Verifying fetal scalp lactate testing results as measured by a portable lactate analyzer
Aim. To determine the reference values of lactate concentration in the blood obtained from the fetal presenting part and measured by a portable lactate analyzer.Prikhod'ko A.M., Tysyachnyi O.V., Romanov A.Yu., Baev O.R.
Materials and methods. A prospective study included 92 pregnant women at delivery who underwent fetal scalp blood sampling to determine the lactate level and umbilical artery sampling to assess the acid-base balance.
Results. An increase in scalp lactate concentration was associated with an increase in umbilical artery pCO2, base deficit, and a decrease in blood pH. Lactate concentration ≥4.9 mmol/l was associated with a higher rate of an emergency delivery. However, the fetal condition was over-diagnosed in 74.5% of cases. Increasing the cutoff threshold of scalp lactate to 5.9 mmol/L allows a 22.8% reduction in unwarranted surgical delivery.
Conclusion. Normal and pre-acidosis fetal scalp lactate concentrations measured by the portable lactate analyzer (Lactate Scout) were ≤4.8 and 4.9–5.8 mmol/L, respectively. Fetal scalp lactate concentration ≥5.9 mmol/L was associated with a high risk of acidosis requiring emergency delivery.
Keywords
According to Jorgensen and Visser, fetal scalp blood sampling during labor to assess acid-base balance (ABB) as a supplement to cardiotocography reduces the rate of operative delivery compared to cardiotocography alone [1, 2].
Lactate is the major specific end-product of anaerobic metabolism. Its concentration can be measured by fetal scalp blood sampling. The presence of lactate in the newborn’s blood is a marker of fetal hypoxia [3], and an increase in its concentration correlates with a decrease in pH [4]. Westgren (1998) showed that the levels of lactate and pH in blood from presenting part of the scalp were comparable in predicting perinatal outcomes. Still, the procedure for measuring lactate was more successful than that for pH [5]. Measurement blood lactate from presenting part of the scalp as a supplement to cardiotocography in the case of a equivocal CTG data may help verify the diagnosis of hypoxia.
Blood lactate measurements using different lactate analyzers produce different absolute values. For example, cutoffs for Lactate Pro™ were <4.2 mmol/L, 4.2–4.8 mmol/L, and ≥ 4.9 mmol/L for normal, preacidosis, and acidosis. However, these cutoffs for Lactate Pro2™ were <6.4 mmol/l, 6.4–7.3 mmol/l, and > 7.3 mmol/l, respectively [6]. These discrepancies between the absolute lactate values by the two devices were associated with a change in the formula that was used by the manufacturer for calculating lactate concentration. Therefore, when using different portable lactometers, it is necessary to take into account possible differences in the results.
Given the above, our study aimed to determine the reference values of blood lactate concentrations measured by a portable lactate analyzer for the diagnosis of fetal hypoxia during labor.
Material and methods
A prospective study was conducted in 2016–2019 at the 1st maternity ward of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. The study included 92 full-term women at delivery, with singletons in the cephalic presentation, having no indications for elective delivery, and having a equivocal or pathological type of CTG in labor.
To evaluate the fetal condition and choose the delivery management strategy, all patients underwent blood sampling from the presenting part of the scalp during labor to determine the lactate level with a Lactate Scout portable lactate analyzer.
The technique of sampling blood from the presenting part of the scalp:
A sampling of fetal-scalp blood can be performed during labor after the rupture of the membranes and dilatation of the cervical os to 2–3 cm.
After the preparation of the external genital area with an antiseptic solution, the presenting part of the scalp is visualized via an amnioscope.
The presenting part of the scalp is carefully dried with a swab to avoid getting blood, mucus, and amniotic fluid into the sample. The test strip, according to the code, is inserted into the portable lactate analyzer before the sample is taken. The lactometer is turned on, and the code of the test strip is entered. One test strip is inserted into the analyzer.
Fetal-scalp blade is used to make a quick stab-like clean incision of the scalp.
Blood is collected into a capillary tube (5 mm of the tube length).
Blood is transferred from the capillary tube to a non-absorbent surface. The edge of the test strip is applied to the drop of blood.
When the analyzer turns on, an acoustic signal will sound. The analysis of one sample takes 10 seconds.
Also, immediately after birth, all the newborns were tested for the acid-base balance (ABB) of arterial blood obtainedfromtheumbilicalcord.Bloodsamplesweretaken by isolating the umbilical cord segment with three clamps. The cord was then cut between the 1st and 2nd clamps, and blood was drawn from the umbilical artery between the 2ndand3rdclamps. ThepH, lactate(lac), basedeficit(BE), oxygen partial pressure (pO2) and carbon dioxide (pCO2) levels were measured using an ABL800 FLEX blood gas analyzer (Radiometer Medical ApS, Denmark).
Considering the differences in the cut-off values of ABB in the diagnosis of metabolic acidosis according to the data of different studies (pH <7.21, <7.2, <7.15) [7], in our work, the cut-off values for the identification of a group with metabolic acidosis was selected based on the results of a previous study of cord blood ABB [8]. Group 1included 78 (85%) newborns without signs of metabolic acidosis (pH ≥ 7.17; BE <12.2 mmol/l); group 2 included 14 (15%) newborns with metabolic acidosis (pH <7.17 and / or BE ≥ 12.2 mmol/L). CTG analysis was performed according to the FIGO consensus guidelines (2015) [9]. Based on the internal protocol of the Center, the cut-off value for decision making and changing obstetric strategy was the fetal scalp blood lactate ≥ 4.9 mmol/L [10]; fetal scalp blood lactate level (slac) was ≤4.8 ( n = 37) and slac ≥ 4.9 (n = 55). If blood sampling for slac during childbirth was carried out several times, then the last result was considered for decision making. When slac was ≥ 4.9 mmol/L, the women underwent emergency delivery within 10–15 minutes.
The study was approved by the Ethics Committee of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia All patients gave informed for sampling blood from the presenting part of the scalp to determine the blood level of lactate and measurement of ABB in the umbilical cord blood.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, USA). The distribution of continuous variables was tested for normality using the generalized D'Agostino-Pearson test. Quantitative variables showing normal distribution were expressed as means (standard deviation); t-test was used to compare them. Data with non-normal distribution were reported as the median (interquartile range), and the Mann-Whitney U test was used to compare them. Categorical variables were reported as counts and percentages and compared via Fisher's exact test with the calculation of the relative risk (RR). Differences were considered statistically significant at p <0.05.
Results
Seventeen (21.8%) and two (14.3%) infants were born by vaginal delivery in groups 1 and 2, respectively. Of these, 10.2% (n = 8) and 14.3% (n = 2) had a vacuum-assisted delivery and 68% (n = 61) and 85.7% (n = 12) were delivered by caesarean section, respectively. The rates of spontaneous delivery (p = 0.53), vacuum-assisted delivery (p = 0.65) and caesarean section (p = 0.46) did not differ between the groups.
The slac concentration of 4.9 mmol/L or more in groups 1 and 2 was observed in 42 (53.4%) and all 14 (100%) women. Of the remaining 36 women in group 1 who had normal slac values, 21 (58.3%) underwent surgical delivery due to questionable CTG type.
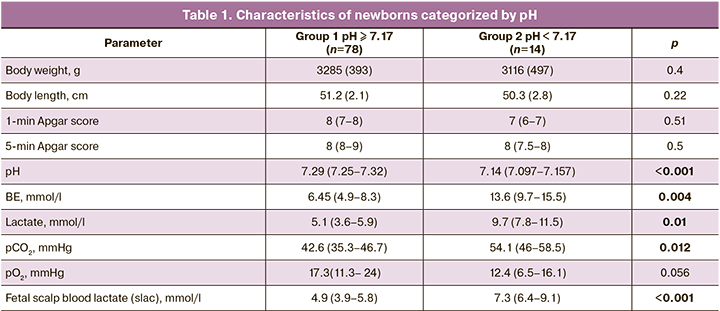
The concentrations of fetal scalp blood lactate and umbilical artery lactate in newborns in group 2 was significantly higher than in group 1(p = 0.0008), which was concurrent with an increase in pCO2 and BE (table 1). At the same time, fetal scalp blood lactate concentration was directly proportional to its level in the umbilical artery. At the same time, there were no significant differences between groups 1 and 2 in the Apgar score, which is probably due to the relative subjectivity of the clinical assessment and the small sample size.
At the next stage of the work, we divided the participants into groups categorized by the level of fetal scalp blood lactate, i.e., a group with slac ≤ 4.8 mmol/L, n = 37 (40.2%) and a group with slac ≥ 4.9 mmol/L, n = 55 (59.8%). In the group slac ≤ 4.8 mmol/L 19 (51.3%), 9 (24.3%), and 9 (24.3%) women had a cesarean section, vacuum-assisted delivery, and spontaneous delivery, respectively.
Analysis of CTG data showed that in the group with slac ≤ 4.8 mmol/l, 35 (94.6%) and 2 (5.4%) patients had an equivocal and pathological type of CTG, respectively. In the group with slac ≥4.9 mmol/l, 41 (74.5%) and 14 (25.5%) patients had an equivocal and pathological type of CTG, respectively. In the group with slac ≤ 4.8 mmol/l, 21 (56.7%) women underwent surgical delivery (of them, 15 (71.4%) had cesarean section, and 6 (28.6%) had vacuum-assisted delivery). Pathological type CTG was statistically significantly more often registered in the group with slac ≥ 4.9 mmol/l, the odds ratio was 4.7 (95% CI = 2.8–20.8, p = 0.03). Equivocal type CTG was recorded equally often in both groups, p = 0.64. In the group with slac ≥ 4.9 mmol/l, 54 (98.2%) patients underwent cesarean section, and 1(1.8%) had vacuum-assisted delivery.
Operative delivery rates were statistically significantly different in the study groups (p = 0.0001 and p = 0.01 for cesarean section in the slac group ≤ 4.8 mmol/l and vacuum-assisted delivery in the slac group ≥ 4.9 mmol/l, respectively). Higher concentrations of lactate were associated with a higher cesarean section rate.
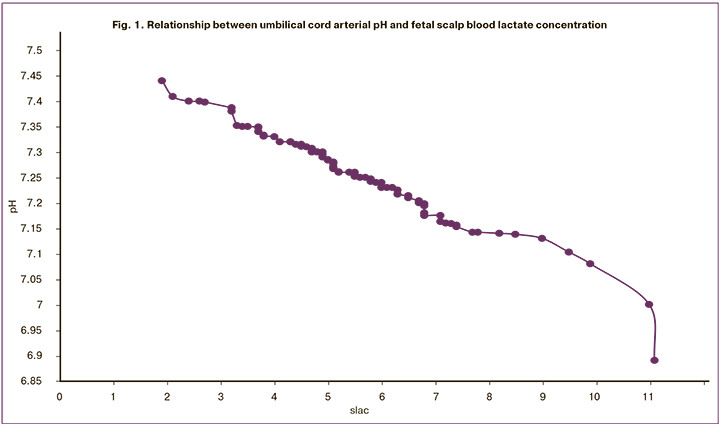
 The concentrations fetal scalp blood lactate and umbilical artery did not differ statistically significantly (p = 0.79) (Fig. 2). In the group with lactate concentration (slac), ≥ 4.9 mmol/L, the Apgar score at the 1st and 5th minutes and the pH values were significantly lower (Table 2). In the slac ≤ 4.8 mmol/L group, only 1 (2.7%) newborn was born with umbilical artery blood pH below 7.17 (slac = 4.7 mmol/L, pH = 7.163). In the slac group ≥ 4.9 mmol/L low pH values were observed in 13 (25.5%) cases (p = 0.0005). Therefore, at a slac level of <4.7 mmol/L, 100% of children were born without signs of acidosis.
The concentrations fetal scalp blood lactate and umbilical artery did not differ statistically significantly (p = 0.79) (Fig. 2). In the group with lactate concentration (slac), ≥ 4.9 mmol/L, the Apgar score at the 1st and 5th minutes and the pH values were significantly lower (Table 2). In the slac ≤ 4.8 mmol/L group, only 1 (2.7%) newborn was born with umbilical artery blood pH below 7.17 (slac = 4.7 mmol/L, pH = 7.163). In the slac group ≥ 4.9 mmol/L low pH values were observed in 13 (25.5%) cases (p = 0.0005). Therefore, at a slac level of <4.7 mmol/L, 100% of children were born without signs of acidosis.
Among newborns who had umbilical cord arterial pH below 7.17 (acidosis), only one had a borderline level of fetal scalp blood lactate (slac = 4.7 mmol/L), while the pH of arterial cord blood was also at the borderline level (7.163). In the group with slac ≥ 4.9 mmol/L, low pH levels were observed in 13 newborns (p = 0.0005). At slac ≥ 4.9 mmol/L, the RR for the development of acidosis in the newborn was 9.2 (95% CI = 1.3–67.0).
At the same time, among newborns with slac ≥ 4.9 mmol/L, acidosis was verified by the umbilical cord blood ABB data only in 25.5% of babies. It is also worth noting that all newborns in this group were born by surgical delivery. In this regard, it can be assumed that with this cut-off , there was an overdiagnosis of fetal disorders in 74.5% of cases. Taking into account the obtained data, sensitivity, and specificity analyses of the fetal scalp blood lactate were conducted using a different cut off values for predicting acidosis in a newborn (cord blood pH <7.17).
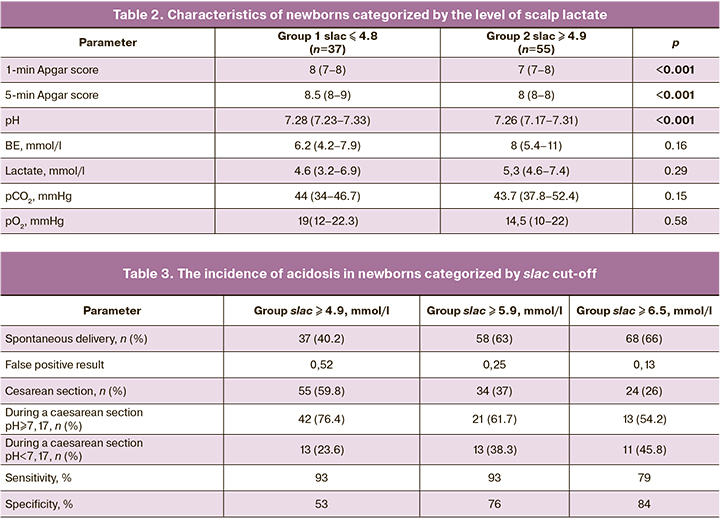
With a scalp lactate (slac) level ≥ 4.9 mmol/L, the sensitivity and specificity of the model was 53% and 93%, respectively. With the cut-off value of 6.5 mmol/L for the fetal scalp blood lactate, the sensitivity and specificity of the model were 84% and 79%, respectively. However, with this cut-off value, every second child will be born with acidosis (in 45.8% of newborns, the umbilical artery pH will be <7.17). (Table 3).
Therefore, the optimal cutoff for making a clinical decision on the labor management strategy based on the fetal scalp blood lactate, at which the prediction of intrapartum fetal hypoxia does not worsen and false-positive rate and, consequently, unwarranted surgical delivery, is reduced, was (slac) ≥ 5.9 mmol/L (sensitivity 76% and specificity 93%). At slac ≥ 5.9 mmol/L, the RR for the development of acidosis in the newborn was 23.2 (95% CI = 3.2–169.8). Therefore, compared to the threshold of 4.8 mmol/L, the prediction of the development of intrapartum fetal acidosis is improved.
With a cutoff for fetal scalp blood lactate of 4.9 mmol/L with suspected fetal hypoxia, 59.8% of women will undergo emergency delivery, while with a cutoff for slac of 5.9 mmol/L only 37%. Therefore, the number of cesarean sections and vacuum extractions will decrease by 22.8%. At the same time, all newborns with verified acidosis will be in the group born by emergency cesarean section, while the number of false positives will drop from 0.52 to 0.25. A further increase in the cutoff for slac of 6.5 mmol/L results in a decrease in the sensitivity and an increase in the number of children with acidosis. At slac ≥ 6.5 mmol/L, the RR for the development of acidosis in the newborn was 10.4 (95% CI = 3.2–34.1).
Discussion
Despite the positive correlation between fetal scalp blood lactate and umbilical cord arterial pH, this relationship is not always proportional [5]. Lactate is not only a marker of hypoxia [11] but also a marker of intrauterine infection or birth stress. Perhaps this accounts for rather high false-positive rates, which was 56.7% in our study with the cutoff of 4.9 mmol/L. Therefore, an essential criterion for selecting patients for intrapartum fetal scalp blood sampling for lactate measurement is the correctly identified type of CTG. In the case of a normal CTG curve, sampling is not recommended. Also, the reference range for lactate depends on the type of device [6]. Only a standardized and approved device shows reliable results. It is essential to understand what lactate concentration is considered as the level of intrapartum hypoxia. According to our data, increasing the cutoff of scalp lactate from 4.9 to 5.9mmol/L improves the validity of the findings and reduces the likelihood of unwarranted surgical delivery by 22.8%.
Conclusion
The study findings showed that normal and pre-acidosis fetal scalp lactate concentrations measured by the portable lactate analyzer (Lactate Scout) were ≤4.8 and 4.9–5.8 mmol/L, respectively. Fetal scalp lactate concentration ≥5.9 mmol/L was associated with a high risk of acidosis requiring emergency delivery.
References
- Jorgensen J.S., Weber T. Fetal scalp blood sampling in labor – a review. Acta Obstet. Gynecol. Scand. 2014; 93(6): 548-55. https://dx.doi.org/10.1111/aogs.12421.
- Visser G.H., Ayres-de-Campos D.; FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: adjunctive technologies. Int. J. Gynecol. Obstet. 2015; 131(1): 25-9. https://dx.doi.org/10.1016/j.ijgo.2015.06.021.
- Engidawork E., Chen Y., Dell’Anna E., Goiny M., Lubec G., Ungerstedt U. et al. Effect of perinatal asphyxia on systemic and intracerebral pH and glycolysis metabolism in the rat. Exp. Neurol. 1997; 145(2, Pt.1): 390-6. https://dx.doi.org/10.1006/exnr.1997.6482.
- Wiberg-Itzel E., Lipponer C., Norman M., Herbst A., Prebensen D. Blood in management of intrapartum fetal distress: randomised controlled multicentre trial. BMJ. 2008; 336(7656): 1284-7. https://dx.doi.org/10.1136/bmj.39553.406991.25.
- Westgren M., Kuger K., Ek S., Grunevald C., Kublickas M., Naka K. et al. Lactate compared with pH analysis at fetal scalp blood sampling: a prospective randomised study. Br. J. Obstet. Gynaecol. 1998; 105(1): 29-33. https://dx.doi.org/10.1111/j.1471-0528.1998.tb09346.x.
- Birgisdottir B.T., Holzmann M., Varli I.H., Graner S., Saltvedt S., Nordström L. Reference values for Lactate Pro 2™ in fetal blood sampling during labor: a cross-sectional study. J. Perinat. Med. 2017; 45(3): 321-5. https://dx.doi.org/10.1515/jpm-2016-0027.
- Yeh P., Emary K., Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51 519 consecutive validated samples. BJOG. 2012; 119(7): 824-31. https://dx.doi.org/10.1111/j.1471-0528.2012.03335.x.
- Приходько А.М., Романов А.Ю., Шуклина Д.А., Баев О.Р. Показатели кислотно-основного равновесия и газовый состав артериальной и венозной пуповинной крови в норме и при гипоксии плода. Акушерство и гинекология. 2019; 2: 93-7. [Prikhodko A.M., Romanov A.Yu., Shuklina D.A., Baev O.R. The indicators of acid-base balance and the gas composition of arterial and venous cord blood in health and fetal hypoxia. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2019; 2: 93-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.2.93-97.
- Ugwumadu A. Are we (mis)guided by current guidelines in intrapartum heart rate monitoring? Case for a more physiological approach to interpretation. BJOG. 2014; 121(9): 1063-70. https://dx.doi.org/10.1111/1471-0528.12900.
- Вихарева О.Н., Баев О.Р., Кан Н.Е., Клименченко Н.И., Тетруашвили Н.К., Тютюнник В.Л., Шмаков Р.Г. Определение лактата в крови из предлежащей части плода. Алгоритм действий во время родов. Краткий протокол. Акушерство и гинекология. 2015; 4: 16. [Vikhareva O.N., Baev O.R., Kan N.E., Klimenchenko N.I., Tetruashvili N.K., Tyutyunnik V.L. et al. Determination of lactate in the blood from the presenting part of a fetus. The action algorithm during childbirth. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2015; 4: 16. (in Russian)]
- Tuffnell D., Haw W.L., Wilkinson K. How long does a fetal scalp blood sample take? BJOG. 2006; 113(3): 332-4. https://dx.doi.org/10.1111/j.1471-0528.2006.00859.x.
Received 17.03.2020
Accepted 18.05.2020
About the Authors
Andrey M. Prikhod’ko, Ph.D., Physician at the 1st Maternity Department, Teaching Assistant at the Department of Obstetrics and Gynecology, Researcher at the Department of Innovative Technologies, Institute of Obstetrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(495)438-30-47. E-mail: a_prikhodko@oparina4.ru.4 Oparina str., Moscow, 117997, Russian Federation.
Oleg V. Tysyachnyi, Ph.D., Researcher at the Department of Innovative Technologies, Institute of Obstetrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(495)438-11-88. E-mail: olti23@mail.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Andrey Yu. Romanov, Ph.D. Student, Specialist at the R&D Department, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel. +7(903)158-94-00.
E-mail: romanov1553@yandex.ru. 4 Oparina str., Moscow, 117997, Russian Federation.
Oleg R. Baev, Dr.Med.Sci., Professor, Head of the 1st Maternity Department, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia, Professor at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First MSMU of Minzdrav of Russia (Sechenov University). Tel.: +7(495)438-11-88.
E-mail: o_baev@oparina4.ru. 4 Oparina str., Moscow, 117997, Russian Federation.
For citation: Prikhod’ko A.M., Tysyachnyi O.V., Romanov A.Yu., Baev O.R. Verifying fetal scalp lactate testing results as measured by a portable lactate analyzer.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 7: 87-92 (in Russian)
https://dx.doi.org/10.18565/aig.2020.7.87-92



