New possibilities for assessing the magnetic resonance characteristics of endometrioid ovarian cysts and their response to dienogest therapy
Chernukha G.E., Solopova A. E., Pronina V.A.
Objective: To evaluate the dynamics of changes in the size of ovarian endometriomas (OMA) in patients receiving the dienogest (DNG) therapy and determine the significant parameters of magnetic resonance imaging (MRI) that affect their reduction.
Materials and methods: This was a prospective study which was conducted at the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, in the period from 2021 to 2023. The study included 24 patients with 37 OMA (average age is 33.54 (7.08) years). The patients received DNG (drug "Zafrilla") at daily dosage 2 mg in a continuous mode. Before the start of therapy and after 6 months of its administration, an MRI of the pelvic organs was conducted with a simultaneous calculation of a number of parameters: the size and volume of cysts with the calculation of the reduction coefficient, the measured diffusion coefficient (MDC), the ratio between the maximum and minimum values of MDC, signal intensity on T2-weighted images. The initial and subsequent levels of AMH and CA-125 were determined in the blood serum.
Results: The analysis of the data showed that DNG therapy leads to a decrease in the volume of OMA by 50% or more in 73% of cases, by 75% or more in every 2nd case of the disease. The full effect of the therapy was noted in every 10th case (100%). The reduction coefficient was significantly influenced by the initial MDC values (r=0.559, p=0.068), the degree of uniformity of the cyst contents (r=0.5491, p=0.0081), the time until its detection (r=-0.4432, p=0.0077), as well as its initial dimensions (<2 cm in diameter, p=0.0015). The higher the initial MDC values correlated with more pronounced effect of therapy. It was shown that at an MDC value of ≥0.792, a decrease in OMA volume by 75% or more with a sensitivity of 90.0% and a specificity of 78.57% can be expected (AUC 0.814 (0.093) [95% CI 0.604;0.942], p=0.0007).
Conclusion: DNG suppressive hormone therapy helps to reduce the volume of OMA by an average of 78.91%. MRI of the pelvic organs is not only a method of diagnosing OMA, but it is also a tool for evaluating the effectiveness of therapy with MDC. MDC value of ≥0.792 may indicate a potential decrease in the volume of OMA by 75% or more, which is important for making a decision about the patient’s management tactics.
Authors' contributions: Pronina V.A. – collecting and processing the material; Pronina V.A., Chernukha G.E. – writing the text; Solopova A.E., Chernukha G.E. – editing the article.
Conflicts of interest: The authors declare no possible conflicts of interest.
Funding: The study was conducted without sponsorship.
Acknowledgements: We express our gratitude to PhD N.D. Simich-Lafitsky for statistical processing and mathematical description of the data obtained in the course of the study.
Ethical Approval: The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients provided an informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Chernukha G.E., Solopova A. E., Pronina V.A. New possibilities for assessing the magnetic resonance characteristics of endometrioid ovarian cysts and their response to dienogest therapy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (6): 114-122 (in Russian)
https://dx.doi.org/10.18565/aig.2024.56
Keywords
One of the most common forms of extragenital endometriosis is ovarian endometrioma (OMA). In terms of prevalence among gynecologic pathologies, this disease may account for up to 50% [1, 2]. According to the recommendations of the European Society of Human Reproduction and Embryology (ESHRE, 2022), OMA can be diagnosed by imaging studies such as echography or magnetic resonance imaging (MRI) of the pelvic organs. Laparoscopy with histologic verification of the diagnosis is not necessary because both ultrasound and MRI have high diagnostic accuracy: the sensitivity of ultrasound is 89–94%, its specificity is 77–86%; sensitivity and specificity of MRI are 88–91% and 92–95%, respectively [3, 4].
To date, the algorithm for the management of patients with OMA is quite clearly defined. If the size of OMA is less than 3 cm in diameter and there are no other indications for surgical treatment, drug therapy can be considered in women of reproductive age [3]. This approach has benefits due to the fact that ovarian surgery leads to irreversible decrease in ovarian reserve with a tendency to its further decrease if there are large OMAs, bilateral endometriomas, and subsequent repeated operations [5, 6]. It is worth noting that the recurrence rate of OMAs after their removal is quite high and can reach 40–50% in cases when subsequent suppressive hormone therapy is not prescribed or when it is administered in short courses [7, 8]. However, due to the lack of patient compliance and low awareness of doctors regarding the need for long-term postoperative therapy, it is quite difficult to follow the principle of «one operation for endometriosis» which was proposed by Chapron C. et al. [9]. It should be noted that the effectiveness of suppressive hormone therapy, the optimal duration of its administration, as well as the possibility of maintaining the effect after withdrawal of hormone therapy are still debatable.
The findings in the literature suggest that not all OMAs respond equally well to hormone therapy with progestogens or gonadotropin-releasing hormone agonists. However, questions about possible predictors of response to therapy are still poorly understood [10–14]. Thus, the study conducted by Matsuura M. et al. showed that almost every second patient (42.3%) had only a 25% or less decrease in OMA size after two months of dienogest (DNG) therapy. Moreover, the authors demonstrated that a decrease in OMA volume was statistically significantly associated with the value of the measured diffusion coefficient (MDC) which was determined by performing MRI of the pelvic organs (correlation coefficient is 0.655, p=0.0003) [14]. Subsequent scientific studies in this area were not found in the literature, but this approach seems to be interesting and promising. Therefore, the objective of the study (interim results are presented in this publication) was to evaluate the dynamics of OMA volume in patients receiving DNG therapy and to assess the significance of MDC for determining the response to therapy.
Materials and methods
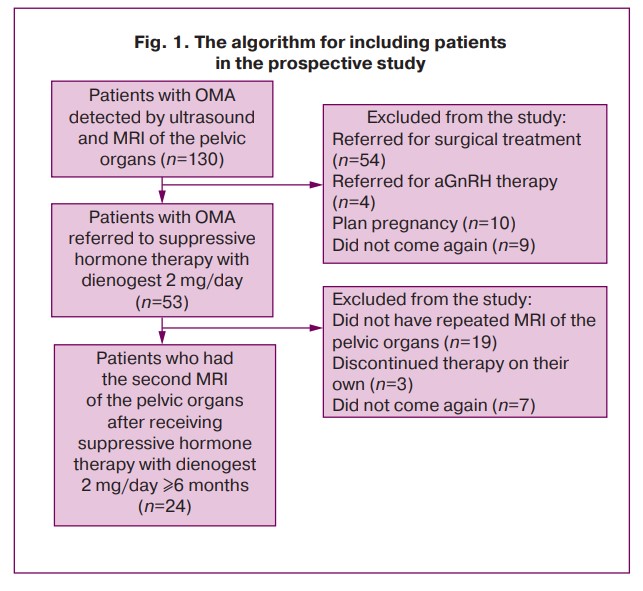
One hundred and thirty reproductive-aged women with OMA revealed by ultrasound and MRI of the pelvic organs were recruited to participate in the study; DNG therapy was administered to 53 women. The final sample of the prospective study included 24 patients with 37 OMAs (average age is 33.54 (7.08) years, average body mass index is 20.17 (18.87; 21.93) kg/m2); the patients had repeated MRI of the pelvic organs when receiving therapy. The algorithm for including the participants in the study is presented in Figure 1.
Statistical analysis
The following parameters were calculated for quantitative indicators: mean (M), standard deviation (SD), median (Me), interquartile range (Q1; Q3). Frequencies (%) were calculated for qualitative and ordinal indicators. All the obtained quantitative parameters were screened for conformity to normal distribution using the Shapiro–Wilk test. Numerical parameters that have normal distribution are presented in M (SD) format, where M is the mean value and SD is the standard deviation of the mean value. The parameters that have a distribution different from normal are presented in the format Me (Q1; Q3). In order to find the differences between the groups of patients, the groups were compared in pairs using the nonparametric Mann–Whitney U test. The differences between the groups of patients before and during therapy were determined with the help of pairwise Wilcoxon test. The correlation analysis was performed using the Spearman’s rank correlation coefficient. MDC threshold was assessed using ROC analysis. The AUC (Area Under Curve) parameter was used to obtain a numerical value of the clinical significance of the test, as well as to compare the two tests, with an indication of the confidence interval; the quality of the test can be estimated using an expert scale for the AUC values. The statistical analysis was performed using MedCalc statistical software (version 20.104 — 64-bit). The results were considered statistically significant at a significance level of 0.05.
Results
The average OMA diameter in the patients included in the study was 2.1 (1.4; 2.5) cm, ranging from 4.6 cm to 0.6 cm. Unilateral localization was noted in 79.2% of cases, with left OMAs occurring five times more frequently than the right ones (p=0.0001). Every fourth patient had recurrence of OMA after previous surgical intervention (6/24, 25.0%). Approximately every second patient had concomitant functional ovarian cysts and every sixth patient had uterine myoma (Table 1).
When comparing the main MR-parameters before inclusion in the study and during the suppressive therapy of DNG, a 5-fold decrease in the OMA volume and a 1.5-fold decrease in the maximum diameter were observed. Its density increased on average by 3 times with the decrease in the volume and diameter of the cyst. Despite the tendency to decrease MDC during therapy, its values did not reach statistical significance, which is probably due to the initial small size of OMAs (Table 2).
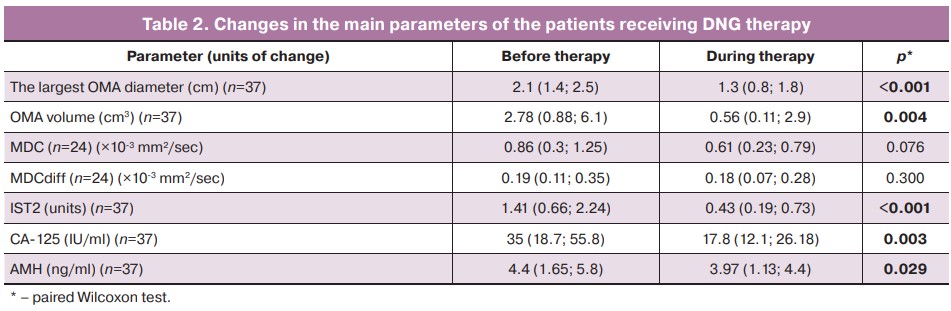
On average, OMA volume was reduced by 78.91% (46.87; 91;08): the volume was reduced by less than 25% in 13.5% (5/37) of cases, less than 50% in 13.5% (5/37), between 50% and 74% in 16.2% (6/37), and 75% or more in 56.8% (21/37) of cases. At the same time, 2.7% (1/37) of cases showed significant negative dynamics (+353.8% of volume), 2.7% (1/37) showed insignificant negative dynamics (+17.8% of volume), 2.7% (1/37) showed no change in OMA volume, and 10.8% (4/37) showed complete effect of the therapy (100%) (Fig. 2).
The targeted analysis of the data showed that the reduction in OMA volume and MDC was influenced by the initial OMA size. Thus, the patients with OMAs <2 cm in diameter showed a reduction in their size by 85.76% (76.09;98.22), and the patients with cysts ≥2 cm in diameter showed only a 63.04% (39.28;81.27) reduction (p=0.0346). The MDC was statistically significantly higher in cysts <2 cm than in cysts ≥2 cm and was 0.76 (0.65;1.04) and 0.23 (0.08;0.50), respectively (p=0.0015). MDCdiff values were also higher in cysts less than 2 cm in diameter, although no statistically significant differences were obtained (0.23 (0.18;0.37) and 0.09 (0.06;0.19), respectively, p=0.0610). The evaluation of the groups of patients who received DNG for 6 and 12 months showed no statistically significant difference in reduction rate (75.41% (48.87;90.38) and 80.56% (44.82;95.67), p=0.7569) and MDC value (1.14 (0.29;1.70) and 0.79 (0.35;0.99), respectively, p=0.4940).
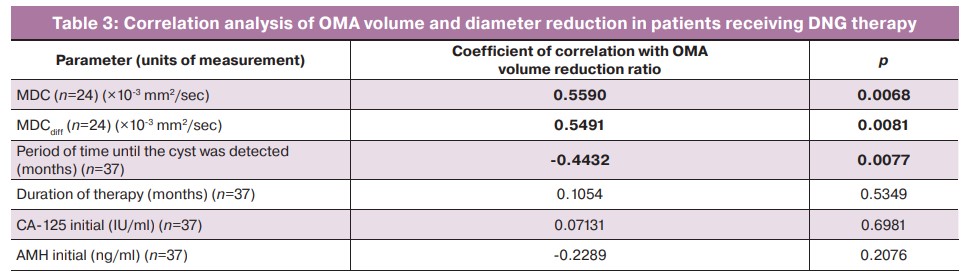
The percentage of MDC volume reduction correlated with MDC: the greater the values of MDC and MDCdiff were, the more the cyst decreased in the patients receiving therapy. The change in OMA volume was also influenced by the period of time before its detection: the older the cyst was, the worse it responded to hormone therapy. The reduction in OMA volume was not affected by baseline CA-125 and AMH levels (Table 3).
A more significant decrease in OMA volume in patients receiving therapy was observed at higher values of MDC. Thus, the initial MDC values were 0.3 (0.2;0.54) ×10-3 mm2/sec in the case of a reduction factor of less than 50%, 0.78 (0.21;1.67) ×10-3 mm2/sec in the case of an OMA reduction of 50-74%, and already 1.23 (0.99;2.01) ×10-3 mm2/sec in the case of a reduction factor of ≥75%. The difference between MDC values at a reduction ratio of less than 50% and greater than or equal to 75% was statistically significant (Mann–Whitney U test, p=0.0002).
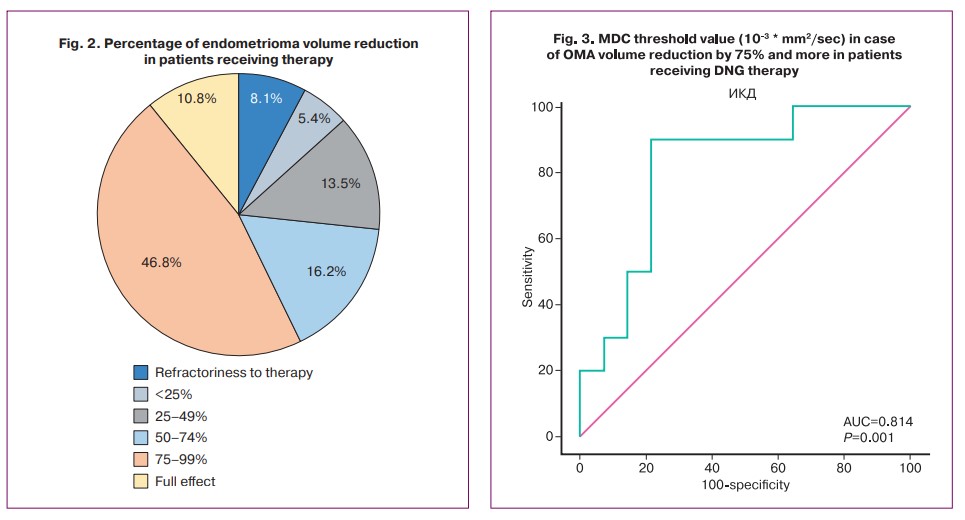
Given the relationship between MDC value and OMA volume reduction ratio, a ROC analysis of baseline MDC values was performed to determine its threshold values for possible prediction of endometrioma response to hormone therapy. It was found that MDC value ≥0.792 could be expected to reduce OMA volume by 75% or more with a sensitivity of 90.0% and specificity of 78.57% (AUC 0.814 (0.093) [95% CI: 0.604–0.942], p=0.0007) (Fig. 3).
Discussion
Despite the fact that OMA can be diagnosed quite accurately by imaging methods, the issue of further management of this group of patients is still debatable [3]. If the tactics for peri- and postmenopausal women are quite clearly defined, including the tendency to increase the risk of ovarian cancer development with age [19], the tactics for women of reproductive age with OMA size less than 3 cm in diameter are quite unclear. In this regard, determining the predictors of the effectiveness of response to hormone therapy is relevant. The results of the study showed that every second case showed a 75% or more reduction in OMA volume in patients receiving DNG for 6 months or more; the mean values of the reduction ratio were 78.91% and were independent of the duration of therapy (correlation coefficient is 0.1054, p=0.5349). In every tenth case, there was a complete effect, which is recorded as the absence of OMA visualization by the control MRI of the pelvic organs. We realize that due to the lack of laparoscopic and histologic follow-up, these results should be interpreted carefully, as there are often difficulties in the differential diagnosis of OMA and other cysts with hemorrhagic contents, such as corpus luteum cysts with hemorrhage. However, MR diagnosis of endometriomas is currently considered to be quite accurate; OMAs, unlike corpus luteum cysts, are characterized by hypointense signal on T1WI without loss of signal intensity in fat suppression mode, as well as «darkening sign» on T2WI due to high concentration of protein and iron inside the cyst caused by repeated cyclic hemorrhage [20].
The issue of preserving the ovarian reserve in patients with endometriomas, including the patients receiving hormone therapy, deserves special attention. There are few literature data on this issue. According to the results of some publications, OMAs can have local toxic and mechanical negative effects on ovarian tissue [21]. Nevertheless, Muzii L. et al. showed that patients who received DNG therapy for 6 months with a significant decrease in OMA volume (on average by 79%) did not demonstrate a statistically significant decrease in AMH (3.40 (2.32) ng/ml baseline and 2.80 (1.90) ng/ml after 6 months, -18%, p=0.27) [22]. According to the present study, AMH reduction was observed only by 9.8% (4.4 (1.65; 5.8) ng/ml baseline and 3.97 (1.13; 4.4) ng/ml during therapy). Although this decrease was statistically significant according to our calculations, it can be assumed that a difference of less than 10% may be due to AMH variability and small sample size, which needs to be clarified in future studies.
According to the results of the study, it was found that the dynamics of OMA sizes in patients receiving DNG therapy can be influenced by the initial values of MDC, MDCdiff, as well as the initial diameter of OMAs and period of time until their detection. MDC and MDCdiff values were shown to correlate with the reduction ratio (r=0.5590 and r=0.5491 respectively, p<0.01). The highest initial MDC values correlated with the more marked effect of therapy. We were able to establish a threshold MDC value on the basis of these results, which allows us to assume the effect of hormone therapy for DNG at the stage of its prescription. The reduction in OMA volume by ≥75% with a sensitivity of 90.0% and specificity of 78.57% (AUC 0.814 (0.093) [95% CI: 0.604–0.942], p=0.0007) can be expected in case of MDC ≥0.792.
The measured diffusion coefficient reflects the degree of mobility of the water molecule in the study area; the lower coefficient refers to the denser contents of the mass. The results of the meta-analysis conducted in 2022 suggest that benign ovarian masses can be differentiated from malignant ovarian masses based on MDC with an average sensitivity and specificity of 0.87 [95% CI: 0.80–0.92] and 0.80 [95% CI: 0.71–0.87] [23]. Our data demonstrate that MDC had a tendency to decrease in patients receiving therapy, which is associated with the suppression of cyclic hemorrhages inside the cysts and a decrease in their size. The statistically significant decrease in cyst signal intensity on T2-weighted images by an average of 3-fold (p<0.001) also confirmed this fact. In addition, the initial larger value of MDCdiff was also found to increase the reduction ratio. Both MDC and MDCdiff were higher in OMAs of smaller diameter compared to OMAs ≥2 cm. This fact in combination with the negative correlation with time to cyst detection (r=-0.4432, p=0.0077) indicates the significance of early verification of endometriosis in women of reproductive age. It can be assumed that the older OMAs are represented by dense viscous content, making up the majority of the cavity of the mass; they respond worse to hormone therapy. Larger cysts are probably characterized by more active blood loss; given some stiffness of the ovarian capsule, this contributes to the formation of a denser and more homogeneous content within.
One patient showed negative dynamics when receiving DNG therapy. In this case, the time from OMA detection to initiation of therapy was 36 months. The cyst volume increased by 4.5 times over 12 months of hormone therapy, and when baseline MDC values were measured, there was a relatively low value of 0.508. Subsequently, the patient was referred for surgical treatment due to the ineffectiveness of conservative therapy, and the diagnosis of OMA was confirmed. Based on this, it can be concluded that for a more accurate assessment of the effect of therapy, it is advisable to analyze a combination of several risk factors, which will be screened in the future on a larger sample of patients. Moreover, there is such a thing as progesterone resistance; this to a certain extent can explain the lack of effect from conservative therapy in a number of patients with endometriosis [24, 25]. DNG is a progestogen with high selectivity for progesterone receptors, that is why the issue of the expediency of replacing one progestogen with another in this situation is controversial and requires randomized clinical trials [16, 17, 26]. Thus, DNG is the drug of choice for the treatment of extragenital endometriosis. In view of the need for long-term hormone therapy, the question arises about the choice of a drug that would be effective and safe along with the economic component, which often significantly affects the compliance of patients. Therefore, an alternative to the original drug is the medication «Zafrilla» (Gedeon Richter OJSC, Hungary). Zafrilla contains a micronized form of progestogen, which ensures its rapid absorption, high bioavailability and long-term maintenance of therapeutic concentration; bioequivalence with the original molecule is 96–99%. Studies have demonstrated high clinical efficacy of the drug and the possibility of its use as prevention of endometriosis recurrences [27, 28].
Thus, it is advisable to perform control MRI 6 months after the start of hormone therapy and then decide whether to continue the therapy or refer the patient for surgical treatment with subsequent long-term anti-relapse suppressive hormone therapy. The study presented preliminary results. At present, patient recruitment and evaluation of OMA volume changes with longer DNG therapy are still in progress, and more accurate data may be obtained; on the basis of these findings, we may be able to assume more precisely the potential reduction of cysts, as well as make a conclusion about the possible presence of a «plateau» in the dynamics of their reduction.
Conclusion
The results of the study confirm the high efficacy of DNG in OMA, which was expressed in the reduction of their volume after 6 months of therapy by 75% and more in every second case. The reduction ratio was significantly influenced by the initial MDC values, the degree of homogeneity of the cyst contents (MDCdiff), the duration of its persistence, and its initial size (<2 cm in diameter). In this case, the higher initial MDC values correlate with more significant effect of therapy. MDC value of ≥0.792 may indicate a potential decrease in the volume of OMA by 75% or more, which is important for making a decision about the patient’s management tactics.
References
- Blum S., Fasching P.A., Hildebrandt T., Lermann J., Heindl F., Born T. et al. Comprehensive characterization of endometriosis patients and disease patterns in a large clinical cohort. Arch. Gynecol. Obstet. 2022; 305(4): 977-84. https://dx.doi.org/10.1007/s00404-021-06200-w.
- von Theobald P., Cottenet J., Iacobelli S., Quantin C. Epidemiology of endometriosis in France: a large, nation-wide study based on hospital discharge data. Biomed. Res. Int. 2016; 2016: 3260952. https://dx.doi.org/ 10.1155/2016/3260952.
- Becker C.M., Bokor A., Heikinheimo O., Horne A., Jansen F., Kiesel L. et al.; ESHRE Endometriosis Guideline Group. ESHRE guideline: endometriosis. Hum. Reprod. Open. 2022; 2022(2): hoac009. https://dx.doi.org/10.1093/hropen/hoac009.
- Bazot M., Lafont C., Rouzier R., Roseau G., Thomassin-Naggara I., Daraï E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil. Steril. 2009; 92(6): 1825-33. https://dx.doi.org/10.1016/j.fertnstert.2008.09.005.
- Moreno-Sepulveda J., Romeral C., Niño G., Pérez-Benavente A. The effect of laparoscopic endometrioma surgery on anti-müllerian hormone: a systematic review of the literature and meta-analysis. JBRA Assist. Reprod. 2022; 26(1): 88-104. https://dx.doi.org/10.5935/1518-0557.20210060.
- Muzii L., Achilli C., Lecce F., Bianchi A., Franceschetti S., Marchetti C. et al. Second surgery for recurrent endometriomas is more harmful to healthy ovarian tissue and ovarian reserve than first surgery. Fertil. Steril. 2015; 103(3): 738-43. https://dx.doi.org/10.1016/j.fertnstert.2014.12.101.
- Maul L.V., Morrision J.E., Schollmeyer T., Alkatout I., Mettler L. Surgical therapy of ovarian endometrioma: recurrence and pregnancy rates. JSLS. 2014; 18(3): e2014.00223. https://dx.doi.org/10.4293/JSLS.2014.00223.
- Seo J.W., Lee D.Y., Yoon B.K., Choi D. The age-related recurrence of endometrioma after conservative surgery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 208: 81-5. https://dx.doi.org/10.1016/j.ejogrb.2016.11.015.
- Chapron C., Marcellin L., Borghese B., Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019; 15(11): 666-82. https://dx.doi.org/10.1038/s41574-019-0245-z.
- Sugimoto K., Nagata C., Hayashi H., Yanagida S., Okamoto A. Use of dienogest over 53 weeks for the treatment of endometriosis. J. Obstet. Gynaecol. Res. 2015; 41(12): 1921-6. https://dx.doi.org/10.1111/jog.12811.
- Uludag S.Z., Demirtas E., Sahin Y., Aygen E.M. Dienogest reduces endometrioma volume and endometriosis-related pain symptoms. J. Obstet. Gynaecol. 2021; 41(8): 1246-51. https://dx.doi.org/10.1080/01443615.2020.1867962.
- Lee J.H., Song J.Y., Yi K.W., Lee S.R., Lee D.Y., Shin J.H. et al. Effectiveness of dienogest for treatment of recurrent endometriosis: multicenter data. Reprod. Sci. 2018; 25(10): 1515-22. https://dx.doi.org/10.1177/1933719118779733.
- Sugimura K., Okizuka H., Kaji Y., Imaoka I., Shiotani S., Mukumoto H. et al. MRI in predicting the response of ovarian endometriomas to hormone therapy. J. Comput. Assist. Tomogr. 1996; 20(1): 145-50. https://dx.doi.org/10.1097/00004728-199601000-00026.
- Matsuura M., Tamate M., Tabuchi Y., Takada S., Tanaka R., Iwasaki M. et al. Prediction of the therapeutic effect of dienogest in ovarian endometrial cysts using the apparent diffusion coefficient. Gynecol. Endocrinol. 2014; 30(8): 597-9. https://dx.doi.org/10.3109/09513590.2014.911277.
- Bazot M., Bharwani N., Huchon C., Kinkel K., Cunha T.M., Guerra A. et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur. Radiol. 2017; 27(7): 2765-75. https://dx.doi.org/10.1007/s00330-016-4673-z.
- Méndez Fernández R., Barrera Ortega J. Magnetic resonance imaging of pelvic endometriosis. Radiologia. 2017; 59(4): 286-96. https://dx.doi.org/10.1016/j.rx.2017.02.002.
- Khashchenko E.P., Uvarova E.V., Fatkhudinov T.K., Chuprynin V.D., Asaturova A.V., Kulabukhova E.A. et al. Endometriosis in adolescents: diagnostics, clinical and laparoscopic features. J. Clin. Med. 2023; 12(4): 1678. https://dx.doi.org/10.3390/jcm12041678.
- Thomeer M.G., Steensma A.B., van Santbrink E.J., Willemssen F.E., Wielopolski P.A., Hunink M.G. et al. Can magnetic resonance imaging at 3.0-Tesla reliably detect patients with endometriosis? Initial results. J. Obstet. Gynaecol. Res. 2014; 40(4): 1051-8. https://dx.doi.org/10.1111/jog.12290.
- He Z.X., Shi H.H., Fan Q.B., Zhu L., Leng J.H., Sun D.W. et al. Predictive factors of ovarian carcinoma for women with ovarian endometrioma aged 45 years and older in China. J. Ovarian. Res. 2017; 10(1): 45. https://dx.doi.org/10.1186/s13048-017-0343-2.
- Thalluri A.L., Knox S., Nguyen T. MRI findings in deep infiltrating endometriosis: A pictorial essay. J. Med. Imaging Radiat. Oncol. 2017; 61(6): 767-73. https://dx.doi.org/10.1111/1754-9485.12680.
- Чернуха Г.Е., Пронина В.А., Солопова А.Е. Современные возможности оптимизации диагностики и терапии эндометриоидных кист яичников. Акушерство и гинекология. 2023; 11: 28-35. [Chernukha G.E., Pronina V.A., Solopova A.E. Modern possibilities for optimizing the diagnosis and therapy of ovarian endometriomas. Obstetrics and Gynecology. 2023; (11): 28-35. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.168.
- Muzii L., Galati G., Di Tucci C., Di Feliciantonio M., Perniola G., Di Donato V. et al. Medical treatment of ovarian endometriomas: a prospective evaluation of the effect of dienogest on ovarian reserve, cyst diameter, and associated pain. Gynecol. Endocrinol. 2020; 36(1): 81-3. https://dx.doi.org/10.1080/09513590.2019.1640199.
- Hu X., Liang Z., Zhang C., Wang G., Cai J., Wang P. The diagnostic performance of maximum uptake value and apparent diffusion coefficient in differentiating benign and malignant ovarian or adnexal masses: a meta-analysis. Front. Oncol. 2022; 12: 840433. https://dx.doi.org/10.3389/fonc.2022.840433.
- Flores V.A., Vanhie A., Dang T., Taylor H.S. Progesterone receptor status predicts response to progestin therapy in endometriosis. J. Clin. Endocrinol. Metab. 2018; 103(12): 4561-8. https://dx.doi.org/10.1210/jc.2018-01227.
- Zhang P., Wang G. Progesterone resistance in endometriosis: current evidence and putative mechanisms. Int. J. Mol. Sci. 2023; 24(8): 6992. https://dx.doi.org/10.3390/ijms24086992.
- Ярмолинская М.И., Флорова М.С. Возможности терапии диеногестом 2 мг у больных наружным генитальным эндометриозом. Проблемы репродукции. 2017; 23(1): 70-9. [Iarmolinskaia M.I., Florova M.S. The possibility of treatment with dienogest 2 mg in patients with genital endometriosis. Russian Journal of Human Reproduction. 2017; 23(1): 70-9. (in Russian)]. https://dx.doi.org/10.17116/repro201723170-79.
- Оразов М.Р., Радзинский В.Е., Орехов Р.Е., Таирова М.Б. Эффективность профилактики рецидивов после хирургического лечения эндометриоза яичников. Вопросы гинекологии, акушерства и перинатологии. 2022; 21(3): 53-62. [Orazov M.R., Radzinsky V.E., Orekhov R.E., Tairova M.B. Effectiveness of medical therapy for preventing ovarian endometriosis recurrence after surgical treatment. Gynecology, Obstetrics and Perinatology. 2022; 21(3): 53-62. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2022-3-53-62.
- Оразов М.Р., Радзинский В.Е., Орехов Р.Е. Эффективность терапии эндометриоз-ассоциированной тазовой боли, резистентной к хирургическому лечению. Гинекология. 2021; 23(4): 314-23. [Orazov M.R., Radzinsky V.E., Orekhov R.E. The effectiveness of therapy for endometriosis-associated pelvic pain resistant to surgical treatment. Gynecology. 2021; 23(4): 314-23. (in Russian)]. https://dx.doi.org/10.26442/20795696.2021.4.201097.
Received 15.03.2024
Accepted 30.05.2024
About the Authors
Galina E. Chernukha, Dr. Med. Sci., Professor, Chief Researcher, obstetrician-gynecologist at the Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,+7(985)999-60-00, c-galina1@yandex.ru, https://orcid.org/0000-0002-9065-5689
Alina E. Solopova, Dr. Med. Sci., Associate Professor, Leading Researcher at the Department of Radiology, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(985)432-68-52, a_solopova@oparina4.ru,
https://orcid.org/0000-0003-4768-115X
Veronika A. Pronina, obstetrician-gynecologist, PhD student, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(916)025-86-26, ver22595@yandex.ru, https://orcid.org/0000-0003-4566-4065
Corresponding author: Galina E. Chernukha, c-galina1@yandex.ru



