Комбинированные пероральные контрацептивы (КОК), содержащие этинилэстрадиол и дроспиренон, используются уже более 20 лет. По данным исследования 2019 г., 22% женщин в мире от 15 до 49 лет использовали гормональную контрацепцию [1]. Дроспиренон обладает антиминералокортикоидным и антиандрогенным действиями, что положительно влияет на здоровье. Следует отметить пероральный контрацептив, содержащий 15 мг эстетрола и 3 мг дроспиренона. Эстетрол (Е4) – это природный эстроген, который синтезируется растениями и естественным образом вырабатывается печенью плода человека во время беременности [2, 3]. Эстетрол, по сравнению с другими эстрогенами, оказывает уникальное дифференцированное действие на ядерные и мембранные эстрогеновые α-рецепторы, что приводит к минимальному влиянию на печень, молочную железу и параметры гемостаза и благоприятному влиянию на эндометрий, влагалище, сердечно-сосудистую систему, кости и головной мозг [4–6]. Комплексное изучение КОК «Эстеретта» представлено в научных исследованиях [7], в которых также показано влияние Е4 на биомаркеры гемостаза, триглицериды, ткань молочной железы. В то же время влияние КОК «Эстеретта» на сексуальную функцию и качество жизни женщин, принимающих контрацептив, изучено недостаточно.
Цель исследования: изучить влияние комбинации 15 мг эстетрола и 3 мг дроспиренона (Эстеретта) на сексуальную функцию и качество жизни у пациенток репродуктивного возраста.
Материалы и методы
В условиях женской консультации осуществлено исследование, включающее 40 пациенток, которым была проведена консультация по вопросам назначения контрацепции. В процессе консультирования был выяснен личный, семейный и акушерский анамнез. Согласно рекомендациям Всемирной организации здравоохранения (ВОЗ) 2015 г. и Национальным медицинским критериям приемлемости методов контрацепции [8], кроме сбора анамнеза, проводились измерение артериального давления на обеих руках и пальпация молочных желез. Выбор женщин, обратившихся для назначения контрацептива, и врача, который проводил консультацию, остановился на КОК, содержащем 15 мг эстетрола и 3 мг дроспиренона (Эстеретта). Прием КОК соответствовал инструкции: 24 дня – активные гормональные таблетки и 4 дня – таблетки плацебо, не содержащие гормоны (режим 24+4).
Перед началом использования Эстеретты и через 6 месяцев пациентки отвечали на вопросы, характеризующие индекс сексуальной функции (Female Sexual Function Index, FSFI) по соответствующему опроснику и качество жизни по опроснику SF-36 (Short Form Medical Outcomes Study) [9]. Оба опросника являются валидированными и содержат стандартные вопросы.
Опросник SF-36 сгруппирован в 8 шкал: физическое функционирование (Physical Functioning, PF); ролевое функционирование, обусловленное физическим состоянием (Role-Physical Functioning, RP); интенсивность боли (Bodily Pain, BP); общее состояние здоровья (General Health, GH); жизненная активность (Vitality, VT); социальное функционирование (Social Functioning, SF); ролевое функционирование, обусловленное эмоциональным состоянием (Role-Emotional Functioning, RE); психическое здоровье (Mental Health, MH). Шкалы сгруппированы в физический (Physical Health, PH) и психологический (Mental Health, MH) компоненты здоровья. Используемый при подсчете шкал знак «”» означает пересчетный балл.
«Индекс сексуальной функции» представлен 19 вопросами, которые объединены в 6 доменов: влечение (1, 2 вопросы), возбуждение (3, 4, 5, 6), любрикация (7, 8, 9, 10), оргазм (11, 12, 13), удовлетворение (14, 15, 16) и боль (17, 18, 19), каждый из которых оценивали в баллах.
Статистический анализ
Статистическая обработка данных 40 пациенток проведена посредством статистического пакета STATISTICA 13.3 (Tibco, USA): для показателей, измеренных по балльной шкале, вычислены среднее арифметическое, медиана до и после приема КОК. Значения показателей до и после приема КОК сравнивали при помощи непараметрического критерия Вилкоксона. Для анализа категориальных показателей использовали частотный анализ. Доли пациенток в разные периоды наблюдения сравнивали при помощи двустороннего критерия Стьюдента. Во всех случаях статистического анализа принят уровень статистической значимости р меньше 0,05.
Результаты и обсуждение
Все обследованные пациентки были в репродуктивном возрасте – от 21 года до 40 лет, средний возраст составил 29,5±4,2 года.
По всем вопросам достигнута значимая разница в сторону улучшения показателей качества жизни. Неизменным осталось отношение к физическим нагрузкам: до использования КОК и через 6 месяцев показатели не отличались.
В соответствии с рекомендациями по анализу ответов опросника качества жизни нами до и через 6 месяцев приема КОК «Эстеретта» рассчитано значение физического функционирования (PF) по формуле:
PFsum=PF3a+PF3б+PF3в+PF3г+PF3д+PF3е+PF3ж+PF3з+PF3и+PF3к,
затем по формуле:
PF=((PFsum-10)/20×100;
PFдо=76,65 балла;
PFпосле=91,1 балла.
Проведена оценка по шкале «Ролевое функционирование, обусловленное физическим состоянием» (RP), которое рассчитывалось по формулам:
RPsum=RP4а+RP4б+RP4в+RP4г и
RP=((RPsum-4)/4)×100;
RPдо=54 балла;
RPпосле=88,5 балла.
Значение по шкале «Интенсивность боли» (BP) рассчитывали по формуле:
ВР=[((ВР7”+ВР8”)-2)/10]×100;
ВРдо=74 балла;
ВРпосле=100 баллов.
Оценку по шкале «Общее состояние здоровья» (GH) считали по формуле:
GHsum=GH1”+GH11а+GH11б”+GH11в+GH11г” и
GH=((GHsum-5)/20)×100;
GHдо=52 балла;
GHпосле=77,5 балла.
Оценку по шкале «Жизненная активность» (VT) рассчитывали по формуле:
VTsum=VT9а”+VT9д”+VT9ж+VT9и,
а затем по формуле: VT=((VTsum-4)/20)×100;
VTдо=40 баллов;
VTпосле=51 балл.
Проведена оценка по шкале «Социальное функционирование» (SF) по формулам:
SFsum= SF6”+SF10 и SF=((SFsum-2)/8×100;
SFдо=59 баллов;
SFпосле=65 баллов.
Оценку по шкале «Ролевое функционирование, обусловленное состоянием» (RE), рассчитывали по формулам:
REsum=RE5а+RE5б+RE5в и RE=((REsum-3)/3)×100;
REдо=47 баллов;
REпосле=85 баллов.
Значение по шкале «Психическое здоровье» (МН) вычисляли по формулам:
МНsum=МН9б+МН9в+МН9г”+МН9е+МН9з” и МН=((МНsum-5)/25×100;
МНдо=51 балл;
МНпосле=69 баллов.
Все вышеперечисленные рассчитанные показатели качества жизни отражены на рисунке 1.
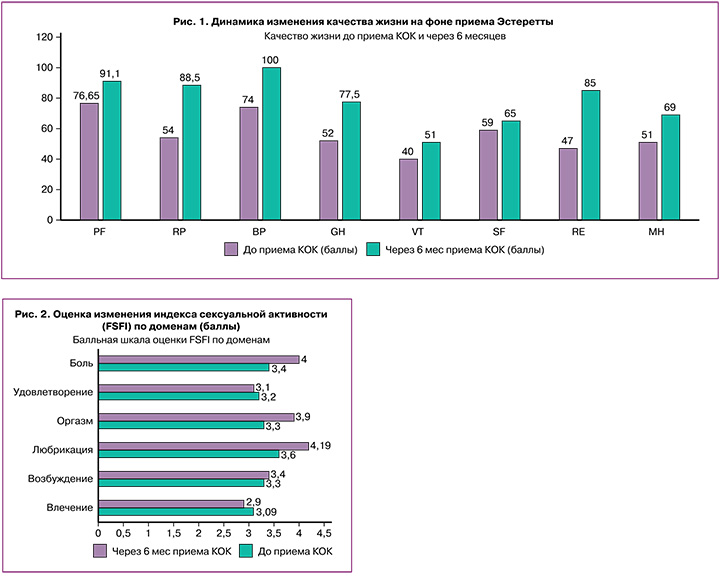
Необходимо учитывать, что SF-36 относится к неспецифическим опросникам качества жизни, однако более высокая оценка в баллах указывает на более высокий уровень качества жизни, что прослеживается по всем шкалам: PF (физическое функционирование) за 6 месяцев приема КОК увеличилось на 14,45 балла, что отражает состояние физической активности, и чем ниже балл, тем более PF ограничено; ролевое функционирование (RP) возросло на 34,5 балла, следовательно, повседневная деятельность пациенток не ограничена; интенсивность боли (ВР) выше в том случае, когда мы получаем низкие баллы, – в нашем исследовании баллы ВР увеличились на 26; более низкая изначально оценка здоровья (GH) возросла с 52 до 77,5 балла; жизненная активность (VT) увеличилась на 11 баллов; социальное функционирование (SF) поднялось на 6 баллов; ролевое функционирование (RE) – на 38 баллов; психическое здоровье, где низкие показатели ассоциируются с наличием депрессивных, тревожных переживаний, психического неблагополучия, увеличилось на 18 баллов (с 51 до 69 баллов). Таким образом, мы получили увеличение в баллах всех показателей, рассчитываемых по опроснику SF-36, что говорит об улучшении качества жизни, а также характеризует препарат «Эстеретта» как контрацептив, способный позитивно влиять на качество жизни, повышая его.
Качество жизни влияет на сексуальные отношения, которые были оценены при помощи индекса сексуальной функции (FSFI), что показано на рисунке 2.
Полный диапазон шкалы баллов продемонстрировал увеличение индекса сексуальной функции за 6 месяцев приема Эстеретты с 16,7 до 21,5 балла. Отмечено, что среди опрошенных пациенток произошло увеличение частоты любрикации, а также оргазма и возбуждения. При этом частота боли не уменьшилась, а даже незначимо увеличилась, а удовлетворение и влечение статистически незначимо уменьшились. Анализ полученных данных показал, что значимые отличия были достигнуты по вопросу 8: «Насколько трудным было достижение увлажнения половых органов в начале полового акта за последние 4 недели?» Оказалось, что количество ответивших «нетрудно» через 6 месяцев приема КОК «Эстеретта» значимо увеличилось, о чем свидетельствует увеличение количества баллов с 4,15±1,35 до 5,05±0,78 (р=0,001). Значимо выросло также количество ответивших на 10-й вопрос, что нетрудно было сохранить увлажнение половых органов до завершения полового акта: соответствующее количество баллов возросло с 3,55±1,52 до 4,45±1,58 (р=0,001). Оба этих вопроса характеризуют повышение любрикации. Повысилось число интервьюированных, которые, отвечая на 12-й вопрос, сказали, что достижение оргазма было нетрудным: исходный показатель составлял 3,63±1,41 балла, а через 6 месяцев он стал 4,85±1,09 балла (р=0,0002). Мы отметили, что через 6 месяцев приема Эстеретты значимо увеличилось количество женщин, которые не испытывали дискомфорт или боль в процессе полового акта (18-й вопрос): количество баллов возросло с 3,28±1,74 до 4,1±1,93 (р=0,005). Заметно увеличилось количество обследованных пациенток, у которых степень дискомфорта/боли во время или после полового акта снизилась с 3,33±1,59 до 4,33±1,75 балла (р=0,001).
Необходимо отметить, что, хотя и без значимой разницы, положительно изменилось отношение женщин к активному «включению» во время полового контакта; повысился уровень полового возбуждения; чаще было отмечено «пробуждение» сексуальности во время полового контакта; повысилось количество удовлетворенных возбуждением; чаще появлялось увлажнение половых органов; чаще достигался оргазм; снизилось количество женщин, которые испытывали дискомфорт или боль в процессе проникновения полового члена во влагалище.
Необходимо указать, что назначенный с целью контрацепции КОК «Эстеретта» показал благоприятное влияние на качество жизни и сексуальную функцию. Наблюдаемые женщины отметили, что сексуальная функция не только не снизилась, но по некоторым показателям улучшилась: легче и у большего количества женщин наступало увлажнение половых органов, которое сохранялось до завершения полового акта. Увеличилось количество женщин, которые достигали оргазма, и уменьшилось число опрошенных, испытывающих дискомфорт или боль в процессе полового акта. В соответствии с опросником качества жизни на фоне приема препарата «Эстеретта» по всем категориям произошло увеличение числа баллов, отвечающих за физическое и психологическое здоровье, что ассоциируется с улучшением качества жизни. Анализ предыдущих исследований [10] показал, что в реальной клинической практике использовали таблетированные гормональные контрацептивы 23,5% интервьюированных женщин. Проведенный опрос через 6 месяцев от начала приема КОК, который содержал 15 мг эстетрола и 3 мг дроспиренона, выявил удовлетворение используемым методом контрацепции у подавляющего большинства женщин (95,0%). Выяснено, что продолжить прием Эстеретты хотят 38 женщин (95,0%), и такое же количество готовы рекомендовать препарат другим пользователям контрацепции.
Аналогичные данные получены и другими исследователями [11, 12].
Заключение
Комбинация 15 мг эстетрола и 3 мг дроспиренона, назначенная изначально для контрацепции, значимо повысила качество жизни и улучшила показатели клинических проявлений сексуальных нарушений.



