Гормональная контрацепция – одна из самых часто применяющихся фармакологических технологий в гинекологии. Ее применение рассчитано не только на купирование патологических состояний, но в первую очередь на планирование беременности даже у абсолютно здоровых женщин. Между тем в применении комбинированных оральных контрацептивов (КОК) кроется риск возникновения тромботических осложнений и флебопатий. Проблеме КОК-индуцированных тромбозов и флебопатий каждый год посвящается много научных публикаций.
В последние 7 лет на основании ряда метаанализов и систематических обзоров сложилось консолидированное мнение, что относительный риск (ОР) развития КОК-индуцированных венозных тромбоэмболических осложнений (ВТЭО) находится в диапазоне от 2 до 4 [1–3]. Однако эти показатели нельзя воспринимать однозначно. Относительный риск развития ВТЭО определяется многими факторами и может быть как ниже, так и выше. Существенное возрастание ОР ВТЭО имеют женщины с врожденными тромбофилиями. В клиническом исследовании (КИ) [4] установлено, что КОК-индуцированные ВТЭО у женщин с наследственными и приобретенными тромбофилиями имели ОР 5,9 (95% доверительный интервал (ДИ) 1,1–32,4; p=0,04), а с отягощенной наследственностью по венозной тромбоэмболии до 50 лет у родственников 1-й линии родства – 5,2 (95% ДИ 1,7–15,6; p=0,004). В ходе КИ, направленного на изучение распространенности наследственной и приобретенной тромбофилии, в когорте из 770 женщин, имевших тромботическое событие в связи с применением КОК (700 женщин с ВТЭО, 70 – с инсультом), наследственная тромбофилия была выявлена у 44,5%. При этом более высокая частота наследственной тромбофилии (42%) наблюдалась в когорте с ВТЭО, а у женщин с инсультом она составила 24% [5]. Еще одно крупное КИ [6], включавшее 1850 женщин фертильного возраста (948 женщин с доказанной тромбофилией в основной группе и 902 женщины – в контрольной группе), показало, что в группе женщин с мутацией Лейдена при приеме КОК OР составил 20,6 (95% ДИ 8,9–58).
По современным представлениям, эстроген-содержащие препараты увеличивают риск как артериального, так и венозного тромбоза. Наиболее распространенной клинической картиной эстроген-связанного тромбоза является венозная тромбоэмболия глубоких вен ног или легочных сосудов, обычно в течение первых нескольких месяцев после начала использования [7, 8]. Тромботический риск зависит и от дозы эстрогена, и от типа прогестагена. Эстроген в сочетании с прогестагенами «нового поколения» в КОК может иметь более высокий тромботический риск, чем эстроген в сочетании с прогестагенами старшего поколения [9]. Тромботический риск, связанный с приемом КОК, содержащими 50 мкг этинилэстрадиола, значительно выше, чем с КОК, содержащими менее 50 мкг [10, 11]. В ряде крупных исследований [12] установлено, что КОК, в состав которых входит 50 мкг этинилэстрадиола, имеют более высокий риск ВТЭО по сравнению с препаратами, содержащими 20 мкг (OР 2,3; 95% ДИ 1,3–4,2) и 30 мкг (OР 2,1; 95% ДИ 1,4–3,2) этинилэстрадиола. КОК, содержащие гестоден, дезогестрел, дроспиренон или ципротерона ацетат, ассоциированы с более высоким риском венозного тромбоза по сравнению с КОК, содержащими левоноргестрел и хлормадинон. В КИ [8] предпринята попытка выяснить, варьирует ли синергетический эффект генетических маркеров, повышающих риск венозного тромбоза и КОК, между различными типами прогестагенов в этих препаратах. Авторы исследовали совместное влияние генетического фактора риска, а именно мутаций F5 rs6025, F2 rs1799963 и FGG rs2066865 и различных прогестагенов, на риск развития ВТЭО. В целом совместный эффект КОК и генетических вариантов был наименьшим для КОК, содержащих левоноргестрел. Для гестодена риск совместного эффекта варьировал от 11,7 (95% ДИ 7,2–19,1) до 30,9 (10,6–89,9). Дезогестрел и ципротерона ацетат имели самые высокие оценки риска: 14,6 (95% ДИ 9,7–21,9) и 32,6 (95% ДИ 13–280,6).
Помимо риска возникновения ВТЭО, у КОК есть класс-специфические сосудистые осложнения – флебопатии в виде развития хронической венозной недостаточности (ХВН) и варикозной болезни. Причиной этих осложнений является то обстоятельство, что эстрогены, стимулируя гипертрофию интимы и медии сосудов (преимущественно вен), вызывают десквамацию эндотелия, что в итоге приводит к дегенеративным изменениям венозной стенки [13, 14]. Повреждение эндотелия является дополнительным фактором развития венозного тромбоза по типу медленно растущего венозного тромба. В современной гинекологии появился термин – менеджмент побочных эффектов комбинированной гормональной контрацепции [15], и в этой связи предприняты попытки объективизировать риски. В исследовании [16] для удобной и оперативной работы гинеколога, ведущего амбулаторный прием, предложен тест-лист вероятности возникновения тромбоза и сосудистых осложнений у женщин, принимающих гормональные контрацептивы, в виде таблицы, на основании которой проводится подсчет баллов вероятности. Разработанный авторами тест-лист контроля сосудистых осложнений при применении гормональной контрацепции может быть также использован для оценки эффективности фармакотерапии ХВН. Стоит отметить, что в настоящее время лечение и профилактика флебопатий хорошо разработаны сосудистыми хирургами-флебологами. В основу лечения флебопатий положены периодический прием венотонических препаратов (на основе диосмина, гесперидина, рутозидов, сапонинов) и ношение компрессионного трикотажа. Разработаны и широко применяются радикальные малотравматичные операции по ликвидации варикоза.
В отличие от флебопатий, проблема КОК-индуцированных ВТЭО еще далека от своего решения. В основе этиологии КОК-индуцированных тромбозов лежит последовательная активация внутреннего каскада свертывания крови с образованием на конечном этапе сетки поперечно-сшитого фибрина с включением форменных элементов крови – венозного тромба. Возможны как минимум два сценария патогенеза КОК-индуцированного тромбогенеза. В одном случае повреждение эндотелия при флебопатии создает условия локального тромбообразования, формируется медленно растущий тромб с постепенной окклюзией регионального венозного бассейна. В другом случае может происходить быстрое развитие тромбоза по типу тромбоэмболии легочной артерии и других крупных сосудов, включая артерии [17–19].
Фармакологические технологии лечения ВТЭО хорошо разработаны и успешно применяются в практической медицине у больных с возникшим и перенесенным тромбозом, для профилактики его возможного развития при установке искусственных клапанов сердца, у больных с тромбофилией. В их основе лежит либо прямая блокада II и X факторов свертывания (новые пероральные антикоагулянты: ривароксабан, апиксабан, дабигатрана этексилат), либо связывание этих факторов с антитромбином III (высоко- и низкомолекулярные гепарины, фондапаринукс натрия), либо уменьшение продукции факторов свертывания (антагонисты витамина К – варфарин). Однако все они непригодны для рутинной профилактики КОК-индуцированных ВТЭО, поскольку требуют систематического, пролонгированного применения. Трудно себе представить ситуацию, когда здоровая женщина, начавшая использовать гормональную контрацепцию, начнет постоянный прием антикоагулянтов в расчете на многолетнее профилактическое применение с очевидным риском геморрагических осложнений. Наиболее комплаентной фармакологической технологией профилактики КОК-индуцированных ВТЭО будет применение лекарственного препарата в режиме краткого курсового приема 1–2 раза в год, и на сегодняшний день есть лекарственный препарат, который можно рассматривать как наиболее удачный для решения данной проблемы.
Существует группа протеолитических ферментов – субтилизинов, которые обладают выраженным фибринолитическим действием. Интерес к этим ферментам растет год от года. В 2018 г. за изучение химических свойств субтилизинов Фрэнсис Арнольд получила Нобелевскую премию. Уникальность этих ферментов заключается в том, что они растворяют только денатурированные или полимеризированные белки. Например, на фибриноген субтилизины не действуют, а поперечно-сшитый фибрин растворяют. Важным обстоятельством является то, что это фибринолитическое действие субтилизинов является прямым, без участия физиологической системы фибринолиза. Главной особенностью тромболитического действия субтилизинов является тот факт, что на нативные белки с сохраненной четвертичной структурой и не агрегировавшие между собой субтилизины не оказывают гидролитического воздействия. Это обстоятельство является принципиальным отличием от тромболитического действия активаторов плазминогена, которые, наряду с разрушением тромба, вызывают плазминовый гидролиз белковых факторов свертывания, прежде всего фибриногена, тем самым формируя риск геморрагических осложнений [20, 21]. В настоящее время сформулирована концепция неплазминового фибринолиза, доказывающая возможность ликвидации тромбоза без участия собственной системы гемостаза [22, 23].
Полученные знания по механизму действия субтилизинов явились стартовой идеей большого проекта по созданию на их основе лекарственного препарата для ликвидации тромбов посредством растворения их фибринового каркаса. В работе над этим проектом приняли участие несколько научно-исследовательских институтов Сибирского отделения РАН (НИИ цитологии и генетики, НИИ ядерной физики, НИИ фармакологии и регенеративной медицины). Важнейшей задачей для реализации этого проекта было создать такую фармацевтическую субстанцию лекарственного препарата, которая обеспечивала бы не только энтеральное всасывание субтилизина, но и отсутствие его аллергогенности после попадания в кровоток. И здесь на помощь пришли физики-ядерщики, предложив технологию электронно-лучевого синтеза. Сущность этой технологии заключается в создании вокруг молекулы субтилизина капсулы из сверхгидрофильного полимера полиэтиленоксида (ПЭО). Этот процесс происходит при облучении раствора субтилизина и ПЭО потоком ускоренных электронов, создаваемых импульсным линейным ускорителем, и называется электронно-лучевой иммобилизацией. В результате в капсуле из ПЭО иммобилизированный субтилизин приобретает способность абсорбироваться и транспортироваться через эпителий кишечника в системный кровоток и лимфатическую систему с биодоступностью до 20%. Помимо обеспечения транспортной функции, ПЭО экранирует молекулу субтилизина от рецепторного аппарата иммунных клеток, что, в свою очередь, обеспечивает отсутствие аллергогенности. При этом активные центры фермента остаются функционально активными. Производство созданного лекарственного препарата было организовано в Новосибирске на биотехнологической фармацевтической фабрике, имеющей лицензию на производство субстанций и готовых лекарственных форм. Зарегистрированное коммерческое название препарата – «Тромбовазим».
«Тромбовазим» имеет два регистрационных удостоверения. Внутривенная форма зарегистрирована для лечения острого инфаркта миокарда с подъемом зубца ST. Пероральная форма имеет показание – лечение ХВН с прямым указанием на тромболитическое действие препарата.
Проведено несколько КИ, которые доказали высокую клиническую эффективность и безопасность применения «Тромбовазима» как перорального тромболитика и подтвердили экспериментально установленную концепцию неплазминового фибринолиза субтилизинами. Также в КИ показана эффективность «Тромбовазима» для лечения флебопатий.
Эффективность применения «Тромбовазима» у больных с флебопатией (ХВН) была установлена еще на этапе регистрационных исследований в большом КИ «РОКИТ-ВН» (119 пациентов) [24]. Интегрированный показатель СЕАР, отражающий клиническое состояние больных, имел достоверную положительную динамику. Средний балл до лечения составлял 4,9±2,3, после курса терапии – 2,8±1,6 (р<0,05). Результаты анализа клинической эффективности полностью согласовывались с динамикой функциональных показателей венозного оттока (реовазографии и лазерной допплеровской флоуметрии). Объемный пульсовой кровоток достоверно увеличивался, а динамическое сопротивление в исследуемых сегментах уменьшилось. Также отмечалось выравнивание исходной асимметрии пульсового кровотока.
В многоцентровом рандомизированном плацебо-контролируемом двойном слепом КИ «VETTER» была поставлена цель – показать тромболитическое действие «Тромбовазима» у больных с острым венозным тромбозом нижних конечностей [25, 26]. Опытная и контрольная группы получали базовую терапию антикоагулянтами. В опытной группе больные получали «Тромбовазим» перорально в трех дозах: 1600, 3200 и 4800 ЕД/сут. Первичной точкой эффективности было восстановление кровотока в бассейне окклюзированных тромбом вен; вторичной – лизис флотирующей части тромба. В таблице 1 представлены результаты лечения при применении «Тромбовазима» в дозе 1600 ЕД/сут.
Эта доза разрешена к клиническому использованию и указана в инструкции по медицинскому применению. Рандомизация в этой группе дала большую часть пациентов, принимающих плацебо. Несмотря на это обстоятельство, статистический анализ достоверно показал (р=0,05), что применение «Тромбовазима» обеспечивает восстановление кровотока в окклюзированной вене в 70% случаев. Антикоагулянтная терапия без применения «Тромбовазима» – в 44%. Флотирующих тромбов также оказалось больше в группе плацебо. Статистический анализ показал существенный прирост эффективности лизиса флотирующей части тромба в группе «Тромбовазима» (57% против 11%). Однако ввиду малого объема выборки этот показатель следует рассматривать как тенденцию (р=0,08). В КИ «VETTER» не отмечено геморрагических осложнений, показатели гемостаза не ухудшались, прием препарата пациенты переносили хорошо.
В исследование [27] были включены 20 пациентов с персистирующей фибрилляцией предсердий, у которых по данным чреспищеводного ультразвукового исследования сердца был выявлен тромб в ушке левого предсердия. После обнаружения тромбоза ушка предсердия всем больным был назначен «Тромбовазим» в дозе 800 ЕД 3 раза в сутки в течение 7 дней в сочетании с антикоагулянтной терапией. При контрольном чреспищеводном ультразвуковом исследовании сердца, выполненном на 8-й день терапии, отсутствие тромбов отмечено у 19 из 20 пациентов с исходно диагностированным тромбозом ушка левого предсердия. Увеличение кровотока в ушке также свидетельствует об освобождении его полости от тромботических масс (табл. 2). Побочных эффектов по время терапии «Тромбовазимом» не наблюдалось.
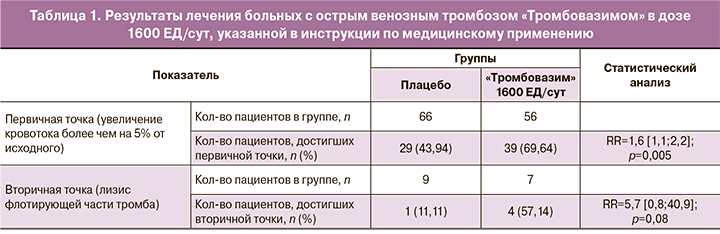
В исследовании [28] приняли участие 13 пациентов с цереброваскулярными расстройствами и выявленными на компьютерной томографии тромбозами венозной системы головного мозга. Локализация визуализированных тромбов: поперечный синус, сигмовидный, венозный, прямой. После проведения курса комплексной терапии с применением препарата «Тромбовазим» 1600 ЕД/сут в течение 12 дней у пациентов наблюдалась следующая картина: из 13 пациентов у 9 (69%) произошла ликвидация тромбоза, у 4 (31%) – тромб визуализировался, но уменьшился в размерах (рис. 1).
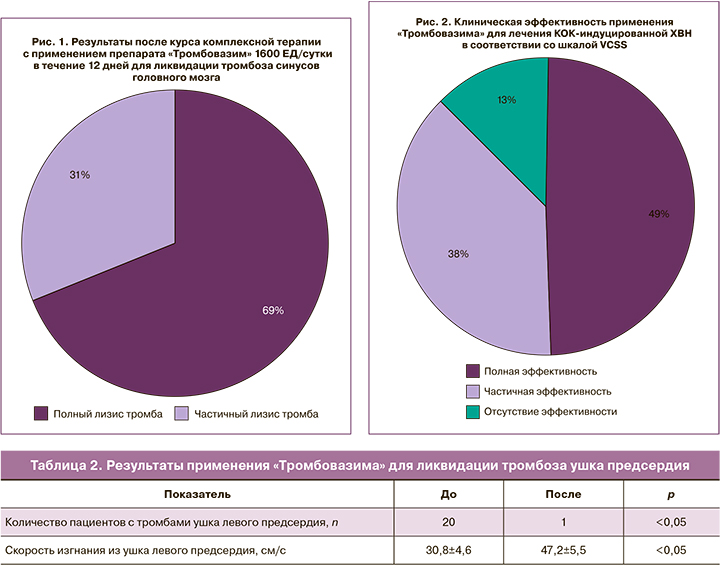
При оценке динамики кровотока в бассейне скомпрометированного кровообращения наблюдалось полное восстановление мозгового кровотока у 6 (46%) пациентов, увеличение в сравнении с исходным – у 7 (54%). Концентрация фибриногена, а также показатели тромбинового и протромбинового времени статистически достоверно не изменялись. Побочных эффектов не отмечено. В результате КИ, проведенного среди женщин с КОК-индуцированной ХВН, установлено, что применение «Тромбовазима» значимо и достоверно улучшает клиническое течение заболевания и является комплаентным и безопасным методом лечения [29]. Для оценки ХВН использовалась шкала VCSS. Полная клиническая эффективность отмечалась в 49% случаев, частичная – в 38%; отсутствие эффективности – в 13% случаев (рис. 2). Частичную эффективность тоже следует считать успехом проводимой терапии, что определяет вероятность эффективного лечения КОК-индуцированной ХВН «Тромбовазимом» в 87% случаев.
Приведенные выше сведения свидетельствуют о чрезвычайной важности проблемы КОК-индуцированных тромбозов и флебопатий и необходимости их профилактики и лечения [30].
Заключение
Поиск и разработка новых методов лечения заболеваний является неотъемлемой частью современной медицины. В части фармакологических технологий практическая медицина может рассчитывать не только на появление инновационных лекарственных препаратов, но и на новое позиционирование уже известных. Убедительным примером этого тезиса являются результаты клинического применения лекарственного препарата «Тромбовазим». Его применение в гинекологии представляет собой новый способ лечения и профилактики КОК-индуцированных ВТЭО и флебопатий. «Тромбовазим» обладает так называемым бинарным эффектом: он устраняет флебопатию и профилактирует тромботические осложнения. Это отражено в инструкции по его медицинскому применению. На сегодняшний день на фармацевтическом рынке России и других стран нет аналогичного лекарственного препарата, который в режиме перорального приема способен самостоятельно растворять тромбы. В случае применения гормональной контрацепции, когда возрастает риск появления тромбов, курсовой прием «Тромбовазима» обеспечивает эффект «генеральной уборки сосудов» и обеспечивает улучшение венозного кровообращения.



