Синдром поликистозных яичников (СПКЯ) относится к одной из наиболее распространенных эндокринопатий у женщин в репродуктивном возрасте с частотой встречаемости от 8 до 13% в зависимости от исследуемой популяции [1]. Согласно Роттердамским критериям, диагноз СПКЯ устанавливается при сочетании двух из трех признаков: олиго-/аменореи, гиперандрогении (клинической и/или биохимической), поликистозной морфологии яичников по ультразвуковым данным [2]. Однако, согласно Международному руководству по диагностике и лечению СПКЯ от 2018 г., определение поликистозной морфологии яичников по ультразвуковым данным не имеет диагностической ценности для пациенток с гинекологическим возрастом менее 8 лет включительно, при этом остается актуальным для определения точного фенотипа СПКЯ. Диагноз рекомендовано устанавливать при наличии подтвержденной клинической и/или биохимической гиперандрогении и нерегулярном менструальном цикле [1]. У подростков обнаруживают фенотипические комплексы СПКЯ, аналогичные для взрослых женщин: классический фенотип А определяется при сочетании трех симптомов: гиперандрогении, олиго-/ановуляции и поликистозной морфологии яичников, фенотип В – при наличии гиперандрогении и олиго-/ановуляции (ГА+АНО); фенотип С – при наличии гиперандрогении и поликистозных яичников по данным УЗИ (ГА+ПКЯ); фенотип D (СПКЯ без гиперандрогении) представлен сочетанием олиго-/ановуляции и поликистозных яичников (АНО+ПКЯ) [3].
Лечение СПКЯ зависит от степени выраженности клинических проявлений и от желания наступления беременности. Прием комбинированных оральных контрацептивов (КОК) демонстрирует свою эффективность в уменьшении выраженности гирсутизма, а также кожных проявлений, ассоциированных с гиперандрогенией (акне) [4]. Таким образом, широкая палитра монофазных КОК, низко- и микродозированных, применяется с целью коррекции гиперандрогении и нормализации менструального цикла у пациенток с СПКЯ.
Известно, что КОК, содержащие этинилэстрадиол (ЭЭ) и прогестин, снижают избыток андрогенов и, как следствие, уменьшают рост волос в андрогензависимых зонах, нетипичных для женщин. ЭЭ снижает продукцию андрогенов яичниками, повышает уровень глобулина, связывающего половые гормоны (ГСПГ), и, как следствие, снижает уровень свободного тестостерона (Т) [5–7]. Повышение уровня ГСПГ наблюдается уже через 3 месяца приема КОК, а через 6 месяцев наблюдается снижение выраженности гирсутизма. Дроспиренон (ДРСП) относят к прогестинам IV поколения с антиандрогенными свойствами. Данный прогестин – синтетическое производное спиронолактона, который обладает антиандрогенным и антиальдостероновым действиями. Исследования демонстрируют увеличение уровня ГСПГ, снижение концентрации тестостерона и выраженности гирсутизма, оцениваемое по шкале Ферримана–Голлвея, среди женщин, которые используют комбинированные таблетки, содержащие 30 мкг ЭЭ в сочетании с 30 мкг ДРСП в течение 6–12 месяцев [7].
Предполагается, что побочные эффекты КОК, такие как влияние на липидный профиль, повышенный риск тромбозов, зависят от сочетания дозы эстрогена и от типа гестагена. В связи с этим была создана новая комбинация КОК (сочетание 20 мкг ЭЭ и 30 мкг ДРСП, при режиме приема 24 активные таблетки + 4 таблетки плацебо) [6]. Более продолжительный прием ДРСП способствует улучшению гормонального фона и снижает частоту побочных эффектов, связанных со стандартным приемом с перерывом в 7 дней. К таким побочным эффектам относят перепады настроения, ассоциированные с периодом менструаций, головные боли, дисменорею и т.д. Помимо этого, влиянием ДРСП на задержку натрия и воды в организме объясняется снижение частоты таких побочных эффектов, как болезненность молочных желез, отечность, предменструальный синдром. Продленное действие контрацептива способствует не только улучшению гормонального фона и снижению частоты побочных эффектов, но также усиливает лечебный антиандрогенный эффект [8].
Наличие побочных эффектов применяемых гормональных препаратов обуславливает необходимость поиска и разработки новых средств терапии с меньшей частотой побочных эффектов при сопоставимой эффективности. Все больше данных свидетельствует о том, что средства на основе L-карнитина, α-липоевой кислоты (АЛК), инозитолов могут быть эффективны в лечении метаболических нарушений пациенток с СПКЯ и иметь патогенетическое действие при определенных фенотипах заболевания в раннем репродуктивном возрасте.
АЛК является эндогенным соединением, продуцируемым митохондриями. Предполагается, что АЛК оказывает инсулиноподобный эффект и влияет на транспорт глюкозы через ряд прямых и непрямых механизмов. АЛК может непосредственно связываться с бета-субъединицей рецептора инсулина и стимулировать ее. Кроме того, АЛК способствует экспрессии IRS-1 (insulin responsive substrate-1), что усиливает передачу сигналов с рецептора инсулина на PI3K/Akt-сигнальный путь, который способствует перемещению и слиянию везикул, которые содержат транспортер глюкозы GLUT4, с цитоплазматической мембраной [9]. Непрямой механизм АЛК опосредован активацией АМФ-активируемой протеинкиназы (AMPK). Увеличение концентрации AMPK, вызванное АЛК, индуцирует фосфорилирование IRS-1, которое облегчает передачу сигналов по пути PI3K/Akt. Кроме того, АЛК непосредственно стимулирует транслокацию GLUT4 к плазматической мембране, имитируя эффект Akt на везикулы, содержащие переносчик GLUT4 [9, 10].
В исследовании Genazzani D. et al. (2014) обоснована эффективность терапии 400 мг АЛК и 1000 мг миоинозитола ежедневно на протяжении 12 месяцев: наблюдалось значимо более выраженное снижение уровня лютеинизирующего гормона (ЛГ), отношения ЛГ/ФСГ (фолликулостимулирующий гормон), однако снижение индекса массы тела (ИМТ) не достигло статистической значимости. В группе пациенток с гиперинсулинемией отмечалось значимое снижение уровня инсулина, индекса инсулинорезистентности HOMA-IR, однако у пациенток с нормальным уровнем инсулина не наблюдалось значимых изменений этих показателей [11]. В исследовании Rago R. et al. (2015) оценивалась эффективность лечения 37 пациенток с СПКЯ без ожирения, у которых не наступила беременность после интрацитоплазматической инъекции сперматозоидов (ИКСИ). Спустя 3 месяца лечения 2000 мг миоинозитола и 800 мг АЛК были выявлены значимые изменения в уровне инсулина, ИМТ, объема яичников, однако же частота наступления беременности значимо не отличалась от группы пациенток, получающих только миоинозитол [12]. В исследовании Morgante G. et al. (2015) приняли участие 30 молодых женщин c СПКЯ с инсулинорезистентностью; одна группа получала 1000 мг миоинозитола, 5 мг монаколина К и 400 мг АЛК, вторая – 2000 мг миоинозитола, 10 мг монаколина К и 800 мг АЛК (6 месяцев). Выявлено улучшение показателей ИМТ, дислипидемии, гиперандрогении и снижение гирсутизма в обеих группах, однако в группе пациенток, получавших комбинацию с АЛК, эти изменения были более выражены [13]. Лечение комбинацией 1000 мг D-хироинозитола и 600 мг АЛК в день 46 женщин репродуктивного возраста с СПКЯ в течение 180 дней в сравнении с отсутствием лечения продемонстрировало значимый клинический эффект в основной группе: отмечено снижения индекса инсулинорезистентности HOMA-IR, инсулина, ИМТ, значимо повысился уровень липопротеинов высокой плотности (ЛПВП), также отмечалось значимое учащение менструаций [14]. По результатам исследования Masharani U. et al., назначение АЛК 600 мг 2 раза в день в течение 16 недель привело к снижению уровня триглицеридов, улучшению чувствительности к инсулину и повышению частоты менструаций у пациенток с СПКЯ в репродуктивном возрасте [15]. По рекомендации ряда авторов, назначение АЛК может быть перспективным в лечении фенотипов СПКЯ на фоне избыточного веса и метаболических нарушений, в том числе в сочетанном назначении с инозитолами и L-карнитином [13–15].
Карнитин, или бетаин 3-гидрокси-4-триметиламиномасляная кислота, – природное вещество, родственное витаминам группы B, представляет собой четвертичное аммониевое соединение. В организм попадает с пищей: мясом, рыбой, молочными продуктами, а также может быть синтезирован эндогенно в печени и почках из двух аминокислот: лизина и метионина. Существует два стереоизомера карнитина: L-карнитин, который является биологически активной формой, и D-карнитин, биологически неактивная форма. Основная физиологическая роль L-карнитина заключается в транспорте длинных цепей жирных кислот из цитоплазмы в митохондрии, где происходит их дальнейшее β-окисление. Эта роль является основополагающей, поскольку ни жирные кислоты, ни эфиры кофермента А (CoA) не могут пройти через внутреннюю митохондриальную мембрану [16, 17]. Особенно актуально изучение влияния карнитина на возможность повышения чувствительности к инсулину, поскольку инсулинорезистентность, как известно, вносит существенный вклад в развитие СПКЯ. В исследовании Salehpour S. et al. прием 3000 мг/сут карнитина на протяжении 3 месяцев привел к значимому снижению уровня глюкозы, инсулина и индекса инсулинорезистентности HOMA-IR [18]. В другой работе прием 250 мг/сут карнитина на протяжении 3 месяцев также привел к значимому снижению этих же показателей (уровня глюкозы, инсулина и индекса инсулинорезистентности HOMA-IR) [19]. В исследовании El Sharkwy I. et al. в группе пациенток, получавших 3000 г/сут карнитина в сочетании с метформином (850 мг/сут, через неделю доза была удвоена – 1700 мг/сут) и кломифена цитратом (150 мг/сут) с 3-го по 7-й день менструации на протяжении 3 месяцев, наблюдалось значимое снижение индекса инсулинорезистентности HOMA-IR в сравнении с группой, получавшей аналогичную комбинацию препаратов вместе с плацебо вместо карнитина [20]. Таким образом, L-карнитин может быть рекомендован в лечении метаболических нарушений у пациенток с СПКЯ.
Влияние карнитина на липидный профиль также освещается в ряде статей. В исследовании Ismail A. et al. пероральный прием карнитина в дозе 3000 мг/сут в сочетании с кломифена цитратом (250 мг/сут с 3-го по 7-й день менструации) в течение 12 недель вызывал значимое снижение уровня холестерина общего, триглицеридов, холестерина липопротеинов низкой плотности (ЛПНП-ХС), а также повышение уровня ЛПВП в сравнении с группой, получающей кломифена цитрат с плацебо [21].
Помимо изучения влияния L-карнитина на метаболические нарушения, большое внимание уделяется оценке изменений гормонального профиля у пациенток с СПКЯ при лечении карнитином. В исследовании Vigerust N. et al. было обнаружено, что уровни свободного и общего карнитина отрицательно коррелировали с уровнем ГСПГ у пациенток с СПКЯ, однако не было выявлено связи между уровнем карнитина в плазме и концентрацией андрогенов [22]. В другой работе было показано, что существует значимая и отрицательная корреляция между уровнем карнитина и индексом свободных андрогенов (ИСА), а также положительная и значимая связь с уровнем ГСПГ [23]. Таким образом, наилучшие результаты в лечении с использованием L-карнитина показаны у пациенток избыточного веса с фенотипами СПКЯ на фоне метаболических нарушений.
Возрастает число данных в пользу эффективности изомеров инозитола в лечении пациенток с СПКЯ. Инозитолы присутствуют в природе в виде девяти стереоизомеров. Из них наиболее распространены миоинозитол (MI) и D-хироинозитол (DCI). Под действием НАД/НАДН-эпимеразы MI превращается в DCI. От активности фермента эпимеразы зависит соотношение MI и DCI в разных тканях. Инозитол поступает в клетку с помощью специальных переносчиков, может присутствовать и в свободной форме, и в форме фосфолипидов клеточной мембраны, в основном в виде фосфатидилинозитолфосфата (PIP) и фосфатидилинозитолбифосфата (PIP2) [24].
MI и DCI являются вторичными мессенджерами сигнала от рецепторов инсулина и принимают участие во внутриклеточном каскаде передачи сигнала. Известно четыре основных сигнальных пути инсулина: быстрые и очень быстрые пути фосфатидилинозитол-3-киназы (PI3K) и митоген-активируемой протеинкиназы (MAPK), медленные и очень медленные пути протеинкиназы С и G-белка. PI3K катализирует превращение PIP2 в PIP3, который, являясь вторичным мессенджером, активирует фосфатидилинозитол-зависимую киназу 1 (PDK-1), которая, в свою очередь, фосфорилирует и активирует Akt-киназу. Этот каскад реакций активирует транслокацию переносчика глюкозы типа 4 (GLUT4) на цитоплазматическую мембрану и инактивирует киназу гликогенсинтазы (GSK), что обуславливает повышение активности фермента гликогенсинтазы (GS) и, как следствие, активирует синтез гликогена и снижает уровень глюкозы в крови.
Следующий сигнальный путь инсулина опосредуется активацией G-белка, при этом происходит активация фосфолипазы D (PLPD), что приводит к гидролизу фосфатидилинозитолов (PIs) и образованию инозитолфосфогликанов (IPGs), расположенных в цитоплазматической мембране. Одним из таких IPG является DCI-IPG, который выступает вторичным мессенджером сигналов от рецепторов инсулина, INS-2. Комплекс с рецептором DCI-IPG/INS-2, связываясь с гетеротримерной 2Ca протеинфосфатазой (PP2Ca), дефосфорилирует и этим активирует GS и PI3K, что запускает синтез гликогена и поступление в клетки глюкозы в инсулинчувствительных тканях. Кроме того, DCI-IPG участвует в стимуляции окислительного метаболизма глюкозы в цикле Кребса и продукции аденозинтрифосфата (АТФ), а также способствует активации гликолиза [24].
Помимо участия в сигнальных путях инсулина, MI и DCI имеют важную роль в передаче сигналов ФСГ/ЛГ. Связывание ФСГ с рецептором активирует Gs-белок, аденилатциклазу и пути цАМФ/протеинкиназы А (ПКА), что опосредует пролиферирующий эффект ФСГ на гранулезу, активацию экспрессии ароматазы и повышение продукции эстрогенов. MI опосредует передачу сигналов с ЛГ и ФСГ через IP3-путь, что связано с высвобождением внутриклеточных запасов кальция из эндоплазматического ретикулума или посредством поступления внеклеточного кальция через мембранные каналы. Этот путь реализуется активацией фосфолипазы С (PLP-C), которая превращает PIP2 в инозитолтрифосфат (IP3) и диацилглицерол. В яйцеклетках в данном пути задействован специфический подтип рецептора, IP3-R1-подтип, чья активация приводит к прогрессии мейоза и играет ключевую роль в оогенезе, когда максимального значения достигает чувствительность ооцитов к кальцию. Кроме того, производные MI участвуют в регуляции уровня антимюллерова гормона (АМГ) в сыворотке крови [24]. Участие MI в сигнальных путях инсулина и ФСГ/ЛГ позволило рассмотреть его в качестве эффективного средства для коррекции метаболических и гормональных нарушений у пациенток с СПКЯ.
В метаанализе Zeng L. et al. (2017) показана эффективность MI в отношении снижения HOMA-IR, снижения уровня ЛГ, а также увеличения уровней эстрадиола и ГСПГ среди пациенток с СПКЯ, однако нет убедительных доказательств того, что MI оказывает влияние на уровень свободного тестостерона. Авторы статьи утверждают, что MI может быть рекомендован для лечения пациенток с СПКЯ с инсулинорезистентностью [25]. В другом метаанализе оценивалась эффективность MI (2000–4000 мг/сут) в сравнении с метформином (1500–2000 мг/сут) на протяжении 12–24 недель. Не было выявлено отличий в лечении пациенток метформином и MI в отношении уровня инсулина, индекса инсулинорезистентности HOMA-IR, Т, андростендиона, ГСПГ и ИМТ. Основным наблюдаемым отличием было отсутствие побочных реакций у пациентов, получавших MI, в сравнении с пациентами, получавшими метформин. Более низкий риск нежелательных явлений MI в сравнении с метформином делает его более безопасным средством, которое можно принимать вместо метформина или в сочетании с более низкими дозами метформина у пациентов, которые не переносят высокую терапевтическую дозу [26]. В метаанализе Pundir J. et al. (2017) показана эффективность MI (1200–4000 мг/сут) в регуляции овуляции, менструального цикла, гормональных и метаболических параметров. Миоинозитол значимо увеличивает частоту овуляции у пациенток с ановуляторными циклами. Частота менструальных циклов возрастает в 6 раз у пациенток с олиго-/аменореей. На фоне терапии значимо снижаются уровни как общего, так и свободного Т, ДГЭА-С, повышается уровень ГСПГ. Влияние на метаболический профиль выражается в снижении уровня инсулина, глюкозы, индекса HOMA-IR, а также повышении соотношения глюкоза/инсулин [27].
В группе подростков с СПКЯ в возрасте от 13 до 19 лет также был продемонстрирован положительный эффект терапии MI, а именно снижение веса, ИМТ, уровня глюкозы в сыворотке крови, С-реактивного белка (СРБ), инсулина и HOMA-IR. В группе пациенток, принимающих комбинированные оральные контрацептивы (КОК), незначительно увеличились масса тела и ИМТ, однако же в группе подростков, получающих комбинированную терапию КОК с миоинозитолом, масса тела и ИМТ не изменились, в то время как значимо снизились уровни СРБ, инсулина и HOMA-IR [28].
Таким образом, особую актуальность приобретают исследования, посвященные ранней диагностике СПКЯ у подростков, клиническим особенностям симптомокомплекса при его формировании в подростковом возрасте и принципам дифференцированного подхода к лечению заболевания с этапа постановки диагноза, в том числе методом негормональной коррекции клинических проявлений.
Учитывая вышеизложенное, целью данного исследования является оценка эффективности лечения подростков с СПКЯ КОК и негормональным средством с учетом фенотипа заболевания, наличия избыточной массы тела и инсулинорезистентности.
Исходя из поставленной цели, были сформулированы следующие задачи:
- сравнить особенности клинической картины, гормональных показателей и эхографической характеристики яичников у пациенток 15–18 лет включительно с классическим фенотипом СПКЯ до назначения микродозированного КОК и через 6 месяцев терапии;
- сравнить особенности клинической картины, гормональных показателей и эхографической характеристики яичников у пациенток 15–18 лет с неклассическими фенотипами СПКЯ до назначения негормональной терапии и спустя 6 месяцев лечения.
Материалы и методы
Оценка эффективности лечения КОК и негормональным средством на основе мио- и D-хиро-инозитола в соотношении 5:1 девочек с СПКЯ в подростковом возрасте проводилась в рамках ретроспективного исследования случай-контроль с 2019 по 2021 гг. В исследование были включены 153 пациентки с СПКЯ от 15 до 18 лет включительно при наличии двух из трех диагностических критериев: олиго-/аменореи, клинической и/или биохимической гиперандрогении и эхографической морфологии поликистозных яичников по данным ультразвукового исследования. В контрольную группу были включены 32 здоровые девочки сопоставимого возраста с регулярным менструальным циклом, первой группы здоровья, не имеющие ни гинекологической, ни эндокринной, ни соматической патологии.
На I этапе проводили сравнительный анализ клинико-анамнестических данных пациенток с СПКЯ (n=153) с группой контроля (n=32). Далее из 153 пациенток с СПКЯ были отобраны 50 пациенток с фенотипом А, которым требовалось лечение КОК по основному заболеванию. Критерием включения фенотипа А служило сочетание гиперандрогении, олиго-/ановуляции и поликистозных яичников по данным УЗИ. Из отобранных пациенток с классическим фенотипом выбыли 17 пациенток в связи с отменой препарата по месту жительства или самостоятельно без предварительного обследования, 13 пациенток выбыли в связи с удаленным местом жительства и амбулаторным наблюдением на местах. Таким образом, итоговую группу для оценки эффективности лечения КОК составили 20 пациенток, которым было проведено клиническое обследование как до назначения микродозированного КОК, так и спустя 6 месяцев на фоне терапии.
Из исходной группы пациенток также были отобраны 35 пациенток с фенотипами B и D, которым была назначена негормональная терапия инозитолами. Выбор негормонального средства был обусловлен сочетанием данных фенотипов СПКЯ с наличием избыточной массой тела и инсулинорезистентности. Критерием включения фенотипа B являлось наличие гиперандрогении и олиго-/ановуляции при отсутствии поликистозных яичников по данным УЗИ. К фенотипу D относили пациенток с олиго-/ановуляцией и поликистозными яичниками по данным УЗИ при отсутствии гиперандрогении. Из отобранных пациенток с фенотипами B и D выбыли 5 пациенток в связи с недостаточной длительностью терапии для оценки эффективности лечения (менее 6 месяцев), оставшиеся 8 пациенток были исключены в связи с удаленным местом жительства и амбулаторным наблюдением на местах. Таким образом, группу негормонального ведения составили 22 пациентки с СПКЯ. Клиническое обследование обеих групп проводилось до лечения, включало общеклинические методы исследования, биохимическое и ультразвуковое исследования. На этом этапе группы сравнивались между собой и с группой контроля. Обследование в том же объеме проводились спустя 6 месяцев. Данные сравнивались с результатами, полученными до лечения, а также с группой контроля. При обследовании учитывали жалобы пациенток на выраженность гирсутизма и сальности кожи, наличие угревых высыпаний. Оценивали особенности становления менструального ритма, характер менструальных выделений и их длительность, а также наличие болезненности, связанной с менструациями. При объективном осмотре измеряли рост (в метрах) и массу тела (в килограммах), определяли ИМТ по Кетле (ИМТ=масса тела/рост2).
В венозной крови у всех участниц исследования определяли концентрации биохимических показателей: билирубина прямого и общего, глюкозы, Mg2+, Ca2+, Fe2+/3+ и СРБ; данные липидного профиля: общего холестерина, ТГ, ЛПНП, ЛПВП, рассчитывали коэффициент атерогенности. Исследования проводили фотометрическим и турбидиметрическим методами на автоматических анализаторах BA-400, A-25 реагентами Biosystems (Испания).
Пациенткам с метаболическими нарушениями был проведен пероральный глюкозотолерантный тест (ПГТТ) с нагрузкой 75 г глюкозы и посчитан НОМА-ИР по формуле:
инсулин натощак (мкЕД/мл)×глюкоза натощак (ммоль/л)/22,5.
На 3–4-й день менструаций и/или на фоне аменореи всем пациентам был произведен забор венозной крови натощак с оценкой уровней ЛГ, ФСГ, андростендиона, дегидроэпиандростерон сульфата (ДГЭА-С), пролактина (ПРЛ), эстрадиола (Э2), АМГ, кортизола, Т, ГСПГ, свободного тироксина (Т4св), тиреотропного гормона (ТТГ). Концентрации гормонов определяли электро- и иммунохемилюминесцентным методом на автоматических анализаторах Cobas е 411 («Ф. Хоффманн-ЛаРош», Швейцария), Immulite 2000, Immulite 1000 (Siemens, США) с использованием реагентов тех же фирм. Определение АМГ, 17-ОН-прогестерона (17-ОНП), ГСПГ осуществлялось методом иммуноферментного анализа на анализаторах DYNEX DSX System и с использованием системы DPC (США) на приборе Immulite. Проводили подсчет индекса свободных андрогенов по формуле:
Тестостерон общий (нмоль/л)/ГСПГ (нмоль/л)×100%.
В первую фазу менструального цикла и/или на фоне аменореи всем пациенткам было проведено УЗИ органов малого таза при наполненном мочевом пузыре трансабдоминальным доступом либо трансректальным методом. Исследование выполнялось на ультразвуковом аппарате Vivid-q фирмы GE HEALTHCARE (Siemens, Германия) с использованием линейного и конвексного датчиков частотой от 1,8–6,0 МГц. В ходе исследования регистрировали размеры тела и шейки матки, толщины эндометрия, определяли размеры и объем яичников, количество фолликулов в срезе и их диаметр.
Статистический анализ
Статистическая обработка данных проведена с использованием программ Excel и Statistica 9 методами дескриптивного анализа (в зависимости от вида распределения переменной). При нормальном виде распределения вычисляли среднее значение (Мean, M) и стандартное отклонение (SD) со стандартной ошибкой среднего (SE). При ненормальном распределении определяли и указывали медиану (Ме) и интерквартильный размах (Q1;Q3). При нормальном распределении переменных для сравнения средних значений величин в двух независимых выборках применяли параметрический t-критерий Стьюдента для независимых выборок, для зависимых выборок использован t-критерий Стьюдента для зависимых выборок. При ненормальном распределении независимых выборок использовался U-критерий Манна–Уитни, для зависимых выборок использовали критерий Уилкоксона. Дисперсионный анализ ANOVA использован для сравнения множества групп при нормальном распределении переменных, при этом попарно сравнение проведено с помощью post hoc метода наименьшей значимой разницы (Least Significant Difference test (LSD)). Критерий Краскела–Уоллиса использовался для сравнения множества групп при ненормальном распределении переменных, попарное межгрупповое сравнение проведено с помощью критерия Данна. Для оценки категориальных переменных вычислены частоты и доли, для сравнения различий использован χ²-тест. При значениях ожидаемых явлений от 5 до 10 использовали поправку Йейтса, при значениях менее 5 применяли точный критерий Фишера.
Результаты и обсуждение
В общую группу пациенток с СПКЯ вошли 153 пациентки, из них большая часть пациенток 100 (65,4%) имели классический фенотип А, 35 (22,9%) пациенток имели фенотип B и меньшая часть – 18 (11,7%) – фенотип D.
Больше половины девочек с СПКЯ (87 пациенток – 56,9%) (р<0,0001; χ²-тест) отмечали повышенный рост волос в андрогензависимых зонах (по верхнему краю губы, на подбородке, вокруг сосков, по средней линии живота, на внутренней поверхности бедер), при этом в группе контроля гирсутизма отмечено не было. Частота жалоб на повышенную сальность кожи и угри также была значимо выше в группе пациенток с СПКЯ в сравнении с группой контроля (64 пациентки – 41,8% против 1–3,1%; p<0,0001).
Сравнительный анализ особенностей становления менструального цикла установил, что ритм менструаций не установился с менархе у 115 (75,2%) пациенток с СПКЯ, что значимо отличалось от группы контроля, в которой только у 1 (3,1%) девочки ритм не был установлен сразу (р<0,0001; χ²-тест). Методами факторного анализа подтверждено, что нерегулярный менструальный цикл с менархе с тенденцией к задержкам менструаций более 90 дней повышает риск развития СПКЯ у девочки в 18,9 раза (р<0,0001; ОШкор = 17,4; 95% ДИ 0,32–0,41).
На момент включения в исследование у большинства пациенток с СПКЯ наблюдалось нарушение ритма менструаций. Задержки менструаций более 45 дней (олигоменорея) были отмечены у 68 (59%) пациенток, нарушения цикла по типу аменореи (отсутствие менструаций более 6 месяцев) были характерны для 47 (41%) девочек.
Пациентки с СПКЯ в сравнении со здоровыми девочками реже отмечали умеренные менструальные выделения (51; 56,7% против 29; 93,6%; р=0,0002; χ²-тест), значимо чаще пациентки отмечали обильные менструации (24; 26,7% против 1; 3,2%; р=0,0116; χ²-тест с поправкой Йейтса). На болезненность менструаций указывали 29 (29,3%) пациенток с СПКЯ, в то время как в группе контроля подобной жалобы не было отмечено ни у одной девочки (p=0,0015, χ²-тест с поправкой Йейтса). Значимо чаще регистрировались менструальные выделения более 7 дней (22; 21,7% против 1; 3,2%; р=0,0362; χ²-тест с поправкой Йейтса).
По антропометрическим данным пациентки с СПКЯ характеризовались более высокими показателями массы тела (62,1 (56,6–66) против 55,7 (50–60) кг; p=0,027991, Манна–Уитни тест) и ИМТ (22,3±4,4 против 20,4±2 кг/м2; p=0,039791, t-критерий Стьюдента). С использованием многофакторного анализа подтверждено, что значение массы тела и ИМТ являются значимыми факторами риска развития СПКЯ у девочек в подростковом возрасте (р=0,0011; ОШ=10,65; 95% ДИ 0,86–0,96 и р<0,0001; ОШ=9,97; 95% ДИ 0,00–0,01).
Исследование гормонального профиля пациенток с СПКЯ в сравнении с группой контроля выявило значимо более высокий уровень:
- ЛГ (9,6 (6,1–13,3) против 4 (2,5–4,9) МЕ/л, р<0,0001);
- ПРЛ (260 (177,5–373) против 189 (144–279) мМЕ/л, р=0,0444);
- кортизола (467,5 (377–548) против 310 (254–440) нмоль/л, р=0,0006);
- Т (1,7 (1,3–2,2) против 0,8 (0,7–1,1) нмоль/л, р<0,0001);
- 17-ОНП (4,7 (3,8–5,7) против 3,5 (2,8–4,1) нмоль/л, р<0,0001);
- ДГЭА-С (7 (4,9–9,1) против 5,1 (3,9–6,3) мкмоль/л, р=0,0006);
- андростендиона (14,9 (11,9–20,5) против 8,5 (6,6–11) нг/мл, р<0,0001);
- АМГ (9,8 (7,8–16,1) против 5,8 (3,8–6,9) нг/мл, р<0,0001).
При этом у пациенток с СПКЯ обнаружен значимо более низкий уровень ГСПГ (33,5 (22–49,3) против 52,9 (39–67,6) нмоль/л; р=0,0007), что обусловило более высокий уровень ИСА (5,4 (3,6–7,8) против 1,6 (1,1–2,3); р<0,0001).
Анализ УЗИ яичников пациенток с СПКЯ выявил:
- увеличение показателей длины (4,2 (3,8–4,7) против 3,6 (3,2–3,9) см, р<0,0001);
- переднезаднего размера (2,9 (2,5–3,3) против 2,4 (2,2–2,6) см, р<0,0001);
- толщины (2,2 (2–2,5) против 1,7 (1,6–2,1) см, р<0,0001);
- среднего объема яичников (14 (10,7–15,9) против 7,7 (5,6–10) см3, р<0,0001); а также
- объемов левого (14,2 (10,5–18) против 7,8 (5,1–10,9) см3, р<0,0001) и правого (14,7 (11,2–19) против 8,2 (5,8–10,2) см3, р<0,0001) яичников.
Анализ биохимического профиля крови не выявил значимых отличий в показателях, за исключением коэффициента атерогенности, который оказался значимо выше у пациенток с СПКЯ в сравнении с группой контроля (2,1 (1,7–2,9) против 1,7 (1,4–1,9); p=0,0136, Манна–Уитни тест).
Таким образом, по данным клинической картины значимыми признаками были жалобы на гирсутизм у 56,9% девочек, угревые высыпания и повышенную сальность кожи – у 41,8% подростков, нарушение менструального цикла с менархе у – 75,2% пациенток, из них по типу олигоменореи – 59%, по типу аменореи – 41%, при этом значимо чаще регистрировались обильные (p=0,0116), болезненные (p=0,0015) менструации длительностью более 7 дней (p=0,0362). По данным лабораторного исследования обращают на себя внимание более высокие уровни ЛГ, Т, ПРЛ, кортизола, 17-ОНП, ДГЭА-С, АМГ, андростендиона, ИСА.
УЗИ яичников пациенток с СПКЯ выявило значимое увеличение длины, толщины, переднезаднего размера и объема яичников.
Дальнейший анализ проводили среди девочек с классическим и неклассическими фенотипами СПКЯ в сравнении со здоровыми девочками.
Исследование биохимического профиля пациенток в сравниваемых группах не выявило значимых отличий по большинству показателей. В сравнении с группой здоровых девочек пациентки с фенотипом А отличались более высокими уровнями коэффициента атерогенности (2,8±1,2 против 1,7±0,6; р=0,0029, LSD-тест), а также билирубина, как общего (20,3 (11,3–19,7) против 11,3 (8,6–13,9) мкмоль/л; р=0,0111, критерий Данна), так и прямого (3,7 (2,7–5,1) против 3,3 (2,4–4,1) мкмоль/л; р=0,0111, критерий Данна).
Сравнительный анализ гормонального профиля пациенток с СПКЯ выявил повышение уровней ЛГ, Т, кортизола, 17-ОНП, ДГЭА-С, андростендиона, АМГ в обеих исследуемых группах в сравнении со здоровыми девочками (табл. 1).
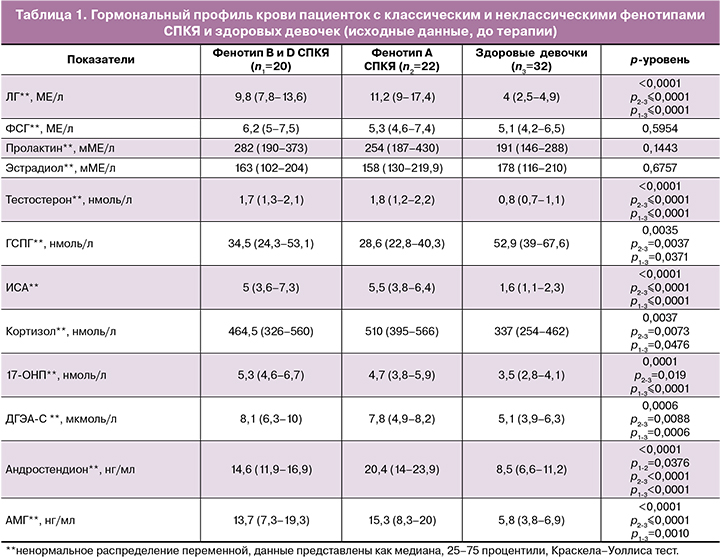
Исследуемые группы с различными фенотипами не отличались значимо между собой по уровню гормонов, за исключением уровня андростендиона, который был значимо выше у пациенток с фенотипом A в сравнении с пациентками с фенотипами B и D. Однако обращали внимание более высокие значения ЛГ, Т, ИСА, АМГ в группе классического фенотипа А в сравнении с девочками с неклассическими фенотипами В и D, которые не достигали статистической значимости, вероятно, в связи с небольшим размером выборки в исследуемых группах. При этом, если сравнивать пациенток во всей выборке девочек с СПКЯ, то фенотип А характеризовался более высоким уровнем ЛГ (10 (6,2–15,2) против 8,6 (5,5–11,5) МЕ/л; р=0,0361, Манна–Уитни тест, Т (1,8 (1,3–2,3) против 1,7 (1,2–2,1) нмоль/л; р=0,0374, Манна–Уитни тест) и ИСА (6±2,9 против 5,5±5,2; р=0,0097, t-критерий Стьюдента) в сравнении с фенотипами В и D.
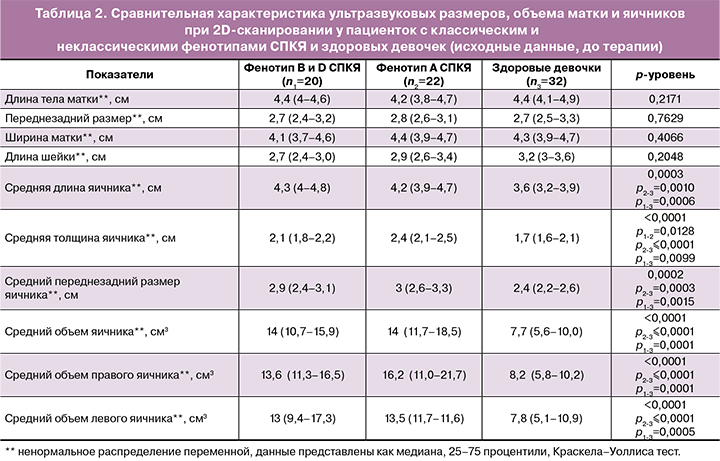
При сравнительном анализе параметров УЗИ выявлено значимое увеличение средней длины, ширины, а также переднезаднего размера яичников в изучаемых группах пациенток с СПКЯ в сравнении с группой контроля. Кроме того, выявлено увеличение как среднего объема яичников, так и левого и правого яичников в отдельности в сравнении с группой здоровых девочек. Однако же значимой разницы между подгруппами пациенток с различными фенотипами выявлено не было (табл. 2).
Все пациентки с классическим фенотипом СПКЯ (n=22) принимали микродозированный КОК с ДРСП по схеме 24+4. Спустя 3 месяца на фоне приема препарата не было выявлено ни одного случая прекращения приема препарата в связи с аллергическими реакциями, указаниями на рвоту, тошноту, упорные головные боли или др. На фоне приема КОК через 6 месяцев закономерная регулярная менструальноподобная реакция наблюдалась у всех пациенток, ни у одной пациентки не было отмечено жалоб на кровяные межменструальные выделения.
Отмечено снижение веса у 6 (30%) пациенток с избыточной массой тела с СПКЯ на 3,7±1,8 кг, еще 10 (50%) пациенток не изменились в весе, при этом у 4 пациенток (20%) отмечена прибавка массы тела на 4,6±2,6 кг, которую девочки связывали с особенностями пищевого рациона.
На фоне приема КОК в течение 6 месяцев 4 из 12 девочек (33,3%), которые предъявляли жалобы на повышенную сальность кожи и угревые высыпания, отметили улучшение состояния и более чистую кожу. Снижение гирсутизма отметили 7 из 13 (53,8%) пациенток, предъявлявших жалобы на избыточный рост волос на теле.
Результат оценки гормонального профиля через 6 месяцев на фоне приема препарата в сравнении с исходными уровнями до начала лечения выявил значимое снижение концентраций ЛГ (3,2 (1,2–5,4) против 11,2 (9–17,4) МЕ/л исходно; p=0,015, тест Уилкоксона, здесь и далее соответственно для непараметрических переменных), Т (1,6 (0,7–2) против 1,8 (1,2–2,2) нмоль/л исходно; p=0,0128, тест Уилкоксона); ИСА (1,4±0,4 против 7,5±3,1 исходно; p=0,0308, t-критерий Стьюдента, здесь и далее для параметрических переменных соответственно); андростендиона (10,2±7,4 против 22,1±6,1 нг/мл исходно; p=0,0165); ДГЭА-С (5,1±1,6 против 7,9±1,7 нг/мл исходно; p=0,0029); при этом отметили повышение уровня ГСПГ (144 (107–180) против 28,6 (22,8–40,3) нмоль/л исходно; p=0,0431).
Однако при сравнении полученных результатов оценки гормонального профиля после лечения было выявлено, что концентрации Т (1,4±0,7 против 0,9±0,3 нг/мл; p=0,008, t-критерий Стьюдента), 17-ОНП (4,5 (4,2–4,6) против 3,5 (2,8–4,1) нг/мл; p=0,0371, Манна–Уитни тест), АМГ (10,9±5,4 против 5,4±2,1 нг/мл; p=<0,0001, Манна–Уитни тест), кортизола (516 (412,5–721,5) против 337 (254–462) нмоль/л; p=0,043095, Манна–Уитни тест) были значимо выше в сравнении с группой здоровых девочек.
Таким образом, уровни Т, 17-ОНП, кортизола, АМГ достоверно снизились после проведенной терапии, однако итоговые значения оставались при этом значимо выше, чем таковые в группе здоровых девочек. Уровни ЛГ, ПРЛ, ДГЭА-С, андростендиона также значимо снизились, однако ни один из приведенных гормонов не достиг референсных значений.
Исходя из полученных результатов, мы можем сделать вывод, что девочкам с СПКЯ необходимо рекомендовать продлить терапию для дальнейшего снижения уровня гормонов до нормативных значений.
По результатам динамического УЗИ органов малого таза размеры яичников оказались значимо меньше, чем до начала приема препарата: объем правого яичника составил 10,6±5,4 см³ против 16,7±6,9 см³ исходно (р=0,0081, t-критерий Стьюдента), объем левого яичника составил 8,1±4,7 против 15,5±7,6 см³ (р=0,0212, t-критерий Стьюдента).
При дальнейшем сравнении полученных результатов с группой здоровых девочек не было выявлено значимых отличий по размерам как левого (9,1 (6,3–10,6) против 7,8 (5,1–10,9) см³; p=0,5724, Манна–Уитни тест), так и правого (10,8 (6,1–12,9) против 8,2 (5,8–10,2) см³; p=0,1554, Манна–Уитни тест) яичника, что говорит о тенденции к нормализации ультразвуковых характеристик яичников у пациенток с классическим фенотипом СПКЯ на фоне лечения.
Пациенткам с неклассическими фенотипами B и D (n=20) была назначена негормональная терапия, на фоне которой значимый клинический эффект в виде полной нормализации менструального цикла достигнут у трети пациенток (7; 31,8% против 0; р=0,0045, точный критерий Фишера), из них до лечения у 6 пациенток наблюдалась олигоменорея, у 1 – аменорея. Также обращает внимание меньшая частота нарушения менструального цикла в группе пациенток с СПКЯ после негормонального лечения в сравнении с исходным уровнем (15; 68,2% против 22;100%; р=0,0045, точный критерий Фишера). У 4 (18,2%) пациенток отмечено возобновление самостоятельного, однако нерегулярного менструального цикла, при этом до лечения у 3 пациенток наблюдалась олигоменорея, у 1 – аменорея. У 11 (50%) пациенток менструальный цикл не нормализовался, из них у 8 (36,4%) пациенток наблюдалась олигоменорея, у 3 (13,6%) сохранялась аменорея.
На фоне лечения у 5 (22,7%) пациенток с СПКЯ с избыточной массой тела было отмечено снижение веса на 6,8±6,6 кг, 13 пациенток (59,1%) не изменились в весе, при этом у 4 пациенток (18,2%) отмечена прибавка веса на 7,9±4,7 кг, которая, со слов пациенток, была обусловлена пищевым поведением и особенностями рациона.
По результатам динамического гормонального исследования выявлено значимое снижение на фоне негормонального лечения инозитолами уровня андростендиона в группе пациенток с фенотипами B и D, при этом итоговая концентрация гормона не отличалась значимо от контрольной группы. Уровни гормонов ЛГ, Т, ИСА, 17-ОНП, ДГЭА-С, АМГ значимо не отличались от исходных концентраций, однако наблюдалась тенденция к снижению этих показателей. При этом итоговые концентрации 17-ОНП, ДГЭА-С не отличались значимо от контрольной группы (табл. 3).
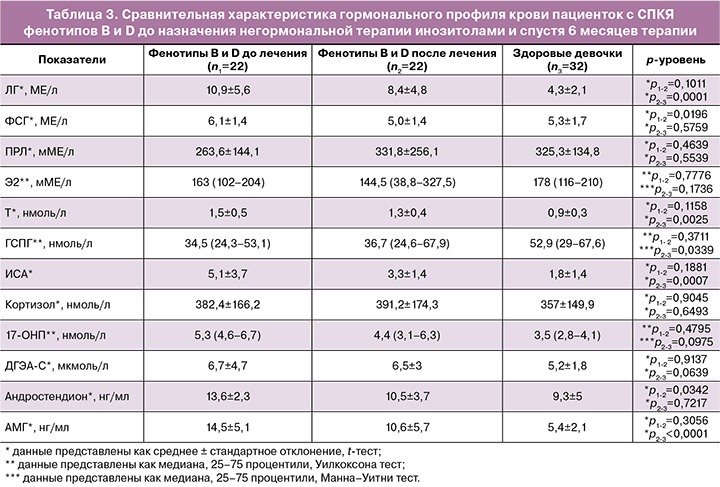
По результатам УЗИ органов малого таза пациенток с СПКЯ фенотипов B и D не было выявлено значимых изменений в объеме левого и правого яичника до и после лечения, однако наблюдается тенденция к снижению объемов обоих яичников. При этом объемы как левого (10,7 (9,1–13,8) против 7,8 (5,1–10,9) см³; p=0,0245, Манна–Уитни тест), так и правого (12,5 (4–18,6) против 8,2 (5,8–10,2) см³; p=0,010, Манна–Уитни тест) яичников остаются значимо выше в сравнении с группой здоровых девочек.
Обращает внимание, что у десятой части пациенток (3; 13,6%) сохранялась аменорея и не отмечен клинический эффект от терапии, из них 2 пациентки характеризовались фенотипом B, одна девочка – фенотипом D. При анализе обследованных пациенток до назначения терапии жалобы на гирсутизм отмечала одна пациентка с фенотипом B и высокими уровнями ДГЭА-С и АМГ, у второй пациентки с фенотипом B отмечено высокое значение ИСА (>4,5). У пациентки с фенотипом D ультразвуковые объемы яичников превышали 15 см3, с большим количеством фолликулов мелкого диаметра, расположенных по всей строме яичников. На фоне негормональной терапии сохранились жалобы на гирсутизм у одной пациентки, при этом было выявлено снижение среднего объема яичников. В связи с отсутствием клинического эффекта пациентки были переведены на гормональную терапию.
Таким образом, на фоне негормональной терапии неклассических фенотипов СПКЯ у половины пациенток через полгода отмечены улучшение клинической картины заболевания по типу восстановления самостоятельных, более регулярных менструальных циклов, тенденция к нормализации основных гормональных показателей и снижению гиперандрогении. Однако длительность терапии в течение 6 месяцев не является достаточной для всей группы пациенток с неклассическими фенотипами СПКЯ для нормализации клинико-лабораторных данных и, несмотря на отмеченную тенденцию к улучшению гормонального фона и ультразвуковых показателей яичников, требует дальнейшего ведения и терапии пациенток. При этом у 13,6% пациенток отмечено отсутствие эффекта от негормональной терапии, что требует дообследования и смены схем лечения.
Заключение
На основании проведенного исследования мы можем сделать следующие выводы.
Для пациенток с СПКЯ в сравнении со здоровыми девочками характерно нарушение менструального цикла с менархе (p<0,0001) по типу олигоменореи (59%) и аменореи (41%), при этом у большинства девочек с СПКЯ менструации отличаются обильностью (p=0,0116) и болезненностью в первые дни (p=0,0015). По результатам анализа гормонального профиля крови подростки с СПКЯ в сравнении со здоровыми девочками характеризуются значимо более высокими уровнями ЛГ, Т, андростендиона, АМГ и ИСА при низком уровне ГСПГ (р<0,0001 для всех показателей), меньшее различие отмечается для 17-ОНР, ДГЭА-С и кортизола (р<0,005 соответственно). Неклассические фенотипы B и D отличаются от классического фенотипа А более высокими уровнями ЛГ, Т, андростендиона и ИСА (р<0,005 для всех показателей).
На фоне лечения пациенток с классическим фенотипом А (АНО+ГА+ПКЯ) микродозированным КОК с ДРСП через 6 месяцев отмечена коррекция косметологических проявлений гиперандрогении (53,8%) и менструального цикла (100%), значимое снижение уровней ЛГ, Т, ИСА, ДГЭА-С, андростендиона (р<0,005 для всех показателей) и ультразвуковых объемов яичников (р<0,005), однако недостаточное для нормализации основных гормональных и ультразвуковых характеристик, что потребовало продолжения терапии.
У половины пациенток с неклассическими фенотипами B (ГА+АНО) и D (АНО+ПКЯ) через 6 месяцев отмечен клинический эффект негормонального лечения средством на основе мио- и D-хироинозитола в соотношении 5:1 в виде восстановления самостоятельного менструального цикла и тенденции к нормализации основных гормональных показателей по уровням ЛГ, Т, ИСА, ГСПГ, не достигших статистической значимости и нормативных значений, что обусловило продолжение негормональной терапии. У 13,6% пациенток с СПКЯ не отмечено клинического эффекта негормональной терапии, что потребовало смены терапии.



