other tissues, increased pro-inflammatory responses, and more severe COVID-19 outcomes [9].
Importantly, the results of this study showed that anti-ACE2 antibodies were detected at a high frequency in infertile patients, irrespective of history of COVID-19, significantly more frequently than in fertile women. They were 1.9 and 2 times more likely to be detected in infertile than in fertile women. However, the highest probability of detecting IgG antibodies against ACE2 (5.6 times higher) was found in patients with mild-to-moderate COVID-19.
Infertile patients differed from the comparison group in terms of higher rates of spontaneous miscarriages, inflammatory gynecological diseases, pelvic surgery, thyroid diseases, and autoimmune thyroiditis. Notably, anti-ACE2 antibodies were significantly associated with primary and secondary infertility, with no significant differences in their detection rates between the two forms of infertility. Seropositive fertile women had a high incidence of allergic diseases, chronic ENT, gastrointestinal diseases, and STI.
Notably, there was a higher level of anti-ACE2 antibodies in patients with infertility than in fertile women, and there was no significant difference in the level of these antibodies in patients who had and did not have a history of COVID-19. This appears to be due to the possibility of anti-ACE2 autoantibodies in other infectious and inflammatory diseases, particularly gynecological diseases such as adnexitis, endometriosis, endometritis, and autoimmune thyroiditis. A predisposition to allergic diseases and history of surgery may have contributed to the formation of these antibodies.
The incidence and levels of anti-ACE2 antibodies were independent of the age of infertile patients and did not differ between ERA and LRA women, but a higher level of IgM anti-ACE2 antibodies was observed in ERA patients with a history of COVID-19.
Of particular interest are the direct correlations between levels of antibodies to ACE2 and hormones, most pronounced with antibodies to FSH. It has been shown in animal models and humans, that the RAS axis formed by ACE2/Ang1-7/Mas is expressed in the ovaries, plays a role in ovarian physiology, and is regulated by gonadotropic hormones [15, 23]. Therefore, a 2.1-fold and 3.3-fold increase in ACE2 and Mas mRNA expression, respectively, was observed in an animal model exposed to chorionic gonadotropins. As an increase in gonadotropic hormone levels increases ACE2 levels, this seems to explain the strong direct correlation between autoantibody levels and ACE2 and FSH levels. The correlation between antibodies to ACE2 and PG may be related to the stimulatory effect of RAS components on steroidogenesis.
The results of this study and evidence of the role of ACE2 in the regulation of RAS and maintaining reproductive health and fertility in women suggest the involvement of antibodies to ACE2 in the development of reproductive failure leading to infertility.
Conclusion
Antibodies to ACE2 have been frequently detected in women with primary and secondary infertility and inflammatory gynecological and thyroid diseases, irrespective of a history of COVID-19. Their detection rates and serum levels were significantly higher than those in fertile women, whereas the detection rate of IgG anti-ACE2 antibodies was higher in patients with a history of COVID-19. Antibodies to ACE2 were often concomitant and correlated with antibodies against hormones, especially FSH. These findings suggest that anti-ACE2 antibodies are significantly associated with primary and secondary infertility and may be involved in the pathophysiology of female infertility.
References
- Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J. et al. Angiotensin-сonverting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020; 126(10): 1456-74. https://dx.doi.org/10.1161/CIRCRESAHA.120.317015.
- Valenzuela R., Pedrosa M.A., Garrido-Gil P., Labandeira C.M., Navarro G., Franco R. et al. Interactions between ibuprofen, ACE2, renin-angiotensin system, and spike protein in the lung. Implications for COVID-19. Clin. Transl. Med. 2021; 11: e371. https://dx.doi.org/10.1002/ctm2.371.
- Pedrosa M.A., Valenzuela R., Garrido-Gil P., Labandeira C.M., Navarro G., Franco R. et al. Experimental data using candesartan and captopril indicate no double-edged sword effect in COVID-19. Clin. Sci. 2021; 135(3): 465-81. https://dx.doi.org/10.1042/CS20201511.
- Beyerstedt S., Casaro E.B., Rangel É.B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021; 40(5): 905-19.https://dx.doi.org/10.1007/s10096-020-04138-6.
- Lanza K., Perez L.G., Costa L.B., Cordeiro T.M., Palmeira V.A., Ribeiro V.T. et al. COVID-19: the renin-angiotensin system imbalance hypothesis. Clin Sci. 2020; 134(11): 1259-64. https://dx.doi.org/10.1042/CS20200492.
- Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection, Eur. J. Intern. Med. 2020; 76: 14-20. https://dx.doi.org/10.1016/j.ejim.2020.04.037.
- Arthur J.M., Forrest J.C., Boehme K.W., Kennedy J.L., Owens S., Herzog C., Harville T.O. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS One. 2021; 16(9): e0257016. https://dx.doi.org/10.1371/journal.pone.0257016.
- Rodriguez-Perez A.I., Labandeira C.M., Pedrosa M.A., Valenzuela R., Suarez-Quintanilla J.A., Cortes-Ayaso M. et al. Autoantibodies against ACE2 and angiotensin type-1 receptors increase severity of COVID-19. J. Autoimmun. 2021; 122: 102683. https://dx.doi.org/10.1016/j.jaut.2021.102683.
- Labandeira C.M., Pedrosa M.A., Suarez-Quintanilla J.A., Cortes-Ayaso M., Labandeira-García J.L., Rodríguez-Pérez A.I. Angiotensin system autoantibodies correlate with routine prognostic indicators for COVID-19 severity. Front. Med. 2022; 9: 840662. https://dx.doi.org/10.3389/fmed.2022.840662.
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020; 46(4): 586-90.https://dx.doi.org/10.1007/s00134-020-05985-9.
- Li M.-Y., Li L., Zhang Y., Wang X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020; 9(1): 45. https://dx.doi.org/10.1186/s40249-020-00662-x.
- Jing Y., Run-Qian L., Hao-Ran W., Hao-Ran C., Ya-Bin L., Yang G., Fei C. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020; 26(6): 367-73. https://dx.doi.org/10.1093/molehr/gaaa030.
- Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., Zhou W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020; 174(7): 722-5. https://dx.doi.org/10.1001/jamapediatrics.2020.0878.
- Cavallo I.K., Dela Cruz C., Oliveira M.L., Del Puerto H.L., Dias J.A., Lobach V.N. et al. Angiotensin-(1-7) in human follicular fluid correlates with oocyte maturation. Hum. Reprod. 2017; 32(6): 1318-24. https://dx.doi.org/10.1093/humrep/dex072.
- Reis F.M., Bouissou D.R., Pereira V.M., Camargos A.F., dos Reis A.M., Santos R.A. Angiotensin-(1-7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil. Steril. 2011; 95(1): 176-81. https://dx.doi.org/10.1016/j.fertnstert.2010.06.060.
- Менжинская И.В., Гладкова К.А., Сидельникова В.М., Сухих Г.Т. Антипрогестероновые антитела в клинике привычной потери беременности. Иммунология. 2008; 29(1): 34-7. [Menzhinskaya I.V., Gladkova K.A., Sidelnikova V.M., Sukhikh G.T. Antiprogesterone antibodies in clinic of habitualloss pregnancy. Immunology. 2008; 29(1): 34-7 (in Russian)].
- Менжинская И.В., Кашенцева М.М., Ванько Л.В., Сухих Г.Т. Иммунохимические свойства аутоантител к хорионическому гонадотропину у женщин с невынашиванием беременности. Иммунология. 2015; 36(1): 30-5. [Menzhinskaya I.V., Kashentseva M.M., Vanko L.V., Sukhikh G.T. Immunochemical properties of autoantibodies aganst chorionic gonadotropin in women with pregnancy loss. Immunology. 2015; 36(1): 30-5. (in Russian)].
- Soro-Paavonen A., Gordin D., Forsblom C., Rosengard-Barlund M., Waden J., Thorn L. et al. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J. Hypertens. 2012; 30(2): 375-83.https://dx.doi.org/10.1097/HJH.0b013e32834f04b6.
- Varagic J., Ahmad S., Nagata S., Ferrario C.M. ACE2: angiotensin II/angiotensin-(1–7) balance in cardiac and renal injury. Curr. Hypertens. Rep. 2014; 16(3): 420. https://dx.doi.org/10.1007/s11906-014-0420-5.
- Park S.E., Kim W.J., Park S.W., Park J.W., Lee N., Park C.Y., Youn B.S. High urinary ACE2 concentrations are associated with severity of glucose intolerance and microalbuminuria. Eur. J. Endocrinol. 2013; 168(2): 203-10.https://dx.doi.org/10.1530/EJE-12-0782.
- Takahashi Y., Haga S., Ishizaka Y., Mimori A. Autoantibodies to angiotensin-converting enzyme 2 in patients with connective tissue diseases. Arthritis Res. Ther. 2010; 12(3): R85. https://dx.doi.org/10.1186/ar3012.
- Pour S.K., Scoville C., Susan S. Tavernier S.S., Aghazadeh‑Habashi A. Plasma angiotensin peptides as biomarkers of rheumatoid arthritis are correlated with anti‑ACE2 auto‑an https://dx.doi.org/tibodies level and disease intensity. Inflammopharmacology. 2022; 30(4): 1295-302. https://dx.doi.org/10.1007/s10787-022-01008-9.
- Pereira V.M., Reis F.M., Santos R.A.S., Cassali G.D., Santos S.H.S., Honorato-Sampaio K., dos Reis A.M. Gonadotropin Stimulation Increases the Expression of Angiotensin-(1–7) and Mas Receptor in the Rat Ovary. Reprod. Sci. 2009; 16(12): 1165-74. https://dx.doi.org/10.1177/1933719109343309.
Received 30.11.2022
Accepted 07.02.2023
About the Authors
Irina V. Menzhinskaya, Dr. Med. Sci., Leading Researcher, Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-11-83, i_menzinskaya@oparina4.ru, 4, Oparina str., Moscow, Russia, 117997.Darya M. Ermakova, Postgraduate Student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, +7(906)555-79-97, daria.ermakova.97@bk.ru, 4, Oparina str., Moscow, Russia, 117997.
Anastasia G. Syrkasheva, PhD, Senior Researcher, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, +7(495)531-44-44, a_syrkasheva@oparina4.ru, 4, Oparina str., Moscow, Russia, 117997.
Yulia S. Drapkina, PhD, Researcher, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)531-44-44, yu_drapkina@oparina4.ru, 4, Oparina str., Moscow, Russia, 117997.
Natalia V. Dolgushina, Dr. Med. Sci., Deputy Director for Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-49-77, n_dolgushina@oparina4.ru, 4, Oparina str., Moscow, Russia, 117997.



