Satisfaction of young women with estradiol valerate/dienogest in real clinical practice in Russia: results of prospective multicenter observational study Q-SWAN
Prilepskaya V.N., Andreeva E.N.
Objective: To evaluate satisfaction in young women (18–35 years old) with a drug containing estradiol valerate/dienogest (E2V/DNG) over a 6-month period in real clinical practice in Russia.
Materials and methods: This was a prospective multicenter study that assessed satisfaction of 504 women (average age is 27.8 years) with the contraceptive containing E2V/DNG. The characteristics of menstrual bleeding, sexual function, and women’s decision to continue using the contraceptive were evaluated in two subgroups: in the presence and absence of abnormal uterine bleeding (AUB). The satisfaction of doctors with this method of contraception was also assessed.
Results: It was found that 98.4% of women and 100% of doctors were “very satisfied” or “satisfied” with this method of contraception, regardless of the presence or absence of AUB. There was a significant decrease in the intensity, duration of menstrual bleeding and pain, and in the frequency of intermenstrual bleeding compared with the baseline indicator. Normalization of the parameters of the FSFI questionnaire was observed in most of the patients (89.7%), although initially these parameters could indicate sexual dysfunction in almost half of the patients (46.0%). The contraceptive E2V/DNG was well tolerated by women, and the overall rate of adverse events was 7.1%. Most women (97.8%) decided to continue taking the medication after completing the study.
Conclusion: The results of the study showed high satisfaction of young Russian women and doctors with the use of E2V/DNG, regardless of the presence or absence of AUB. The medication has a positive effect on the characteristics of bleeding/cycle control and on the quality of sexual function, therefore, the women demonstrated a high adherence to this method of contraception.
Authors’ contributions: Prilepskaya V.N., Andreeva E.N. – developing the concept and design of the study, collecting and processing the data, writing the text, editing the article, approving the final version of the article.
Conflicts of interest: Bayer AG, as the sponsor of the study, was involved in the design, collection and analysis of research data, according to the statistical analysis plan which was previously approved. The authors who prepared the article had unlimited access to all research data, meet all ICMJE authorship criteria and vouch for the accuracy and completeness of the analytical results presented by them.
Funding: The article was prepared without financial support.
Ethical Approval: The study was approved by the Independent Interdisciplinary Committee on Ethical Review for Clinical Studies and was conducted in accordance with the regulatory documents of the Russian Federation.
Patient Consent for Publication: The patients provided an informed consent for the participation in the study and publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Prilepskaya V.N., Andreeva E.N. Satisfaction of young women with estradiol valerate/dienogest in real clinical practice in Russia: results of prospective multicenter observational study Q-SWAN.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (3): 108-117 (in Russian)
https://dx.doi.org/10.18565/aig.2024.21
Keywords
According to the report “The World Population in 2022” of United Nations Population Fund (UNFPA), almost half of pregnant women in the world reported that they did not plan their pregnancy [https://www.unfpa.org/sites/default/files/pub-pdf/RU_SWP22%20report.pdf]. This is an extremely alarming signal, because more than 60% of unplanned pregnancies in the world are unwanted and, as a rule, are terminated: 56% in the world, 77–78% in the countries of Eastern Europe, including the Russian Federation (RF) [1]. According to the guidelines of the World Health Organization (WHO), abortion cannot be considered as a method of birth control in modern society. The modern method of birth control is considered to be contraception. Hormonal contraception takes a leading place among the methods of birth control. When it is used correctly, its contraceptive effectiveness is significant. Contraception is reversible, acceptable, it enables to prevent abortion and choose the time for desired and healthy motherhood, that meets the goals of our сountry to preserve women’s reproductive health and to increase birth rates [2].
Current guidelines for optimal patient counseling in choosing a contraceptive method recommend that the clinicians should use the concept of person-centered care and shared decision making [3–5]. Receiving timely and reliable information from the doctor, understanding the potential advantages suggests active involvement of women in the process of choosing the right contraception that best meets her individual needs and expectations, and will help to increase adherence to the chosen method and satisfaction with the obtained results.
Combined oral contraceptives (COCs) appeared in the market about 60 years ago, and since then this is the most widely used hormonal method of contraception. The importance of this method for women’s reproductive health can be hardly overestimated [6]. Currently, according to global surveys, over 40% of women of reproductive age choose this type of contraception [1, 7]. Most often, the reason for refusal to use COCs (in 42.1% of cases) is dissatisfaction with this method mainly due to side effects [8]. Moreover, the women often express concerns about possible weight gain, negative impact on emotional state and libido, as well as on bleeding profile [8, 9]. Therefore, it seems especially important that this medicine is well tolerated by patients and enables long-term use until the completion of reproductive plans or until menopause and adequate follow-up.
The combination of estradiol valerate (E2V), which is identical to natural estrogen17β-estradiol (E2), and dienogest (DNG) – a selective fourth-generation progestin in the dynamic dosing regimen (E2V/DNG, Qlaira) with prolonged estrogen treatment phase up to 26 days and a short hormone-free interval (HFI) merely of 2 days, significantly reduces the incidence of adverse reactions and leads to high adherence to this method of contraception [10, 11].
The official indication for using E2V/DNG is oral contraception for women after the onset of menarche and treatment for prolonged and/or heavy menstrual bleeding (HMB) without organic causes of abnormal bleeding in women, who want to use oral contraceptives [12]. At present time, E2V/DNG is one of most studied COCs in the world. More than 15,000 women participated in multiple randomized clinical trials (RCTs) and in the trials in real clinical practice, which assessed contraceptive reliability, cycle control, the effects on primary dysmenorrhea, and hormone withdrawal-associated symptoms during HFI [8, 13–16], cardiovascular safety profile during almost 7 years of patient follow-up [17], as well as evaluation of reduction of blood loss in treatment of heavy and/or prolonged menstrual bleeding which is not associated with organic pathology [18, 19].
However, since the time the contraceptive pill containing E2V/DNG became available in our country, many gynecologists continue to consider it as a method of oral hormonal contraception preferable for women in late reproductive stage. This is largely due to the proven favorable safety profile of metabolic parameters and reduced risk of venous thromboembolism (by 60% versus other COCs) [17, 20]. This preference unreasonably deprives young women of many benefits of E2V/DNG associated with its composition and unique dosing regimen of hormonal components.
Combined oral contraceptive E2V/DNG is quite widely used in our country in real clinical practice, but satisfaction of Russian patients and doctors with this method of contraception has not yet been properly evaluated, despite the key importance of this indicator for ensuring contraceptive reliability, duration of use and women’s quality of life in general. In addition, no studies have been conducted in the world or in Russia to assess the attitudes toward this oral contraceptive in the target population of young women.
The objective of the study was to evaluate satisfaction in young women aged 18–35 years with oral contraceptive containing estradiol valerate and dienogest (E2V/DNG) over a 6-month period in real clinical practice in Russia.
Materials and methods
The prospective multicenter Q-SWAN study (Q-SWAN – Qlaira, Satisfaction, WomAN) [ClinicalTrials.gov: NCT04901377] was carried out in 28 Russian research centers from June 2021 to April 2023. The study was approved by the Independent Interdisciplinary Committee for Ethical Expertise in Clinical Research and was carried out in accordance with acting regulatory documents in the Russian Federation. The study included young women who required contraception at the time of selection and enrollment in the study and sought care in the appropriate research centers. All patients have signed informed consent to participate in the study.
Inclusion criteria were the patients aged 18–35 years; indication for using oral contraception; the patients not taking the combination of E2V/DNG at least one month before enrollment in the study.
Major exclusion criterium was contraindication to use combined E2V/DNG oral contraceptive pill based on the instruction for its use [12].
Patient characteristics
At baseline, 504 women aged 18–35 years (the mean age 27.8 (±4.7) years) were enrolled in the study. Duration of follow-up period was 6 months. All participants started taking E2V/DNG and were included in the group of security analysis. Of them, 2% of women (10/504) stopped taking the medicine before the planned end of follow-up period. A total of 494/504 women (98.0%) were valid for sampling of patients, who completed participation in the study in accordance with per protocol set (PPS). Further, research results only this population of women were represented. The number of women who completed or discontinued participation in the study due to various reasons is represented in Figure.
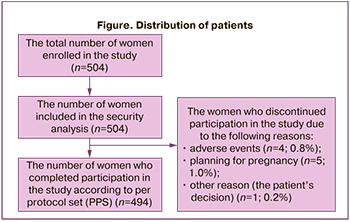
The study envisaged that there were 3 visits to healthcare facility: the visit for enrollment in the study (day 0); intermediate visit (after 3 menstrual cycles) and last visit (after 6 cycles). During the visit for patient enrollment, the researchers conducted interviews or surveyed medical records, and collected the following data: the year of birth/age, main vital signs (blood pressure, heart rate), medical history (comorbidities) to confirm eligibility criteria or exclusion criteria in the study, the methods contraception that were used during the year before enrollment in the study.
During the first visit, E2V/DNG combination (Qlaira, Bayer) was indicated for all participants of the study in the dynamic dosing regimen 26/2 (E2V 3 mg, on day 1-2, E2V 2 mg/ DNG 2 mg on day 3–7, E2V 2 mg/ DNG 3 mg on day 8–24, E2V 1 mg on day 25–26 and placebo on day 27–28 of the menstrual cycle). The results were registered in the individual Case Report Form (CRF) for each patient. Information was collected, and patient condition was assessed during routine visits to doctor in the appropriate medical facility, but at terms that responded closely to the requirements of the protocol of the study.
Given that assessment of satisfaction with using the medicine by the participants of the study could be influenced by the initial characteristics of menstrual bleeding, the results obtained in two subgroups of patients were analyzed separately. Subgroup 1 included women who during the first visit reported about HMB for more than 8 days (HMB group) (n=223, 45.1%; 223/494). Subgroup 2 included the remaining participants (n=271, 54.9%; 271/494), who had not HMB at baseline.
The methods of study
The main variable (the primary endpoint) was satisfaction with E2V/DNG over the 6-month follow-up. For this purpose, the satisfaction survey was conducted among the participants and overall satisfaction was measured using the 5-point Likert scale. The scoring of the answers to the question “Are you satisfied with the method of contraception that was used in the study?” were categorized as 1 – “not satisfied at all”; 2 – “not satisfied”; 3 – “I can’t ascertain, whether I am satisfied or not”; 4 – “satisfied”; 5 – “fully satisfied”. The overall satisfaction indicator was determined as the percentage of women who chose the category 4 and 5.
Secondary variable (the secondary endpoint) and survey methods (questionnaires):
- To assess the doctor's satisfaction with the above described method of contraception for women.
- To assess menstrual bleeding profile. During 3 months before commencement of the study and against the background of taking the medicine, the participants themselves assessed their menstrual bleeding characteristics according to the following indicators: regularity (regular, irregular, or absence); maximum duration of bleeding (0–2 days, 3–5 days, 6–7 days, ≥8 days); maximum intensity of bleeding (spotting, light, normal, heavy); painful bleeding (yes/no); intermenstrual bleeding (yes/no).
- To describe sexual function at the time of commencement of the study and over the 6-month period of taking E2V/DNG. For this purpose, two questionnaires widely recognized in clinical researches, were used. The participants self-reported on the Female Sexual Function Index (FSFI) questionnaire, that included 19 questions to evaluate sexual function measuring six domains: desire, arousal, lubrication, orgasm, satisfaction and pain) [21]. The FSFI total score of ≤26.55 points indicated sexual dysfunction. Also, The Atrophy Symptom Questionnaire (ASQ) was used to assess vulvovaginal symptoms that could directly affect sexual function. The presence and severity of each symptom (vaginal dryness, tenderness, irritation, dyspareunia discharge) was scored from 0 (absence of symptoms) to 3 points (severe symptoms). The total score was calculated as the sum of the scores for each symptom divided by 5.
- To assess the reasons for stopping to take E2V/DNG.
- To assess patient’s intention to continue taking E2V/DNG.
Statistical analysis
Statistical data analysis was presented as exploring descriptive statistics. It was described in detail in Statistical Analysis Plan (SAP), version 1.0 of November 09, 2021 and did not envisage testing of preformulated hypothesis. Software program MS Excel was used for statistical data processing.
Results
The primary endpoint: satisfaction of women with the method of contraception
The study showed that the patients were highly satisfied with contraceptive efficacy of E2V/DNG that was taken by the patients for contraceptive use during the 6-month follow-up in real clinical practice. In general, 98.4% of women “were satisfied” or “very satisfied” with using the medicine already after the 3-month follow-up, and at the time of their last visit to the doctor after the 6-month follow-up, and the percentage of women was the same. None of the patients were neither “not satisfied” or “not satisfied at all” with using E2V/DNG during the both visits. Similar results were obtained in the subgroups of patients regardless of the presence or absence of HMB before the commencement of the study: 97.8%; 99.1% and 98.9%; 97.8% after the 3-month and 6-month follow-up in the subgroups of women with or without HMB, respectively.
The secondary endpoints
Satisfaction of the doctors with the method of contraception
The level of satisfaction with this method of contraception was also high among the doctors: 99.6% and 100% of doctors were “satisfied” or “very satisfied” with the results obtained after using E2V/DNG that was reported by their patients during intermediate and last visits, respectively, regardless of having or not having heavy menstrual bleeding at baseline.
Assessment of bleeding profile
During intake of combined oral contraceptive containing E2V/DNG, the intensity, duration of bleeding and severity of dysmenorrhea reduced (Table).
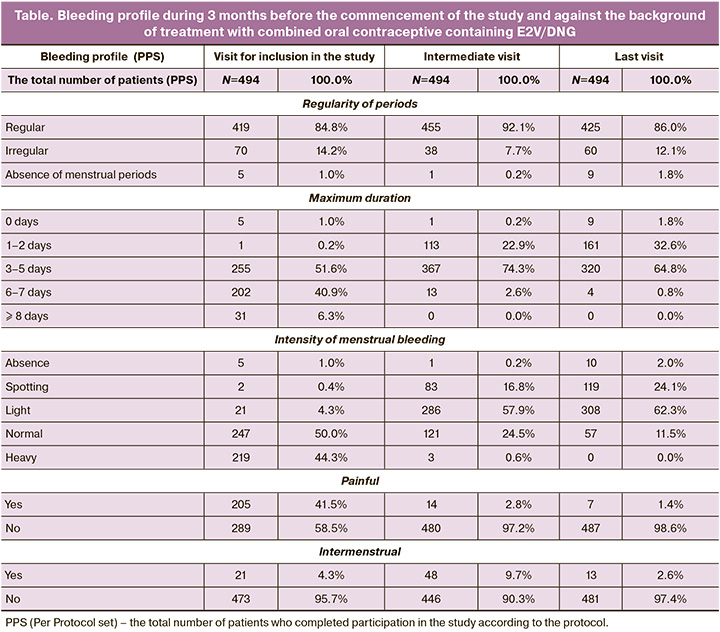
At the beginning of the study 94.3% of participants reported normal or heavy menstrual bleeding. By the end of the study 86.4% (427/494) of women had spotting or light menstrual-like discharge. At baseline, period length was 3–7 days in majority of women – in 92.5% (457/494). By the end of follow-up, the percentage of women with 6 or 7-day menstrual bleeding reduced from 40.9% (202/494) to 0.8% (4/494). Menstrual-like bleeding during 1–5 days was in the majority of women – 97.4% (481/494). At the beginning of the study, almost half of the participants – 45.1% (223/494) had HMB. Three months later only 3 women (0.6%) had HMB, and by the end of follow-up none of the patients were registered with heavy or prolonged menstrual bleeding. At baseline, the largest number of women with dysmenorrhea was in the subgroup with HMB – 55.6% (124/223), and their number significantly reduced to 0.9% (2/223) by the end of the study. Similar dynamics in reduction of dysmenorrhea was noted in the subgroup of women without HMB. At baseline, dysmenorrhea was in 29.9% (81/271) of women, and in 1.8% (5/271) by the end of the study. During the first visit to doctor, 4.3% (21/494) of women reported intermenstrual bleeding. During the intermediate visit the number of women slightly increased – 9.7% (48/494). During the last visit, the percentage of women with intermenstrual bleeding was lower compared with the initial percentage – 2.6% (13/494).
Evaluation of sexual function
This study evaluated the quality of sexual function using 2 questionnaires – FSFI и ASQ. Initially, almost half of the total number of participants – 227 women (46.0%) had low total FSFI scoring ≤26.55, and this could indicate sexual dysfunction. Against the background of taking E2V/DNG by the end of the 3rd and 6th month, the proportion of these women reduced significantly to 19.6% (96/494) and 10.3% (51/494), respectively. By the end of the study, the total FSFI scoring ˃26.55 was for the majority of women – 89.7% (443/494). Similar dynamics was observed during 3 visits in the subgroups of women with HMB – 4.4%; 19.7%; 9.3% and in women without HMB – 47.2%; 19.6%; 10.3%, respectively. Both in the general population and in both subgroups, a positive trend was observed in each of 6 indicators of the FSFI questionnaire from baseline values to the end of follow-up.
According to ASQ questionnaire, initially the total score was on average 0.5 (±0.6) points in the entire group of participants, that can be regarded as the absence of symptoms or mild manifestation. In general, a positive dynamics was observed in the whole population of women. The total scores were on average 0.2 (±0.3) and 0.1 (±0.2) points during the intermediate and last visits, respectively, and there were no significant differences between the subgroups of women with and without HMB. At baseline, only few women had moderate or severe symptoms of vulval and vaginal disorders: dryness (14.7%; 73/494), pain (9.1%; 45/494), irritation (8.9%; 44/494), dyspareunia (9.3%; 46/494) and vaginal discharge (12.5%; 62/494). By the end of the study the values of these indicators reduced to 0.8% (4/494), 0.2% (1/494), 0.4% (2/494), 0.6% (3/494) and 1.2% (6/494), respectively.
Assessment of the reason for stopping to take E2V/DNG oral contraceptive
The overall incidence of adverse events (AEs) in the study was in 7.1% (36/504) of women. Among them, in 3% (15/504) of participants AEs were associated with taking E2V/DNG oral contraceptive. Serious AEs including occurrence of pregnancy were not registered. Only 2% (10/504) of women decided to discontinue taking E2V/DNG oral contraceptive: 0.8% (4/504) of participants due to AEs (diarrhea, intermenstrual bleeding, low libido, abnormal uterine bleeding), 1% (5/504) due to pregnancy planning, and 0.2% (1/504) due to patient’s own decision.
Assessment of patients’s intention to continue taking E2V/DNG oral contraceptive
In general, after completion of the study 97.8% (483/494) of patients intended to continue taking E2V/DNG oral contraceptive, both in the subgroup of women with HMB (95.5%) and without HMB (99.6%). Only 11 patients had no intention to continue taking E2V/DNG after completion of patient follow-up.
Using contraception during the year before the study
At baseline, 12.1% (61/504) of women used combined hormonal contraceptives during one year before enrollment in the study. Duration of taking contraceptive pills was the following: <3 months – 13.3% (8/61), 3–6 months – 26.2% (16/61), 6–12 months – 23% (14/61), 1–3 year – 24.6% (15/61), 3–5 years – 8.2% (5/61), >5 years – 4.9% (3/61), and 87.9% of women (443/504) used at least one of other methods of contraception. These women reported about using 646 methods of contraception during one year before the study. Most often the women used barrier contraceptives (condoms, diaphragms) – 45.0 % (291/646), and 28.2% (182/646) of women used the withdrawal method of contraception; 4.8% (31/646) of women used intrauterine device (IUD); 15.2% (98/646) of women did not use any method of contraception.
Limitations of the study
Since Q-SWAN is an observational study, it cannot be ruled out that there were methodological limitation in the design of the study associated with wrong patient selection error, incomplete data transfer, which are particularly non-obvious to researchers.
Patient’s own assessment of the results (the quality of life/satisfaction) is a subjective method, and bias cannot be ruled out. The intervals between subsequent follow-up visits are more variable than in controlled clinical trials, where fixed scheduling of visits is ensured. Our study was not comparative. The comparisons were made only with the previous clinical or observational studies, that can be associated with systemic errors and distortion.
Discussion
For the first time in Russia, a multicenter observational prospective study was conducted, the main purpose of which was to assess satisfaction of young women aged 18–35 years with prescribed E2V/DNG combined oral contraceptive over the 6-month follow-up in routine medical practice. Although Q-SWAN study was not comparative, the results of the study fully correlate with previously data obtained in multiple clinical and observational studies including RCTs and real clinical practice, where the issues regarding satisfaction of women and their adherence to E2V/DNG combined oral contraceptive [11, 18, 19, 22–24] in comparison to other COCs were studied. The results of the study demonstrated high level of satisfaction with the use of E2V/DNG combination among the patients and doctors. The researchers believed that since the analysis could include the patients with HMB, which was not diagnosed at baseline, they might demonstrate higher satisfaction with E2V/DNG compared to women without this disorder. For this reason, two subgroups of women with and without HMB were analyzed additionally. For the first time it was found that regardless of whether the women had or not had HMB at baseline, similar high level of satisfaction with the use of E2V/DNG was among the doctors and patients. Apparently, other factors can have a significant impact on this indicator.
The results of the large-scale multicenter observational study CONTENT (n=3,150), which was also conducted in real clinical practice, are of great interest. The purpose of the study was to investigate the experience of women who willingly changed taking COCs containing ethinyl estradiol (EE) due to various reasons for E2V/DNG (n=2.558) or the progestogen-only pill (mini pill) (n=592) [22]. The following indicators were evaluated over the course of one year: satisfaction with medication, continuation of use and acceptability of bleeding profile, that correlated with the objectives of this study. The researchers convincingly demonstrated not only greater adherence of women regardless of their age to COC containing E2V/DNG, but also a higher degree of satisfaction compared to mini pill already by the 3rd – 5th month of follow-up. At the time of the last visit, about three fourths of women reported improvement in physical well-being (75.7%) and emotional state (71.8%) after switching to E2V/DNG. The authors of CONTENT believe that the reasons for better adherence and satisfaction with contraceptive pill containing E2V/DNG are more positive changes in bleeding profile compared to mini-pill: reduction in bleeding intensity, duration of bleeding and the severity of dysmenorrhea. By the end of the study, against the background of taking E2V/DNG, 54% of women reported withdrawal bleeding as “insignificant/scanty”; in 48.7% of women duration of discharge was only 1–2 days. The same changes were observed in Q-SWAN study. By the end of the study, the majority of women had painless, short (1-5 days) and light (one pad a day was enough) menstrual-like bleeding, that undoubtedly contributes to high level of satisfaction with E2V/DNG and, as it turned out, was especially appreciated by women, regardless of the initial intensity of menstruation. E2V/DNG combination envisages a gradual reduction in the dose of estrogen component along with increased dose of progestin, which has pronounced antiproliferative properties with subsequent short HFI. This dosing regimen (26/2) of hormonal components ensures stability of endometrium and can normalize the menstrual cycle, as well as reduce duration and volume of menstrual blood loss [11, 23].
It is important to note that by the end of the study, no menstrual disorders were registered in the subgroup of participants with prolonged or heavy menstrual bleeding, that is particular important for young women in the context of future pregnancy. Heavy periods are associated with iron deficiency and anemia, and cause enormous harm during pregnancy, both for the mother and her future child [25, 26]. Unfortunately, there is a growing number of women with anemia in early pregnancy in Russia. The effectiveness of E2V/DNG with regard to reduction of blood loss and positive influence on improvement of hemoglobin and ferritin levels in women with HMB was proved in several RCTs. Currently, E2V/DNG combination is the only COC that is officially indicated for medical use [12, 19]. It is noteworthy that the patients who needed only contraception without therapeutic purposes were enrolled in the study. Despite this fact, almost half of the population had HMB, which supposedly was not identified during collection of anamnesis even before the doctor’s decision to enroll the patient in the study, and the presence of HMB was identified only during the visit for enrollment in the study, when the women answered the questionnaire about the characteristics of menstruation. This indicates the importance of the detailed survey of all women about the severity of menstrual blood loss during counseling, as well as regarding the choice of the method of contraception.
The issue of menstrual cycle control using COCs with estrogen, which is identical to natural 17β-estradiol is being discussed quite often. The results of menstrual cycle control were positive: previously, 4.3% of women reported intermenstrual discharge. By the 3rd month of follow-up, the percentage of these women increased to 9.7%, that was expected and appropriate to the period of adaptation to hormonal medicine. By the end of the study, only 2.6% of women reported such changes. Previously, the RCT, where participated >800 women proved satisfactory bleeding profile. This trial compared various parameters of cycle control between the groups of women receiving E2B/DNG or COCs with 20 mcg ethinyl estradiol (EE)/levonorgestrel (LNG) [18]. The percentage of women with intermenstrual bleeding was low and comparable between the groups (14% and 12%, respectively). The results of pooled retrospective analysis of primary data obtained in 12 clinical trials, where this contraceptive was used by women in two age groups: ≤ 25 (n=1309) and >25 (n=2132) years, showed that by the 12th menstrual cycle, the percentage of women aged ≤25 years and aged >25 years with intermenstrual bleeding was similar (13.4% and 12.8%, respectively). Similar results were obtained in women aged ≤20 years (12.7%) [27]. In another RCT, only 2.5% of women refused taking E2V/DNG due to irregular bleeding [28]. In Q-SWAN study, only 2 (0.4%) of women discontinued taking E2V/DNG due to impaired bleeding profile.
Maintaining sexual function is directly related to satisfaction and adherence to contraception, although most women do not raise themselves this sensitive issue during their visit to the doctor. Q-SWAN study paid special attention to this issue, and despite the young age of the participants, almost half of women (46.0%) previously had low total FSFI scoring ≤26.55 points, that indicated sexual dysfunction. The obtained results demonstrated improvement of sexual function with taking E2V/DNG during the whole follow-up period. By the end of the study, most women (89.7%) had the scores of FSFI questionnaire within normal range. This correlates with the results obtained in the study by Davis S.R. et al., where the women, who reported low sexual desire against the background of taking COC (n=213), were randomized for receiving E2V/DNG (n=106) or EE/LNG (n=107) with purpose to compare possible effects of antiandrogenic and androgenic progestins on sexual function [29]. After changing to either a nonandrogenic or androgenic progestin-containing oral contraceptive preparation, the authors reported improvements of the scores in all domains of the FSFI, and the average increase in the scores for the domains, which most commonly were associated with worsening of sexual function (desire and arousal) – 5.90 points when taking E2V/DNG and 5.79 points when taking EE/LNG, compared with baseline scores (P<0.0001 for both medicinal preparations. In real clinical practice, Caruso S. et al. used Short Personal Experience Questionnaire (SPEQ) and noted that cyclicity of sexual behavior for such indicators as desire, arousal, orgasm, pleasure was preserved against the background of taking E2V/DNG, that is, sexual activity was the same as in the natural cycle [30]. The authors associate the results with dynamic dosing regimen of sex steroids in E2V/DNG formulation, their lesser effect on the synthesis of sex hormone-binding globulin, and short HFI.
Improvement of sexual function can be associated with the beneficial effect of E2V, which dissociates into natural metabolites – estradiol and estriol, at the local level improves differentiation of the multi-layered squamous epithelium, increases blood flow and innervation of vaginal mucosa, increases lactobacillus population, decreases vaginal pH level, etc. [31, 32]. As it was expected, in the majority of young participants in our study the parameters of vulvovaginal health measured by the Atrophy Symptom Questionnaire (ASQ) were normal. Only in few women, moderate or severe vulvovaginal symptoms were identified at baseline, which improved by the end of follow-up, and could contribute to improvement of sexual function. The results of Q-SWAN study showed that the patient may not be aware of having sexual dysfunction, and only specially developed methods can detect deviations from norm. Therefore, the doctor should bring up this sensitive issue, particularly during counseling of women on choosing the method of contraception [33, 34].
Based on the results of the study, it was not surprising that the majority of women expressed their intention to continue taking the medicine after it was finished regardless of baseline menstrual bleeding profile.
Conclusion
In recent years, researchers focus on assessment of evidence of the effectiveness and safety of medicinal preparations obtained first in RCTs, and then on confirmation of the findings in well-designed clinical research studies, such as Q-SWAN study, which the doctors face in their work. The results obtained in real clinical practice can be not only an important source of additional information about the characteristics of the medical preparation, but also can help the doctor to communicate with the patient and convincingly substantiate the choice of the most appropriate method of contraception for the woman. The right choice largely depends on adequate counseling of women, since tolerability and duration of using contraceptives, including COCs, directly depend on the recommendations given by health care professional.
The results of prospective, multicenter, observational Q-SWAN study convincingly demonstrated high tolerability and satisfaction with using E2V/DNG combined oral contraceptive by women aged 18–35 years over the 6-month follow-up in real clinical practice, including due to positive effect on menstrual bleeding profile and quality of sexual function. High degree of satisfaction among young women with Qlaira contraceptive pill suggests its long-term use for contraceptive purposes, starting from young age and up to menopause with appropriate follow-up.
References
- Дикке Г.Б. Потребности, ожидания и сомнения у пользователей гормональными контрацептивами. Гинекология. 2020; 22(1): 33-7. [Dikke G.B. Needs, expectations and doubt users of hormonal contraceptives. Gynecology. 2020; 22(1): 33-7. (in Russian)]. https://dx.doi.org/10.26442/20795696.2020.1.200044.
- Шереметьева Е.В., Андреева Е.Н. Индивидуальное консультирование молодых женщин по вопросам контрацепции в реальной клинической практике. Проблемы репродукции. 2022; 28(4): 89-96. [Sheremetyeva E.V., Andreeva E.N. Individualized contraceptive counseling for young women in clinical practice. Russian Journal of Human Reproduction. 2022; 28(4): 89-96. (in Russian)]. https://dx.doi.org/10.17116/repro20222804189.
- Nappi R.E., Vermuyten N., Bannemerschult R. Missed opportunities in contraceptive counselling: findings from a European survey-based study with simulated patient consultation. Eur. J. Contracept. Reprod. Health Care. 2022; 27(2): 85-94. https://dx.doi.org/10.1080/13625187.2021.2010040.
- Bitzer J., Marin V., Lira J. Contraceptive counselling and care: a personalized interactive approach. Eur. J. Contracept. Reprod. Health Care. 2017; 22(6): 418-23. https;//dx.doi.org/10.1080/13625187.2017.1414793.
- Cavallaro F.L., Benova L., Owolabi O.O., Ali M. A systematic review of the effectiveness of counselling strategies for modern contraceptive methods: what works and what doesn't? BMJ Sex. Reprod. Health. 2020; 46(4): 254-69. https://dx.doi.org/10.1136/bmjsrh-2019-200377.
- FSRH Clinical Effectiveness Unit. FSRH clinical guideline: combined hormonal contraception (January 2019, Amended October 2023). https://dx.doi.org/10.1136/bmjsrh-2018-CHC.
- Прилепская В.Н., Абакарова П.Р., Яроцкая Е.Л. Современная контрацепция и качество жизни женщины. Доктор Ру. 2017; 3: 37-42. [Prilepskaya V.N., Abakarova P.R., Yarotskaya Ye.L. Modern Contraception and Women’s Quality of Life. Doctor.Ru. 2017; (3): 37-42. (in Russian)].
- Дикке Г.Б. Отказ от ранее выбранного метода контрацепции и стратегии повышения приверженности. Проблемы репродукции. 2017; 23(5): 54-60. [Dikke G.B. Failure from the previously selected method of contraception and strategy for improving commitment. Russian Journal of Human Reproduction. 2017; 23(5): 54-60. (in Russian)]. https://dx.doi.org/10.17116/repro201723554-60.
- Le Guen M., Schantz C., Règnier-Loilier A., de La Rochebrochard E. Reasons for rejecting hormonal contraception in Western countries: A systematic review. Soc. Sci. Med. 2021; 284: 114247. https://dx.doi.org/10.1016/j.socscimed.2021.114247.
- Graziottin A. The shorter, the better: a review of the evidence for a shorter contraceptive hormone-free interval. Eur. J. Contracept. Reprod. Health Care. 2016; 21(2): 93-105. https://dx.doi.org/10.3109/13625187.2015.1077380.
- Андреева Е.Н., Шереметьева Е.В. Режимы контрацепции: повышение приверженности женщин современным комбинированным оральным контрацептивам с максимально коротким безгормональным интервалом. Гинекология. 2020; 22(2): 46-50. [Andreeva E.N., Sheremetyeva E.V. Contraception modes: increasing women’s commitment to modern combined oral contraceptives with the shortest possible hormone-free interval. Gynecology. 2020; 22(2): 46-50. (in Russian)]. https://dx.doi.org/10.26442/20795696.2020.2.200128.
- Актуальная версия инструкции препарата «Клайра» от 31.05.2023. Номер РУ: ЛП-№ (002448)-(РГ-RU). [The current version of the instructions of the drug Qlaira dated 05/31/2023. RU number: LP-No. (002448)-(RG-RU). (in Russian)].
- Nelson A., Parke S., Mellinger U., Zampaglione E., Schmidt A. Efficacy and safety of a combined oral contraceptive containing estradiol valerate/dienogest: results from a clinical study conducted in North America. J. Womens Health (Larchmt). 2014; 23(3): 204-10. https://dx.doi.org/10.1089/jwh.2013.4320.
- Yu Q., Huang Z., Ren M., Chang Q., Zhang Z., Parke S. Contraceptive efficacy and safety of estradiol valerate/dienogest in a healthy female population: a multicenter, open-label, uncontrolled Phase III study. Int. J. Womens Health. 2018; 10: 257-66. https://dx.doi.org/10.2147/IJWH.S157056.
- Petraglia F., Parke S., Serrani M., Mellinger U., Römer T. Estradiol valerate plus dienogest versus ethinylestradiol plus levonorgestrel for the treatment of primary dysmenorrhea. Int. J. Gynaecol. Obstet. 2014; 125(3): 270-4. https://dx.doi.org/10.1016/j.ijgo.2013.11.017.
- Macìas G., Merki-Feld G.S., Parke S., Mellinger U., Serrani M. Effects of a combined oral contraceptive containing oestradiol valerate/dienogest on hormone withdrawal-associated symptoms: results from the multicentre, randomised, double-blind, active-controlled HARMONY II study. J. Obstet. Gynaecol. 2013; 33(6): 591-6. https://dx.doi.org/10.3109/01443615.2013.800851.
- Dinger J., Möhner S., Heinemann K. Combined oral contraceptives containing dienogest and estradiol valerate may carry a lower risk of venous and arterial thromboembolism compared to conventional preparations: Results from the extended INAS-SCORE study. Front. Womens Health. 2020; 5: 1-8. https://dx.doi.org/10.15761/FWH.1000178.
- Ahrendt H.J., Makalová D., Parke S., Mellinger U., Mansour D. Bleeding pattern and cycle control with an estradiol-based oral contraceptive: a seven-cycle, randomized comparative trial of estradiol valerate/dienogest and ethinyl estradiol/levonorgestrel. Contraception. 2009; 80(5): 436-44. https://dx.doi.org/10.1016/j.contraception.2009.03.018.
- Fraser I.S., Parke S., Mellinger U., Machlitt A., Serrani M., Jensen J. Effective treatment of heavy and/or prolonged menstrual bleeding without organic cause: pooled analysis of two multinational, randomised, double-blind, placebo-controlled trials of oestradiol valerate and dienogest. Eur. J. Contracept. Reprod. Health Care. 2011; 16(4): 258-69. https://dx.doi.org/10.3109/13625187.2011.591456.
- Haverinen A.H., Luiro K.M., Szanto T., Kangasniemi M.H., Hiltunen L., Sainio S. et al. Combined oral contraceptives containing estradiol valerate vs ethinylestradiol on coagulation: A randomized clinical trial. Acta Obstet. Gynecol. Scand. 2022; 101(10): 1102-11. https://dx.doi.org/10.1111/aogs.14428.
- Meston C.M., Freihart B.K., Handy A.B., Kilimnik C.D., Rosen R.C. Scoring and interpretation of the FSFI: What can be learned from 20 years of use? J. Sex. Med. 2020; 17(1): 17-25. https://dx.doi.org/10.1016/j.jsxm.2019.10.007.
- Briggs P., Serrani M., Vogtländer K., Parke S. Continuation rates, bleeding profile acceptability, and satisfaction of women using an oral contraceptive pill containing estradiol valerate and dienogest versus a progestogen-only pill after switching from an ethinylestradiol-containing pill in a real-life setting: results of the CONTENT study. Int. J. Womens Health. 2016; 8: 477-87. https://dx.doi.org/10.2147/IJWH.S107586.
- Barnett C., Dinger J., Minh T.D., Heinemann K. Unintended pregnancy rates differ according to combined oral contraceptive – results from the INAS-SCORE study. Eur. J. Contracept. Reprod. Health Care. 2019; 24(4): 247-50. https://dx.doi.org/10.1080/13625187.2019.1629412.
- Прилепская В.Н., Довлетханова Э.Р. Гормональная контрацепция с применением комбинированного перорального контрацептива, содержащего эстрадиола валерат/диеногест. Медицинский оппонент. 2022; 2: 46-52. [Prilepskaya V.N., Dovletkhanova E.R. Hormonal сontraception using a combined oral contraceptive containing estradiol valerate/dienogest. Medical. Opponent. 2022; (2): 46-52. (in Russian)].
- Young M.F., Oaks B.M., Tandon S., Martorell R., Dewey K.G., Wendt A.S. Maternal hemoglobin concentrations across pregnancy and maternal and child health: a systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019; 1450(1): 47-68. https://dx.doi.org/10.1111/nyas.14093.
- Куценко И.И., Кравцова Е.И., Холина Л.А., Томина О.В. Терапия латентного железодефицитного состояния у беременных. Гинекология. 2022; 24(6): 512-7. [Kutsenko I.I., Kravtsova E.I., Kholina L.A., Tomina O.V. Latent iron deficiency therapy in pregnant women. Gynecology. 2022; 24(6): 512-7. (in Russian)]. https://dx.doi.org/10.26442/20795696.2022.6.202023.
- Jensen J.T., Bitzer J., Nappi R.E., Ahlers C., Bannemerschult R., Parke S. Pooled analysis of bleeding profile, efficacy and safety of oral oestradiol valerate/dienogest in women aged 25 and under. Eur. J. Contracept. Reprod. Health Care. 2020; 25(2): 98-105. https://dx.doi.org/10.1080/13625187.2020.1731734.
- Palacios S., Wildt L., Parke S., Machlitt A., Römer T., Bitzer J. Efficacy and safety of a novel oral contraceptive based on oestradiol (oestradiol valerate/dienogest): a Phase III trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010; 149(1): 57-62. https://dx.doi.org/10.1016/j.ejogrb.2009.11.001.
- Davis S.R., Bitzer J., Giraldi A., Palacios S., Parke S., Serrani M. et al. Change to either a nonandrogenic or androgenic progestin-containing oral contraceptive preparation is associated with improved sexual function in women with oral contraceptive-associated sexual dysfunction. J. Sex. Med. 2013; 10(12): 3069-79. https://dx.doi.org/10.1111/jsm.12310.
- Caruso S., Agnello C., Romano M., Cianci S., Lo Presti L., Malandrino C., Cianci A. Preliminary study on the effect of four-phasic estradiol valerate and dienogest (E2V/DNG) oral contraceptive on the quality of sexual life. J. Sex. Med. 2011; 8(10): 2841-50. https://dx.doi.org/10.1111/j.1743-6109.2011.02409.x.
- De Seta F., Restaino S., Banco R., Conversano E., De Leo R., Tonon M. et al. Effects of estroprogestins containing natural estrogen on vaginal flora. Gynecol. Endocrinol. 2014; 30(11): 830-5. https://dx.doi.org/10.3109/09513590.2014.936847.
- Di Carlo C., Gargano V., De Rosa N., Tommaselli G.A., Sparice S., Nappi C. Effects of estradiol valerate and dienogest on quality of life and sexual function according to age. Gynecol. Endocrinol. 2014; 30(12): 925-8. https://dx.doi.org/10.3109/09513590.2014.975688.
- Юренева С.В., Ильина Л.М. Комбинированные оральные контрацептивы с натуральным эстрогеном и сексуальная функция: оптимальный метод контрацепции для женщин различного возраста. Гинекология. 2019; 21(1): 33-7. [Yureneva S.V., Ilina L.M. Combined oral contraceptives with natural estrogen and sexual function: the optimal method of contraception for women of different ages. Gynecology. 2019; 21(1): 33-7. (in Russian)]. https://dx.doi.org/10.26442/20795696.2019.1.190184.
- de Castro Coelho F., Barros C. The potential of hormonal contraception to influence female sexuality. Int. J. Reprod. Med. 2019; 2019: 9701384. https://dx.doi.org/10.1155/2019/9701384.
Received 30.01.2024
Accepted 07.02.2024
About the Authors
Vera N. Prilepskaya, Dr. Med. Sci., Professor, Honored Scientist of the Russian Federation, Head of the Scientific Polyclinic Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4 Akademika Oparina str., Moscow, 117997, Russia,vprilepskaya@mail.ru, https://orcid.org/0000-0003-3993-7629
Elena N. Andreeva, Dr. Med. Sci., Professor, Deputy Director – Director of the Institute of Reproductive Medicine, National Medical Research Center for Endocrinology, Ministry of Health of Russia; Professor at the Department of Obstetrics, Gynecology and Reproductive Medicine, Russian University of Medicine, Ministry of Health of Russia, 117036, Russia, Moscow, Dmitry Ulyanov str., 11, gynec@endocrincentr.ru



