В настоящее время отечественная фармацевтическая промышленность находится в прямой зависимости от зарубежных поставок, поскольку около 40% всех лекарственных препаратов, реализуемых на рынке Российской Федерации, ввозятся из-за границы [1], а значительная часть лекарственных средств производится из импортного сырья.
В условиях текущего санкционного давления высокую степень зависимости от импорта зарубежных лекарств можно с уверенностью назвать одним из серьезных рисков для национальной безопасности нашей страны. В этой связи лекарственное импортозамещение становится одной из приоритетных задач государственной политики в сфере здравоохранения [2, 3].
Замещение импортных лекарственных средств осуществляется путем создания отечественных инновационных (оригинальных) препаратов, а также путем производства воспроизведенных лекарственных препаратов – аналогов оригинальных лекарств зарубежного производства. Выпуск на рынок воспроизведенных лекарственных средств происходит после завершения действия патентной защиты оригинальных препаратов. Доля воспроизведенных препаратов на отечественном рынке неуклонно растет [2].
Воспроизведенный лекарственный препарат – лекарственный препарат для медицинского применения, который имеет эквивалентный референтному лекарственному препарату качественный и количественный состав действующих веществ в эквивалентной лекарственной форме, биоэквивалентность или терапевтическая эквивалентность которых соответствующему референтному лекарственному препарату подтверждена исследованиями [4].
Одним из основных критериев определения лекарственного препарата в качестве воспроизведенного является подтверждение его биоэквивалентности оригинальному (референтному) препарату. Биоэквивалентность лекарственных препаратов предполагает их терапевтическую эквивалентность. Исследование биоэквивалентности является основным видом медико-биологического контроля качества воспроизведенных препаратов и регулируется нормативными документами [5].
В настоящее время в Российской Федерации в условиях необходимости импортозамещения зарубежных оригинальных контрацептивных препаратов российской фармацевтической компанией ООО «Фармасинтез-Тюмень» на основании результатов исследований биоэквивалентности зарегистрированы новые воспроизведенные контрацептивные препараты линейки ПланиЖенс: «ПланиЖенс лево» (левоноргестрел+этинилэстрадиол), таблетки, покрытые оболочкой, 0,15 мг+0,03 мг; «ПланиЖенс дезо 20» (дезогестрел+этинилэстрадиол), таблетки 0,15 мг+0,02 мг; «ПланиЖенс дезо 30» (дезогестрел+этинилэстрадиол), таблетки 0,15 мг+0,03 мг; «ПланиЖенс номе» (номегэстрол+эстрадиол), таблетки, покрытые пленочной оболочкой, 2,5 мг+1,5 мг. Данные препараты являются воспроизведенными по отношению к соответствующим оригинальным (референтным) препаратам: «Микрогинон», таблетки, покрытые оболочкой, 0,15 мг+0,03 мг («Байер Фарма АГ», Германия); «Мерсилон», таблетки 0,15 мг+0,02 мг («Н.В. Органон», Нидерланды); «Марвелон», таблетки 0,15 мг+0,03 мг («Н.В. Органон», Нидерланды); «Зоэли», таблетки, покрытые пленочной оболочкой, 2,5 мг+1,5 мг («Н.В. Органон, Нидерланды).
Более 60 лет назад был зарегистрирован первый эстроген-гестагенный препарат для предохранения от нежеланной беременности. С момента создания первого комбинированного контрацептива наблюдаются значительные изменения компонентов, содержащихся в таблетках [6]. Что касается эстрогенной части комбинированных оральных контрацептивов (КОК), достигнуто снижение дозы эстрогенов; в настоящее время большинство препаратов содержат 20–30 мкг этинилэстрадиола. В течение последних 15 лет появились и новые эстрогены, например, эстрадиол, эстрадиола валерат и эстетрол [7]. Составляющим КОК также является прогестагенный компонент. Прогестагены можно разделить по химическому составу: производные прогестерона – хлормадинона ацетат, ципротерона ацетат, номегэстрола ацетат; производные тестостерона (19-нортестостерона) – левоноргестрел, этоногестрел, дезогестрел, норгестимат и гестоден, а также производные спиронолактона – дроспиренон [8–10]. Каждый из прогестагенов оказывает влияние на организм, которое реализуется через их возможность взаимодействовать с рецепторами, приводя как к положительным системным воздействиям (антиандрогенное, антиальдостероновое и т.д.), так и к нежелательным побочным эффектам (прибавка массы тела, появление акне, отечности и т.д.) [8–10].
Препарат «ПланиЖенс лево» представляет собой комбинацию гестагена (левоноргестрела) и эстрогена (этинилэстрадиола). Левоноргестрел – один из наиболее изученных прогестагенов с хорошим профилем безопасности риска тромбозов, имеет антиэстрогенную и относительную андрогенную активность [10–12]. Высокая эффективность комбинации левоноргестрела и этинилэстрадиола доказана в ряде клинических исследований [13, 14].
В состав препаратов «ПланиЖенс дезо 20» и «ПланиЖенс дезо 30» входят гестаген (дезогестрел) и эстроген (этинилэстрадиол). Дезогестрел является высокоселективным прогестагеном с низкой андрогенной активностью. Описаны позитивные эффекты дезогестрела на ткань молочной железы. Он снижает активность эстрогеновых рецепторов альфа, не влияя при этом на активность ферментов синтеза эстрогенов в молочной железе [15, 16]. Применение КОК без андрогенной активности может снижать риск развития фиброзно-кистозной болезни молочных желез, сопровождающейся масталгией [17].
Препарат «ПланиЖенс номе» содержит гестаген (номегэстрол) и эстроген (эстрадиол). Номегэстрол – высокоселективный прогестаген, близкий по природе к естественному прогестерону. За счет длительного периода полувыведения ПланиЖенс номе обеспечивает контроль менструального цикла (отсутствие межменструальных кровянистых выделений) на фоне влияния натурального эстрогена [12, 18, 19]. Описано несколько механизмов, вследствие которых КОК эффективны при акне: уменьшение синтеза андрогенов яичниками (подавление продукции лютеинизирующего гормона гипофизом), снижение периферического синтеза андрогенов (ингибирование 5-альфа-редуктазы в волосяных фолликулах и коже), снижение уровня свободного тестостерона за счет повышения глобулина, связывающего половые гормоны и др. [15, 20]. Антиандрогенный эффект номегэстрола позволяет повысить приверженность к КОК у пациенток с андрогенозависимыми симптомами: акне, себореей, гирсутизмом и андрогенной алопецией [15, 21].
Материалы и методы
Для подтверждения биоэквивалентности новых воспроизведенных контрацептивных препаратов линейки ПланиЖенс соответствующим оригинальным препаратам проведены исследования биоэквивалентности у здоровых добровольцев женского пола.
Исследования биоэквивалентности контрацептивных препаратов линейки ПланиЖенс были проведены в Российской Федерации в соответствии с принципами, изложенными в Хельсинкской декларации Всемирной медицинской ассоциации «Рекомендации для врачей, занимающихся биомедицинскими исследованиями с участием людей» (Бразилия, Форталеза, 2013 г.); принципами Надлежащей клинической практики [22, 23]; международными правилами проведения клинических исследований (ICH GCP); требованиями к проведению исследований биоэквивалентности российского законодательства и ЕАЭС [24–26], а также в соответствии с утвержденными в установленном порядке протоколами исследований биоэквивалентности.
В исследования биоэквивалентности включались здоровые женщины в возрасте 18–45 лет, добровольно изъявившие желание участвовать в исследовании, прошедшие физикальное и лабораторно-инструментальное обследования, подписавшие письменное информированное согласие и соответствующие всем критериям включения/невключения в исследование. В исследование биоэквивалентности препарата левоноргестрел+этинилэстрадиол (ПланиЖенс лево) были рандомизированы 36 здоровых добровольцев (завершили исследование 35 добровольцев); в исследования биоэквивалентности препаратов дезогестрел+этинилэстрадиол были рандомизированы– 34 здоровых добровольца (завершили исследование 33 добровольца) (ПланиЖенс дезо 20) и 36 здоровых добровольцев (завершили исследование 33 добровольца) (ПланиЖенс дезо 30); в исследование биоэквивалентности препарата номегэстрола+эстрадиол (ПланиЖенс номе) – 38 здоровых добровольцев (завершили исследование 36 добровольцев).
Цель проведенных исследований биоэквивалентности: изучение сравнительной фармакокинетики и оценка биоэквивалентности воспроизведенных контрацептивных препаратов линейки ПланиЖенс у здоровых добровольцев женского пола при однократном приеме их натощак.
Дизайн исследований
Исследование биоэквивалентности было открытым сравнительным рандомизированным перекрестным с двумя периодами и двумя последовательностями исследованием с однократным приемом натощак каждого из препаратов исследования здоровыми добровольцами.
Исследования биоэквивалентности были открытыми в отношении приема исследуемых препаратов, но были заслеплены для биоаналитической лаборатории при работе с биообразцами.
Обоснование выбора препаратов сравнения
Выбор препаратов сравнения (R) для исследуемых препаратов (Т) обоснован следующим: препараты сравнения «Микрогинон», «Мерсилон», «Марвелон», «Зоэли» являются оригинальными препаратами по отношению к исследуемым препаратам (T): «ПланиЖенс лево», «ПланиЖенс дезо 20», «ПланиЖенс дезо 30», «ПланиЖенс номе» соответственно; исследуемые препараты (T) и препараты сравнения (R) имеют одинаковую лекарственную форму; содержат одинаковую дозу действующих веществ; вспомогательные вещества, входящие в состав сравниваемых препаратов, хорошо изучены и не оказывают влияния на фармакокинетику данных препаратов.
График исследований биоэквивалентности
Клиническая фаза исследований состояла из периода скрининга, двух периодов исследования, когда осуществлялся прием исследуемых препаратов (по 1 таблетке однократно), отбор образцов крови и «отмывочного» периода между периодами исследования.
Длительность периода скрининга в исследованиях биоэквивалентности препарата «ПланиЖенс лево» составила 7 дней; в исследованиях биоэквивалентности препаратов «ПланиЖенс дезо 20» и «ПланиЖенс дезо 30» – до 14 дней; в исследовании биоэквивалентности препарата «ПланиЖенс номе» – от 4 до 9 дней.
Длительность каждого периода исследования составляла около 3,5 суток (приблизительно 84 ч), в которых осуществлялись прием исследуемых препаратов (по 1 таблетке натощак однократно) и отбор образцов крови. Длительность «отмывочного» периода во всех исследованиях составила 28 суток от момента первого приема одного из исследуемых препаратов.
Каждый доброволец был госпитализирован в клинический центр для проведения первого периода исследования не позднее чем за 12 ч до момента приема одного из исследуемых препаратов. В первый день первого периода исследования добровольцы были рандомизированы в соотношении 1:1 с присвоением номера, который определял последовательность приема исследуемого и референтного препаратов (TR или RT).
Госпитализация на первом периоде исследования длилась около 36 ч, после чего каждый доброволец был отпущен домой. Амбулаторные визиты осуществлялись через 48 и 72 ч после приема препарата. После проведения визита через 72 ч доброволец был отпущен домой до контрольного визита до начала второго периода исследования.
За 1–3 дня до начала второго этапа исследования добровольцы прибывали на амбулаторный визит в клинический центр для сдачи промежуточных анализов крови и мочи, осмотра гинеколога (для оценки критериев включения/невключения). В случае получения нормальных результатов анализов, при наличии критериев включения и отсутствии критериев невключения, добровольцы приглашались для госпитализации на второй этап. Процедуры второго периода исследования были идентичны первому. После проведения всех процедур на втором этапе исследования каждому добровольцу был проведен завершающий осмотр, после которого исследование для добровольцев считалось завершенным.
Общая продолжительность исследований биоэквивалентности для добровольца в исследованиях составила: ПланиЖенс лево – 39 дней; ПланиЖенс дезо 20 и ПланиЖенс дезо 30 – до 46 дней; ПланиЖенс номе – 36–41 день.
Фармакокинетические параметры
Для каждого добровольца рассчитывались следующие фармакокинетические параметры, необходимые для оценки биоэквивалентности сравниваемых лекарственных препаратов: AUC0–72 – площадь под кривой «плазменная концентрация-время» с момента приема препарата до 72 ч; AUC0–∞ – площадь под кривой «плазменная концентрация-время» с момента приема препарата до бесконечности; Cmax – максимальная плазменная концентрация действующего вещества; Tmax – время достижения Cmax; T½ – период полувыведения из плазмы; f’ – относительная биодоступность исследуемого препарата по отношению к препарату сравнения AUC0–72(T)/ AUC0–72(R); f’’ – относительная степень всасывания, определяемая отношением Cmax(T)/Cmax(R); Cmax/AUC – относительная скорость всасывания; kel – константа скорости терминальной элиминации; MRT – среднее время удерживания препарата в организме.
Биоаналитический метод
Для определения левоноргестрела, номегэстрола, эстрадиола и этинилэстрадиола в плазме крови здоровых добровольцев был использован биоаналитический метод высокоэффективной жидкостной хроматографии с тандемной масс-спектрометрией.
Определение активного метаболита дезогестрела – этоногестрела и этинилэстрадиола в биологических образцах проводили методом высокоэффективной жидкостной хроматографии с масс-селективным детектором в режиме MRM (positive).
Статистический анализ
Рассчитывались следующие статистические параметры: среднее арифметическое значение (Mean), среднее геометрическое значение (Geom), стандартное отклонение среднего результата (SD), коэффициент вариации (CV), медиана (Median), максимальные (Max) и минимальные (Min) значения. Различия первичных фармакокинетических параметров оценивались с помощью дисперсионного анализа (ANOVA; параметрический метод).
Расчет фармакокинетических параметров и статистический анализ полученных данных выполнены в предположении о логнормальном распределении параметров AUC и Cmax.
Критерии оценки биоэквивалентности
Вывод о биоэквивалентности сравниваемых препаратов был сделан на основе оценки 90% доверительных интервалов для отношений средних геометрических значений параметров AUC0–72 и Сmax исследуемых и референтных препаратов. После проведения логарифмического преобразования эти показатели анализировались с помощью дисперсионного анализа.
Препараты считались биоэквивалентными, если границы оцененного 90% доверительного интервала для отношений средних геометрических значений параметров AUC0–72 и Cmax исследуемых и референтных препаратов находились в пределах 80,00–125,00%.
Биоэквивалентность исследуемых комбинированных препаратов считалась доказанной при подтверждении биоэквивалентности по обоим компонентам.
Оценка безопасности
Оценка безопасности проводилась на основании оценки нежелательных явлений (НЯ), результатов физикального обследования и измерения жизненно важных показателей, данных электрокардиографии и результатов лабораторных анализов.
Результаты и обсуждение
Исследования биоэквивалентности были проведены путем определения концентраций левоноргестрела, этоногестрела (активного метаболита дезогестрела), номегэстрола, эстрадиола и этинилэстрадиола в плазме крови здоровых добровольцев женского пола.
После однократного перорального приема препаратов левоноргестрела+этинилэстрадиол в дозе 0,15 мг+0,03 мг установлены близкие значения максимальной концентрации и относительной биодоступности левоноргестрела и этинилэстрадиола. Доверительные интервалы для отношений средних геометрических значений AUC0–72 и Cmax левоноргестрела и этинилэстрадиола исследуемого и референтного препаратов соответствуют допустимым пределам 80,00–125,00% (табл. 1).

Индивидуальные и усредненные профили фармакокинетических кривых левоноргестрела и этинилэстрадиола исследуемого препарата «ПланиЖенс лево» и препарата сравнения «Микрогинон» совпадают (рис. 1, 2).
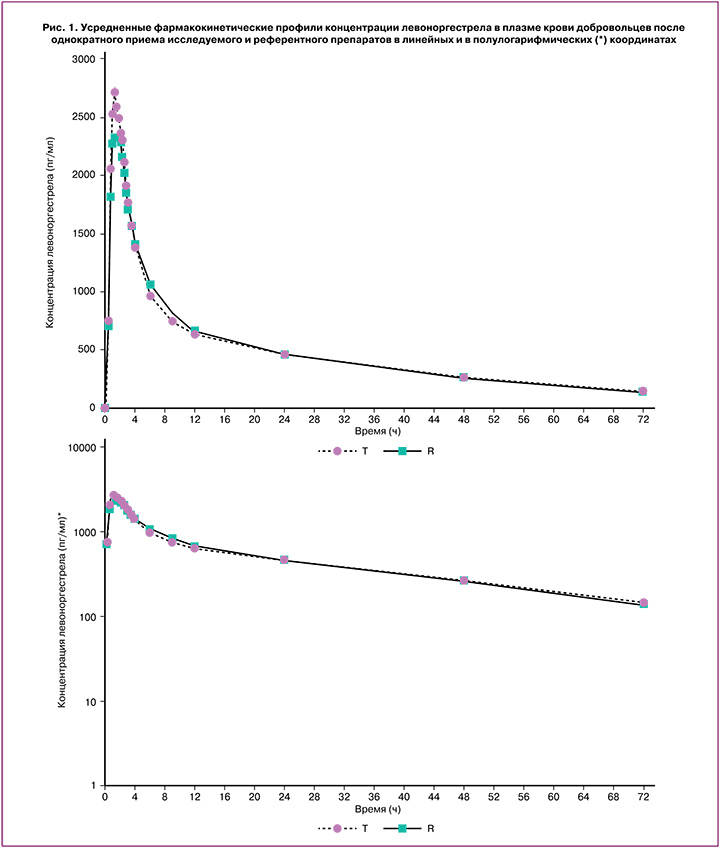

На основании полученных результатов исследования можно сделать вывод, что препараты «ПланиЖенс лево» (левоноргестрел+этинилэстрадиол), таблетки, покрытые оболочкой, 0,15 мг+0,03 мг (ООО «Фармасинтез-Тюмень», Россия) и «Микрогинон», таблетки, покрытые оболочкой, 0,15 мг+0,03 мг («Байер Фарма АГ», Германия) являются биоэквивалентными.
После однократного перорального приема препаратов дезогестрела+этинилэстрадиол в дозах 0,15 мг+0,02 мг и 0,15 мг+0,03 мг установлены близкие значения максимальной концентрации и относительной биодоступности этоногестрела (активного метаболита дезогестрела) и этинилэстрадиола. Доверительные интервалы для отношений средних геометрических значений AUC0–72 и Cmax этоногестрела и этинилэстрадиола исследуемого и референтного препаратов соответствуют допустимым пределам 80,00–125,00% (табл. 2, 3).
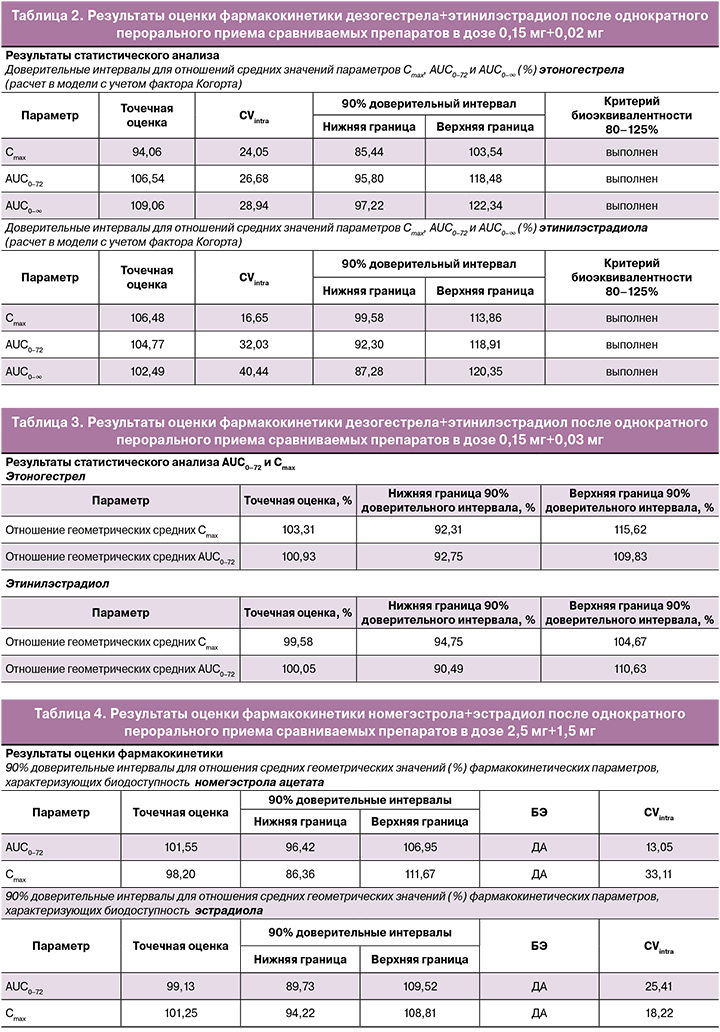
Индивидуальные и усредненные профили фармакокинетических кривых этоногестрела (активного метаболита дезогестрела) и этинилэстрадиола исследуемого препарата «ПланиЖенс дезо 20» и препарата сравнения «Мерсилон» совпадают (рис. 3).
На основании полученных результатов исследования можно сделать вывод, что препарат «ПланиЖенс дезо 20» (дезогестрел+этинилэстрадиол), таблетки 0,15 мг+0,02 мг (ООО «Фармасинтез-Тюмень», Россия), биоэквивалентен препарату «Мерсилон», таблетки 0,15 мг+0,02 мг, («Н.В. Органон», Нидерланды); препарат «ПланиЖенс дезо 30» (дезогестрел+этинилэстрадиол), таблетки 0,15 мг+0,03 мг (ООО «Фармасинтез-Тюмень», Россия), биоэквивалентен препарату «Марвелон», таблетки 0,15 мг+0,03 мг («Н.В. Органон», Нидерланды).
После однократного перорального приема препаратов номегэстрола+эстрадиол в дозе 2,5 мг+1,5 мг установлены близкие значения максимальной концентрации и относительной биодоступности номегэстрола и эстрадиола. Доверительные интервалы для отношений средних геометрических значений AUC0–72 и Cmax номегэстрола и эстрадиола исследуемого и референтного препаратов соответствуют допустимым пределам 80,00–125,00% (табл. 4).
Индивидуальные и усредненные профили фармакокинетических кривых номегэстрола и эстрадиола исследуемого препарата «ПланиЖенс номе» и препарата сравнения «Зоэли» совпадают (рис. 4, 5).
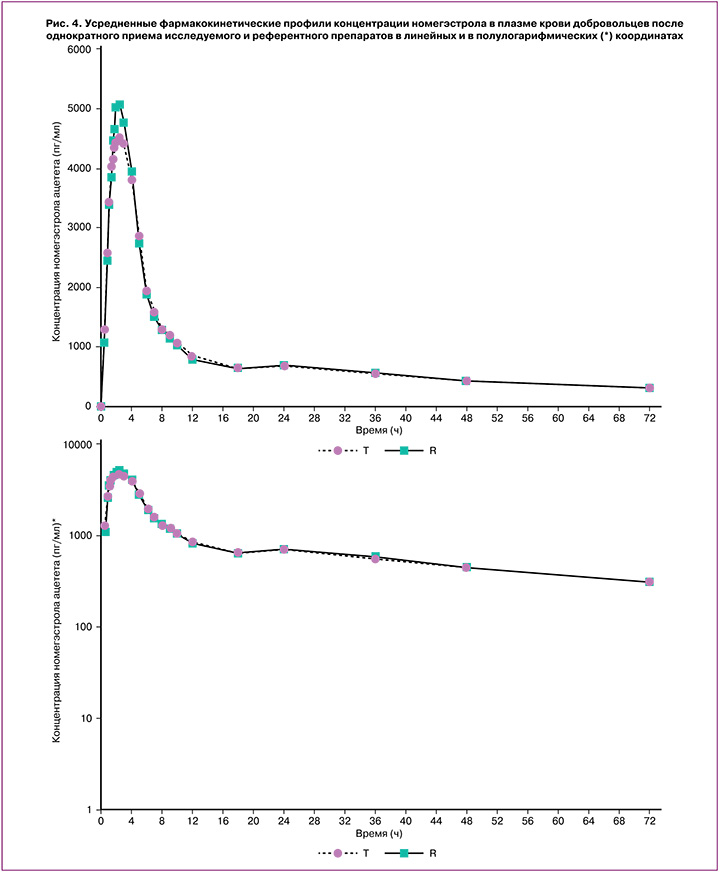
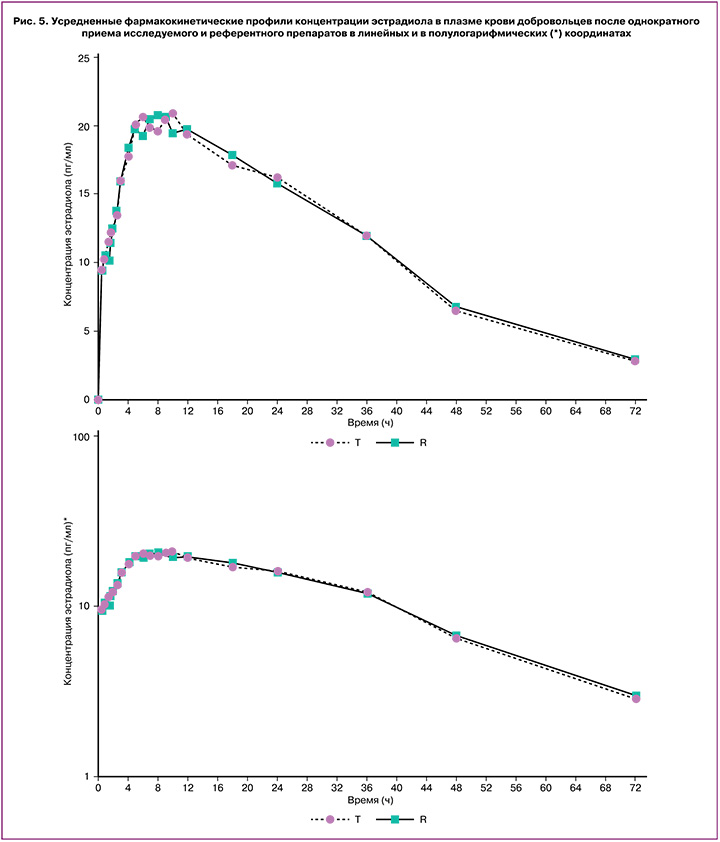
На основании полученных результатов исследования можно сделать вывод, что препараты «ПланиЖенс номе» (номегэстрол+эстрадиол), таблетки, покрытые пленочной оболочкой, 2,5 мг+1,5 мг (ООО «Фармасинтез-Тюмень», Россия) и «Зоэли», таблетки, покрытые пленочной оболочкой, 2,5 мг+1,5 мг («Н.В. Органон, Нидерланды), являются биоэквивалентными.
Оценка безопасности
В исследовании биоэквивалентности препаратов левоноргестрела+этинилэстрадиол после приема исследуемого препарата зарегистрировано 3 НЯ; в 2 случаях связь НЯ с приемом препарата отмечена как возможная, в 1 случае – связь сомнительная. После приема препарата сравнения «Микрогинон» зарегистрировано 1 НЯ. Во всех случаях тяжесть НЯ оценена как легкая. Исходом всех НЯ являлось разрешение без последствий для здоровья, дополнительных действий не предпринималось.
В исследовании биоэквивалентности препаратов дезогестрела+этинилэстрадиол после однократного применения их в дозе 0,15 мг+0,02 мг возникло 27 НЯ, из них 13 НЯ – после приема исследуемого препарата и 14 НЯ – после приема препарата сравнения «Мерсилон». Все НЯ были легкой степени тяжести, прошли самостоятельно, связь с приемом препарата расценена как сомнительная.
В исследовании биоэквивалентности препаратов дезогестрела+этинилэстрадиол после однократного применения их в дозе 0,15 мг+0,03 мг возникло 42 НЯ: 21 после приема исследуемого препарата и 21 после приема препарата «Марвелон». У 3 добровольцев НЯ (связь которых с препаратом расценена как сомнительная) стали причиной досрочного прекращения участия в исследовании. Все НЯ были легкой или средней степени тяжести. Частота и тяжесть НЯ после приема исследуемого и референтного препаратов были одинаковыми.
В исследовании биоэквивалентности препаратов номегэстрола+эстрадиол зарегистрировано 5 НЯ: после приема исследуемого препарата возникло 3 НЯ, после приема препарата «Зоэли» – 2 НЯ. Все НЯ имели легкую степень тяжести и возможную связь с препаратом. Исходом всех НЯ было выздоровление без последствий. Действий в отношении НЯ не предпринималось.
Во всех исследованиях биоэквивалентности серьезных НЯ, а также случаев возникновения беременности выявлено не было. Отклонений в лабораторных показателях крови и мочи добровольцев во время исследования не выявлено.
По результатам исследований биоэквивалентности можно сделать вывод о схожем профиле безопасности исследуемых препаратов и препаратов сравнения.
Заключение
Результаты проведенных исследований биоэквивалентности воспроизведенных контрацептивных препаратов линейки ПланиЖенс показали, что границы оцененных 90% доверительных интервалов для отношений средних геометрических значений фармакокинетических параметров AUC0–72 и Cmax левоноргестрела, этоногестрела (активного метаболита дезогестрела), номегэстрола, эстрадиола и этинилэстрадиола после однократного приема натощак исследуемых и референтных препаратов левоноргестрела+этинилэстрадиол, дезогестрела+этинилэстрадиол, номегэстрола+эстрадиол находятся в пределах 80,00–125,00%.
Полученные данные подтверждают, что воспроизведенные лекарственные препараты производства ООО «Фармасинтез-Тюмень», Россия: «ПланиЖенс лево», таблетки, покрытые оболочкой, 0,15 мг+0,03 мг; «ПланиЖенс дезо 20», таблетки 0,15 мг+0,02 мг; «ПланиЖенс дезо 30», таблетки 0,15 мг+0,03 мг; «ПланиЖенс номе», таблетки, покрытые пленочной оболочкой, 2,5 мг+1,5 мг биоэквивалентны соответствующим оригинальным препаратам: «Микрогинон», таблетки, покрытые оболочкой, 0,15 мг+0,03 мг («Байер Фарма АГ», Германия); «Мерсилон», таблетки 0,15 мг+0,02 мг («Н.В. Органон», Нидерланды); «Марвелон», таблетки 0,15 мг+0,03 мг («Н.В. Органон», Нидерланды); «Зоэли», таблетки, покрытые пленочной оболочкой, 2,5 мг+1,5 мг («Н.В. Органон, Нидерланды).
Профили безопасности всех исследуемых препаратов сопоставимы с профилями безопасности соответствующих оригинальных препаратов.



