A differential approach to the administration of combined oral contraceptives in polycystic ovary syndrome
Aim. To investigate the effect of combined oral contraceptives (COC) containing ethinyl estradiol/drospirenone (EE/DRSP) and estradiol valerate/dienogest (EV/DNG) on the endocrine and metabolic profiles of patients with polycystic ovary syndrome (PCOS). Materials and methods. One hundred patients with PCOS were randomized into two groups to receive either EV/DNG (group I, n=50, 24.1±2.8 years) or EE/DRSP (group II, n=50, 25.6±2.1 years). All patients underwent clinical, laboratory, and diagnostic investigations at baseline and after 12 months of treatment. Results. Both EE/DRSP and EV/DNG were effective in improving clinical and biochemical HA. Mean levels of TT decreased by 17% and 20%, FT - by 19% and 29% in group I and II, respectively (p> 0.05). There was a 17% and 28% reduction in the levels of A in group I and II, respectively (p> 0.05). Mean levels of SHBG showed a 2.2 and 5.7 fold increase in group I and II, respectively (p <0.05). In the absence of a negative effect on most metabolic parameters, EV/DNG reduced the rates of impaired glucose tolerance (IGT) and hyperinsulinemia (HI) by 12% and 14%, respectively; EE/DRSP occasionally caused HI and IGT. There was an increase in mean TG levels by 16% and 29% in patients of group I and II, respectively (p <0.05). Conclusion. The study findings suggest the feasibility of a differential approach to the administration of COCs. In patients with metabolic disorders and thrombotic risk (in the absence of high-risk thrombophilia), the treatment of choice may be EV/DNG. In PCOS patients with signs of hyperandrogenism but without significant metabolic disorders, EE/DRSP should be preferred.Chernukha G.E., Tabeeva G.I., Dumanovskaya M.R.,. Marchenko L.A.
Keywords
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in reproductive age women showing clinical and biochemical hyperandrogenism (HA), chronic anovulation, irregular menstrual cycle, infertility, polycystic ovary transformation, and increased ovarian volume [1,2]. Currently, PCOS is considered as a reproductive metabolic syndrome with the rates of dyslipidemia, hyperinsulinemia (HI), and impaired glucose tolerance (IGT) reaching 60-70%, 50-60%, and 20-30%, respectively [3]. This highlights the need to define the balance between benefits and harms of hormonal therapy [4]. According to international and numerous national guidelines, first-line treatment of women with PCOS who are not planning to become pregnant is combined oral contraceptives (COCs) [5,6]. Many researchers agree that the choice of an estrogen-progestin agent to regulate the menstrual cycle and treat symptomatic HA does not depend on the type of contraceptive. This point of view may be attributed to the lack of multicenter comparative studies investigating COCs, including naturally occurring estrogens. The therapeutic effect of COCs mediated not only by suppressing gonadotropin-releasing hormone, inhibiting luteinizing hormone (LH) and androgen synthesis in the ovaries and adrenal glands but also by increasing liver production of sex hormone-binding globulin (SHBG) resulting in reduced testosterone bioavailability (T) [7, 8]. Anti-androgenic effect of COCs is largely determined by their progestogenic component. Several progestogens, such as cyproterone acetate (CPA) and drospirenone (DRSP) also can inhibit the activity of 5-α-reductase and competitively bind to androgen receptors, which increases the anti-androgenic effect. Previous studies showed that the CPA shows strongest antiandrogen activities. [9]. However, the 2018 international guidelines do not recommend CPA-containing COCs as first-line treatment of PCOS due to adverse effects, including adverse metabolic outcomes and venous thromboembolic risks [6]. The need for long-term correction of clinical and biochemical HA in PCOS [10] indicates the importance of choosing the most effective and safe COC, taking into account the estrogenic and gestagenic components included in their composition.
COCs are not always “metabolically neutral”, some of them may adversely affect lipid and carbohydrate metabolism [11]. According to available evidence, DRSP-containing COCs produce a more favorable effect on the metabolic status of PCOS patients, compared with CPA - containing agents [12]. However, the literature has reported cases of their negative impact on carbohydrate metabolism associated with an increased incidence of HI [4]. It is also noteworthy that PCOS increases the risk of thrombotic complications associated with both metabolic disorders and the presence of hereditary thrombophilia, in particular with the polymorphism in the plasminogen activator inhibitor (PAI) gene. Among patients with PCOS, the incidence of this genetic defect that impairs fibrinolysis reaches 90% [13-15]. Long-term use of COCs contributes to endogenous hypofibrinolysis, inhibits natural blood anticoagulants, and increases resistance to activated protein C (APC) [16-20]. The extent of COC - associated changes in hemostasis depends directly on the estrogenic and progestin components included in their composition. A considerable lack of research addressing the effect of COC containing synthetic estrogens in combination with DRSP and naturally occurring estrogen in combination with dienogest (DNG) on the androgenic and metabolic profile of patients with PCOS motivated us to conduct this study. This study aimed to investigate the effect of combined oral contraceptives (COC) containing ethinyl estradiol/drospirenone (EE/DRSP) and estradiol valerate/dienogest (EV/DNG) on the endocrine-metabolic parameters of patients with PCOS.
Materials and methods
The study comprised 100 women with PCOS. The inclusion criteria for the study patient selection were as follows: age 18-35 years, the presence of PCOS diagnosed according to the Rotterdam criteria [5], lack of interest in pregnancy at the time of treatment, and no hormone therapy in the last three months before enrollment in the study. Exclusion criteria: concomitant endocrine, severe non-gynecologic comorbidities and contraindications for COCs [21].
All patients underwent clinical, laboratory and diagnostic investigations at baseline and after 12 months of treatment. The severity of hirsutism was assessed by the Ferriman-Gallwey scale; a total score of more than eight was considered as hirsutism [22]. The body mass index (BMI) was calculated using the formula BMI = weight (kg)/height2 (m2). The hormonal profile was assessed on the 2nd or 3rd day of a natural or progesterone-induced menstrual cycle. Serum levels of anti-Muller hormone (AMH), LH, follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), prolactin, total testosterone (TT), free testosterone (FT), androstenedone (A), dihydrotestosterone (DHT), SHBG, and 17 oxyprogesterone was determined by a fluorescence immunoassay with an Immulite 2000 automatic analyzer (Siemens, USA). The free androgen index (FAI) was calculated by the formula: FAI = TT (nmol/l) / SHBG (nmol/l) * 100. A two-hour glucose-tolerant test was performed with monitoring of fasting insulin secretion and 1 and 2 hours after 75 g glucose load. The blood lipid profile was assessed by total cholesterol (TC), triglycerides (TG), high-density lipoproteins (HDL) and low-density lipoproteins (LDL), and atherogenic coefficient (AC) using a spectrophotometric method. Insulin resistance (IR) was diagnosed by the HOMA index calculated by the formula: fasting glucose (mmol/l) × fasting insulin (μE/ml)/22.5.
Pelvic ultrasonography was performed on day 5-7 of the menstrual cycle with a 2000 Toshiba SSA-240 machine (Japan) using a 7.5 MHz transvaginal convex transducer to measure the ovarian volume and antral follicle count (AFC) in the ovarian volume. The patients were divided into two groups to receive either COCs containing EV 3 mg, EV 2 mg + DNG 2 mg, EV 2 mg + DNG 3 mg, EV 1 mg (Klayra) (group I, n = 50), or COCs with 20 µg EE + 3 mg DRSP (Yaz) (group II, n = 50). The study was approved by the ethical committee and was conducted at the Department of Gynecologic Endocrinology, V.I. Kulakov NMRC for OG & P of Minzdrav of Russia.
The effectiveness of therapy was evaluated in 87 women. Thirteen patients dropped out of the study for reasons unrelated to COC adverse effects. Eight and five women were excluded from the group I and II, respectively, due to low compliance.
Statistical analysis was performed using the SPSS (IBM Statistical Package for the Social Sciences, version 21). All data are presented as a mean ± standard deviation; the comparison was performed using the Mann-Whitney U-test. Correlations were assessed with a Spearman correlation coefficient. Results were considered statistically significant at p <0.05.
Results
Age of patients in group I (24.1 ± 2.8 years) and group II (25.6 ± 2.1 years) were comparable (p> 0.05). They had similar BMI (20.6 ± 3.8 vs. 21.4 ± 2.9 kg/m2, respectively) (p> 0.05). The prevalence of overweight and obesity was 8% and 2% vs. 12% and 2.5% among patients in group I and II, respectively (both p> 0.05). Patients in both groups had menstrual disorders from oligomenorrhea (OM) (90% in group I and 84% in group II), to secondary amenorrhea (10 and 16%), respectively (p> 0.05).
No significant differences were observed between the groups regarding the basic hormonal and metabolic characteristics (table). The level of AMH ranged from 6.0 to 23.3 ng/ml; AMH levels in patients of both groups were higher than the previously established threshold values for different age groups [23, 24]. LH level> 10µU/ml was observed in 1/3 patients of both groups, and the LH/FSH ratio> 3 was found in 24% (12/50) of women in the group I and 20% (10/50) in group II. The levels of prolactin and TSH in patients of both groups did not exceed the reference values.
Clinical HA was observed in 48% (24/50) and 40% (20/50) of patients in the form of hirsutism and 16% (8/50) and 20% (10/50) in the form of acne in group I and II, respectively.
Biochemical HA was detected in 56% (28/50) and 50% (25/50) women in the group I and II, respectively. Increased TT levels were observed in 38% (19/50) and 32% (16/50), FT - in 10% (5/50) and 12% (6/50), A - in 30% (15/50) and 26% (13/50) of women in the group I and II, respectively. The level of SHBG ranged from 20.6 to 120 nmol/l; its level below 50 nmol/ml was observed in 50% and 48% of patients in group I and II, respectively.
 As seen from the table, the mean ovarian volumes and AFC in both groups exceeded the threshold values (10.0 cm3, 12 follicles) considered as diagnostic for PCOS, according to the Rotterdam criteria [5].
As seen from the table, the mean ovarian volumes and AFC in both groups exceeded the threshold values (10.0 cm3, 12 follicles) considered as diagnostic for PCOS, according to the Rotterdam criteria [5].
Based on the study findings, the patients were stratified by PCOS phenotypes. In group I, 26 (52%), 3 (6%), 2 (4%), and 19 (38%) patients had the classical phenotype A (HA + OM + PCO), phenotype B (HA + OM), phenotype C (HA + PCO), and non-androgenic phenotype D (OM and PCO), respectively. In group II, 29 (58%), 1 (2%), 2 (4%), and 24 (36%) patients had phenotype A, phenotype B, phenotype C, and phenotype D, respectively.
After 12 months of treatment, the study groups demonstrated different changes in several clinical, hormonal, and metabolic parameters. Improvement in the condition of skin with a disappearance or a significant improvement of acne at 12 months of treatment was observed in 62.5% (5/8) and 80% (8/10) of women in the group I and II, respectively (p> 0, 05). A decrease in the severity of hirsutism at 12 months of treatment was observed in 42% (10/24) and 55% (11/20) of women in the group I and II, respectively (p> 0.05).
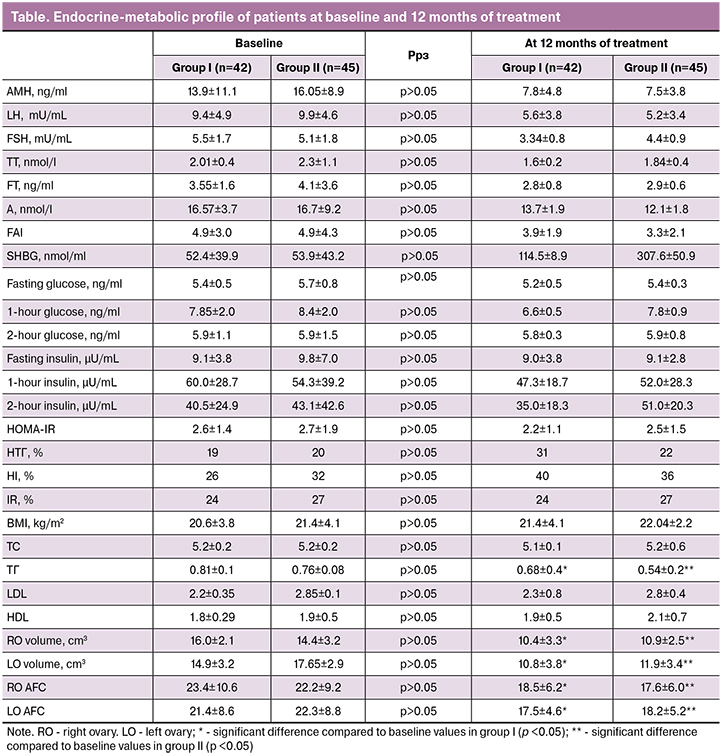
The changes in hormonal parameters in the setting of COC therapy are shown in Fig. 1. Mean levels of LH decreased by 40% and 48% in group I and II, respectively (p> 0.05). Correction of biochemical HA resulted in reduced levels of TT, FT, and A. Mean levels of TT decreased by 17% and 20%, FT - by 19% and 29% in group I and II, respectively (p> 0.05). The 12-month treatment course also resulted in 17% and 28% reduction in the levels of A in group I and II, respectively (p> 0.05). Mean levels of SHBG showed a 2.0 and 5.7 fold increase in group I and II, respectively (p <0.05).
After 12 months of treatment, mean levels of AMH decreased by 44and 53% in group I and II, respectively (p> 0.05), but they still did not reach the standard values.
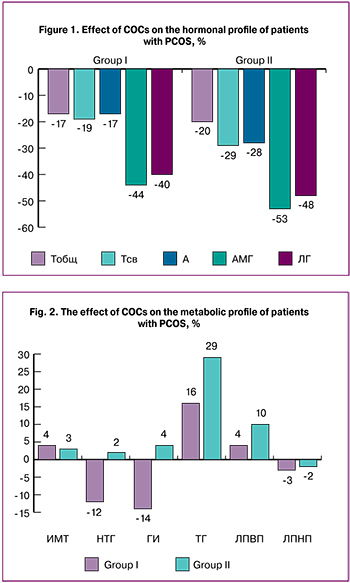
COC therapy in both groups did not have a significant effect on the patients’ body weight (Fig. 2). Before and after treatment, BMI was 20.6 ± 3.8 and 21.4 ± 4.1 kg/m2 in group I and 21.4 ± 2.9 and 22.04 ± 2.2 kg/m2 in group II (p> 0.05). At the end of the 12-month of COC therapy, the patients in group I demonstrated a mild decrease in the rates of IGT (n = 5, 12%) and HI (n = 6, 14%) (Fig. 2). In group II, no significant changes were observed in the glucose tolerance test parameters; IGT and HI increased in 1 (2%) and 2 (4%) patients, respectively.
Significant differences between the study groups were found in blood lipid profiles regarding the levels of TG. COC therapy was associated with an increase in mean TG levels by 16% and 29% in patients of group I and II, respectively (p <0.05). Despite this trend, all lipid profile parameters remained within the reference range. The use of COC was associated with a slight upward trend in the mean HDL levels, which increased by 4% and 10% in group I and II, respectively; mean LDL levels decreased by 3% and 2%, respectively (p> 0.05) (fig. 2). The mean level of TC remained unchanged during the treatment course.
As seen from the table. 2, after 12 months of therapy, ultrasound-measured ovarian volumes decreased in both groups (p <0.05).
Adverse effects in group I patients included breakthrough bleeding (6%) and breast pain (5%); the patients in group II reported nausea (4%), headache (4%), and breakthrough bleeding (4%). In all cases, adverse effects did not require COC therapy discontinuation.
Discussion
According to modern concepts, PCOS is viewed as a reproductive metabolic syndrome conferring risk for developing cardiovascular diseases and type 2 diabetes mellitus (DM). The high prevalence of metabolic abnormalities in PCOS patients, the inconsistency of data on the effect of COCs on insulin sensitivity and blood lipid profile [25] stimulated interest in investigating the effects of COCs containing various types of estrogens and progestogens on metabolic parameters. The study findings showed that EE/DRSP and EV/DNG do not adversely affect carbohydrate metabolism. After 12 months of EE/DRSP therapy, only one additional case of IGT and 2 cases of HI was observed. The use of EV/DNG resulted in a decrease in the rates of IGT and HI by 12% and 14%, respectively. In the available literature, we did not find comparative studies investigating the effect of these types of COCs on the metabolic and lipid profile of patients with PCOS. However, previous studies showed a less pronounced effect of EE/DRSP on carbohydrate metabolism, in contrast to COCs that contained chlormadinone acetate (CPA). The authors attributed this observation to antimineralocorticoid properties of DRSP [26]. Aldosterone can induce IR, whereas DRSP has a positive effect on tissue insulin sensitivity [27]. The 12-month course of COC therapy did not significantly affect the lipid profile of patients with PCOS, except for a 29% and 16% increase in the mean TG level in patients receiving EE/DRSP and EV/DNG, respectively (p> 0.05). The rates of hypertriglyceridemia did not significantly exceed the baseline values. It remains controversial whether COCs impact BMI [28]. Our findings suggest that both types of COCs do not have a significant effect on BMI.
The results of the study showed that a 12-month course of COC therapy effectively reduced clinical manifestations of HA and improved its biochemical control. The changes in TT during the use of EV/DNG and EE/DRSP were similar; its mean levels decreased by 17% and 20%, respectively. Although EE/DRSP therapy reduced mean FT and A levels by 29% and 28% compared to only 19% and 17% in patients receiving EV/DNG, no significant differences were found between the groups. The lack of significant differences in antiandrogenic activity can probably be explained by the equivalent effect of the drugs on LH levels, and by a similar effect on the activity of 5α-reductase. Despite the lack of studies comparing DRSP-containing and DNG-containing COCs, its comparison with other COCs that have potential antiandrogenic activity did not reveal significant advantages of DRSP in terms of reducing androgen level and hirsutism severity [29].
The most interesting aspect of our study results is data regarding changes in the levels of serum SHBG. The 12-month course of COC therapy resulted in a 5.7-fold increase in the mean level of SHBG in patients receiving EE/DRSP while the EV/DNG users showed only a 2.2-fold increase. The marked increase in the level of SHBG can be considered both from the positive side, because it reduces the bioavailability of androgens, and from the negative side, due to its effect on the blood thrombogenic potential. Such a significant difference in changes of SHBG levels in patients receiving two types of COCs can be explained by a more pronounced effect of EE on the synthesis of SHBG, which, as is well known, more strongly potentiates its increase, compared to endogenous estradiol. In turn, progestogens with antiandrogenic activity, unlike those with androgenic activity, exacerbate the estrogen-induced increase in the level of SHBG [30]. Several studies have demonstrated a positive correlation of SHBG with the index of resistance to APC in patients receiving COCs [30]. This indirectly indicates an increase in blood thrombogenic potential. In women with a regular menstrual cycle, APC enhances fibrinolysis by neutralizing PAI-1. Increased resistance to APC during EE/DRSP therapy may result in hypofibrinolysis, increasing the risk of thrombotic complications in patients with PCOS [31]. According to the literature, EV/DNG, contrary to EE-containing COCs, has a negligible effect on procoagulant and platelet hemostasis, as evidenced by the absence of changes in the levels of prothrombin 1 + 2 and D-dimer [16]. The results of a large-scale INAS-SCORE study (The International Active Surveillance study “Safety of Contraceptives: Role of Estrogens”) have demonstrated a twofold decrease in the incidence of venous thromboembolism and the absence of differences in the incidence of arterial thromboembolism during the administration of EV/DNG compared with other COCs [32, 33]. It should be noted that in our study, there was not a single case of venous thromboembolism among PCOS patients receiving COCs.
Conclusion
Based on the findings of our study, it can be concluded that 12-month courses of EE/DRSP and EV/DNG are effective in improving clinical and biochemical HA in patients with PCOS. In the absence of a significant effect of both COC types on the atherogenic potential of the blood, EV/DNG contributes to a slight decrease in the rates of IGT and HI. EE/DRSP occasionally causes HI and IGT. A less pronounced increase in the level of SHBG in EV/DNG users may imply its lower thrombogenic potential. This condition associated with the risk of cardiovascular complications should be considered when choosing a long-term hormone therapy for PCOS. Our findings suggest the feasibility of a differential approach to the administration of hormone therapy. In patients with disorders of carbohydrate and/or lipid metabolism and those with thrombotic risk (in the absence of high-risk thrombophilia), the treatment of choice may be EV/DNG. In PCOS patients with signs of hyperandrogenism but without significant metabolic disorders, EE/DRSP should be preferred.
References
- Cascella T., Palomba S., De Sio I., Manguso F., Giallauria F., De Simone B. et al. Visceral fat is associated with cardiovascular risk in women with polycystic ovary syndrome. Hum. Reprod. 2008; 23(1): 153-9.
- Orio F., Vuolo L., Palomba S., Lombardi G., Colao A. Metabolic and cardiovascular consequences of polycystic ovary syndrome. Minerva Ginecol. 2008; 60(1): 39-51.
- Anwar S., Shikalgar N. Prevention of type 2 diabetes mellitus in polycystic ovary syndrome: A review. Diabetes Metab. Syndr. 2017; 11(Suppl. 2): S913-7.
- Halperin I.J., Kumar S.S., Stroup D.F., Laredo S.E. The association between the combined oral contraceptive pill and insulin resistance, dysglycemia and dyslipidemia in women with polycystic ovary syndrome: a systematic review and meta-analysis of observational studies. Hum. Reprod. 2011; 26(1): 191-201.
- Rotterdam ESHRE/ASRM–Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004; 81: 19-25.
- Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L. et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018; 33(9): 1602-18.
- Martin K.A., Chang R.J., Ehrmann D.A., Ibanez L., Lobo R.A., Rosenfield R.L. et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008; 93(4): 1105-20.
- Zimmerman Y., Eijkemans M.J., Coelingh Bennink H.J., Blankenstein M.A., Fauser B.C. The effect of combined oral contraception on testosterone levels in healthy women: a systematic review and meta-analysis. Hum. Reprod. Update. 2014; 20(1): 76-105.
- Bhattacharya S.M., Jha A. Comparative study of the therapeutic effects of oral contraceptive pills containing desogestrel, cyproterone acetate, and drospirenone in patients with polycystic ovary syndrome. Fertil. Steril. 2012; 98(4): 1053-9.
- Ehrmann D.A. Polycystic ovary syndrome. N. Engl. J. Med. 2005; 352(12): 1223-36.
- Lopez L.M., Grimes D.A., Schulz K.F. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst. Rev. 2014; (4): CD006133.
- Sitruk-Ware R., Nath A. Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Pract. Res. Clin. Endocrinol. Metab. 2013; 27(1): 13-24.
- Пшеничникова Т.Б., Пшеничникова Е.Б. Генетическая и приобретенная формы тромбофилии у больных с метаболическим синдромом в сочетании с синдромом поликистозных яичников. Акушерство и гинекология. 2006; 5: 29-31. [Pshenichnikova T.B., Pshenichnikova Y.B. Genetic and acquired forms of thrombophilia in patients with metabolic syndrome in combination with polycystic ovary syndrome. Akusherstvo i ginekologiya/Obstetrics and gynecology. 2006; 5: 29-31. (In Russian)]
- Burchall G.F., Piva T.J., Linden M.D., Gibson-Helm M.E., Ranasinha S., Teede H.J. Comprehensive assessment of the hemostatic system in polycystic ovarian syndrome. Semin. Thromb. Hemost. 2016; 42(1): 55-62.
- Idali F., Zareii S., Mohammad-Zadeh A., Reihany-Sabet F., Akbarzadeh-Pasha Z., Khorram-Khorshid H.R. et al. Plasminogen activator inhibitor 1 and methylenetetrahydrofolate reductase gene mutations in iranian women with polycystic ovary syndrome. Am. J. Reprod. Immunol. 2012; 68(5): 400-7.
- Линников В.И., Бондаренко Н.И. Влияние оральных контрацептивов на систему гемостаза и метаболизм липидов. Таврический медико-биологический вестник. 2011; 14(3,1): 141-3. [Linnikov V.I., Bondarenko N.I. The effect of oral contraceptives on the hemostatic system and lipid metabolism. Tavrichesky biomedical journal. 2011; 14 (3, part 1): 141-3. (in Russian)]
- Хамани Н.М. Клиническое значение контроля гемостаза у женщин, принимающих гормональные контрацептивы: дисс. … канд. мед. наук. М.; 2018. [Hamani N.M. The clinical significance of controlling hemostasis in women taking hormonal contraceptives: Diss. ... Cand. med. sciences. M.; 2018. (in Russian)]
- Макацария А.Д., ред. Тромбогеморрагические осложнения в акушерско-гинекологической практике. Руководство для врачей. М.: МИА; 2007: 17-76. [Makatsariya A.D., ed. Thrombohemorrhagic complications in obstetric and gynecological practice. A guide for doctors. M.: MIA; 2007: 17-76.(in Russian)]
- Макацария А.Д., Саидова Р.А. Гормональная контрацепция и тромбофилические состояния. М.: Триада Х; 2004. [Makatsariya A.D., Saidova R.A. Hormonal contraception and thrombophilic conditions. M.: Triada X; 2004. (in Russian)]
- van Vliet H.A., Bertina R.M., Dahm A.E., Rosendaal F.R., Rosing J., Sandset P.M., Helmerhorst F.M. Different effects of oral contraceptives containing different progestogens on protein S and tissue factor pathway inhibitor. J. Thromb. Haemost. 2008; 6(2): 346-51.
- Cravioto M.D. New recommendations from the World Health Organization (WHO) for the use of contraceptive methods. Gac. Med. Mex. 2016; 152(5): 601-3.
- Lizneva D., Gavrilova-Jordan L., Walker W., Azziz R. Androgen excess: investigations and management. Best Pract. Res. Clin. Obstet. Gynaecol. 2016; 37: 98-118.
- Найдукова А.А., Каприна Е.К., Иванец Т.Ю., Чернуха Г.Е. Значение АМГ в диагностике синдрома поликистозных яичников. Акушерство и гинекология. 2017; 1: 46-52. [Naidukova A.A., Kaprina E.K.,Ivanets T.Yu., Chernukha G.E. The value of AMH in the diagnosis of polycystic ovary syndrome. Akusherstvo i ginekologiya/Obstetrics and gynecology. 2017; 1: 46-52. (in Russian)]
- Найдукова А.А., Каприна Е.К., Иванец Т.Ю., Чернуха Г.Е. Возрастные аспекты оценки уровня антимюллерова гормона при синдроме поликистозных яичников. Акушерство и гинекология. 2017; 3: 95-100. [Naidukova A.A., Kaprina E.K., Ivanets T.Yu., Chernukha G.E. Age aspects of assessing the level of anti-Mullerian hormone in polycystic ovary syndrome. Akusherstvo i ginekologiya/Obstetrics and gynecology. 2017; 3: 95-100. (in Russian)]
- Чернуха Г.Е., Блинова И.В., Купрашвили М.И. Эндокринно-метаболические характеристики больных с различными фенотипами синдрома поликистозных яичников. Акушерство и гинекология. 2011; 2: 70-6. [Chernukha G.E., Blinova I.V., Kuprashvili M.I. Endocrine and metabolic characteristics of patients with different phenotypes of polycystic ovary syndrome. Akusherstvo i ginekologiya/Obstetrics and gynecology. 2011; 2: 70-6. (in Russian)]
- Yildizhan R., Gokce A., Yildizhan B., Cim N. Comparison of the effects of chlormadinone acetate versus drospirenone containing oral contraceptives on metabolic and hormonal parameters in women with PCOS for a period of two-year follow-up. Gynecol. Endocrinol. 2015; 31(5): 396-400.
- Remde H., Hanslik G., Rayes N., Quinkler M. Glucose metabolism in primary aldosteronism. Horm. Metab. Res. 2015; 47(13): 987-93.
- Gallo M.F., Lopez L.M., Grimes D.A., Carayon F., Schulz K.F., Helmerhorst F.M. Combination contraceptives: effects on weight. Cochrane Database Syst. Rev. 2014; (1): CD003987.
- Amiri M., Ramezani Tehrani F., Nahidi F., Kabir A., Azizi F. Comparing the effects of combined oral contraceptives containing progestins with low androgenic and antiandrogenic activities on the hypothalamic-pituitary-gonadal axis in patients with polycystic ovary syndrome: systematic review and meta-analysis. JMIR Res. Protoc. 2018; 7(4): e113.
- Raps M., Helmerhorst F., Fleischer K., Thomassen S., Rosendaal F., Rosing J. et al. Sex hormone-binding globulin as a marker for the thrombotic risk of hormonal contraceptives. J. Thromb. Haemost. 2012; 10(6): 992-7.
- Raps M., Rosendaal F., Ballieux B., Rosing J., Thomassen S., Helmerhorst F., van Vliet H. Resistance to APC and SHBG levels during use of a four-phasic oral contraceptive containing dienogest and estradiol valerate: a randomized controlled trial. J. Thromb. Haemost. 2013; 11(5): 855-61.
- Dinger J., Minh T.D., Heinemann K. Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception. 2016; 94(4): 328-39.
- Григорян О.Р., Андреева Е.Н. Влияние эстрадиола валерата и диеногеста в составе КОК на систему гемостаза (обзор литературы). Проблемы репродукции. 2015; 21(2): 14-7. [Grigoryan O.R., Andreeva E.N. Effect of estradiol valerate and dienogest in the composition of the HEC on the hemostasis system (literature review). Problemy reproduktsii/Reproduction problems. 2015; 21 (2): 14-7. (in Russian)]
Received 07.12.2018
Accepted 22.02.2019
About the Authors
Chernukha G.E., MD, Professor, Head of the Department of Gynecologic Endocrinology. V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology of Minzdrav of Russia, Moscow, Russia. E-mail: g_chernukha@oparina4.ru. Phone: 8(916)3110521.Address: Moscow 117997, Ac. Oparina str. 4.Tabeeva G.I., Ph.D., Senior Researcher at the Department of Gynecologic Endocrinology, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology of Minzdrav of Russia, Moscow, Russia.Address: Moscow 117997, Ac. Oparina str. 4.
Dumanovskaya M.R., Ph.D., Researcher at the Department of Gynecologic Endocrinology ,V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology of Minzdrav of Russia, Moscow, Russia/Address: Moscow 117997, Ac. Oparina str. 4.
Marchenko L.A., MD, Professor, Leading Researcher at the Department of Gynecologic Endocrinology, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology of Minzdrav of Russia, Moscow, Russia.Address: Moscow 117997, Ac. Oparina str. 4.
For citations: Chernukha G.E., Tabeeva G.I., Dumanovskaya M.R., Marchenko L.A. A differential approach to the administration of combined oral contraceptives in polycystic ovary syndrome. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (7): 56-62 (in Russian).
http://dx.doi.org/10.18565/aig.2019.7.56-62



