Specific features of molecular mechanisms of vaginal secretion in women with decline in sexual function in assisted reproductive technology programs
Women experiencing infertility compared to fertile women are more likely to have sexual dysfunction, including lack of genital arousal, dyspareunia. Sex steroids have a key effect on the morphological and functional condition of the urogenital tract. Aim. To explore specific features of gene expression of vaginal transcellular secretion factors (AQP3, ESR1, VEGF121, and VEGF165) in ovarian stimulation (OS) for IVF and in natural cycle IFV (NC-IVF) in women with decline in sexual function. Materials and methods. The study included 47 women with decline in sexual function: 24 women underwent OS for IVF, and 23 women underwent treatment for NC-IVF. Female sexual function was assessed using clinical interviews and the FSFI questionnaire. Results. The patients in group 1 (OS for IVF) were younger (p <0.001) and duration of their infertility was shorter (p<0.001), the level of serum anti-Mullerian hormone (AMH) was higher (p<0.001) and the level of follicle-stimulating hormone (FSH) was lower (p<0.001) compared to patients in group 2 (NC-IVF). Group 1 showed higher median values for the female sexual function index (FSFI) in 3 domains “erousal” (p=0.05), “lubrication” (p=0.04), and “desire” (p=0.09). PCR analysis in patients with OS found a high level of ESR1 expression (p=0.012), VEGF121 (p=0.01), a tendency for high AQP3 values (p=0.09) compared to patients in group 2 (NC-IVF); the level of VEGF165 expression was comparable in both groups. High levels of ESR1, VEGF 121, AQP3 expression in patients with OS were associated with higher FSFI scores in domains “erousal”, “lubrication”, “desire”. Conclusion. The obtained results allowed to determine some pathogenetic mechanisms of impairment of vaginal secretion, genital sexual arousal, as well as development of dyspareunia.Stenyaeva N.N., Krasnyi A.M., Khritinin D.F., Burduli A.G., Sadekova A.A., Kostava M.N., Kalinina E.A.
Keywords
Infertility has a negative effect on the marital relationships and in general, on the quality of life of married couples [1–3]. Infertile women, in comparison with fertile women, are more likely to experience a decline in sexual function, which manifests not only by low sexual desire, but also by difficulties in sexual arousal, low orgasmicity, lubrication impairment and dyspareunia [4, 5].
Psychophysiological processes associated with the realization of sexual response in women are provided by the sequential activation of the morphofunctional complexes of the copulatory cycle, to which the genital segmental component is attributed and includes receptor secretory structures, the neuromuscular apparatus of the genital organs and cerebrospinal nervous centres and neural pathways [6]. Sexual arousal manifests by neurovascular event, which involves an increase in vaginal and clitoral blood flow, vasocongestion, genital engorgement, and lubrication [7]. Impaired genital response to sexual stimulation is often associated with dyspareunia.
Sex steroids have a key effect on the morphofunctional status of the urogenital tract. Various effects of estrogens on urogenital tissue in women, which are involved in the processes of cell maturation, activation of vaginal blood flow, and lubrication are the most studied issues. Estrogens affect the maintenance of vaginal smooth muscle density, blood vessel morphology and the density of nerve endings in the mucosal epithelium [8]. Nuclear estrogen receptors ERα, which are encoded by ESR1 gene, are present in vaginal epithelial cells. ERα regulates the expression of various protein molecules, including aquaporins (AQPs) and vascular endothelial growth factors [9, 10]. Also, it is known that estrogens can directly affect the expression of ESR1 gene. The studies show that estrogens can both increase and decrease ESR1 expression in various tissues [11].
AQPs are proteins that allow transportation of water, glycerol and other small neutral solutes through cell membranes. Kim S.O. et al. have studied the localization and expression of AQP1-9 in vaginal tissues in perimenopausal women, their study showed that AQP1 was mainly expressed in capillaries and venules, AQP2 was expressed in the cytoplasm of the epithelial cells; AQP3 was mainly associated with the plasma membrane of the vaginal epithelium, while AQP5 and AQP6 was expressed in the cytoplasm of all vaginal epithelial cells [12]. Thus, according to Lee H.S. et al., it is AQP3, that could be considered as a potential component of the vaginal lubrication mechanism [13].
Vascular endothelial growth factor (VEGF) is a cytokine with at least 8 isoforms produced by cells to stimulate vasculogenesis and angiogenesis. VEGF-A significantly increases vascular permeability, thereby allowing extravasation of proteins and other molecules from the blood vessels [14]. It is interesting to note that in addition to the determining role in angiogenesis, the data on the neurotrophic and neuroprotective activity of VEGF-A have been accumulated over recent years. [15, 16].
VEGF121 and VEGF165 are signaling proteins that perform the functions necessary for the normal vaginal secretions; moreover, it is known that the VEGF121 isoform mainly regulates vascular permeability, while VEGF165 induces angiogenesis [17].
During natural menstrual cycles, serum estrogen levels increase in parallel with folliculogenesis and usually decrease after initial release of luteinizing hormone (LH) [18]. Controlled ovarian stimulation (OS) is used in IVF cycles, in this regard, the changes in estrogen levels are an important component in assessment of the ovarian and endometrial response to stimulation [19].
Treatment of infertility in women using assisted reproductive technologies (ART) showed that estrogen levels were higher than their levels during spontaneous ovulation. Carosso A. et al studied the influence of supraphysiological estrogen levels on the vaginal and endometrial microbiota. They found that OS significantly alters the composition of the vaginal and endometrial microbiota [20].
Currently, the properties of vaginal secretion with reduced sexual function in women, as well as the influence of OS on the molecular mechanisms of vaginal transcellular secretion, have not been studied.
Aim of the study: to explore specific features of gene expression of vaginal transcellular secretion factors (AQP3, ESR1, VEGF121, and VEGF165) in ovarian stimulation (OS) for IVF and in natural cycle IFV (NC-IVF) in women with decline in sexual function.
Materials and methods
Examination and treatment of patients, as well as material collection were carried out in Professor B.V. Leonov Department of Assisted Reproductive Technologies and in the Outpatient Department of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. The study included 47 women with decline in sexual function. Group 1 included 24 women, who underwent OS for IVF, and group 2 included 23 women, who underwent treatment in natural cycle IFV (NC-IVF).
The study included the women aged 21–46 years with tuboperitoneal factor in infertility and/or with male factor in infertility, who signed informed consent to participate in the study. Mandatory inclusion criteria were permanent sexual partner, normal karyotype of spouses/partners; normal body mass index (BMI) of a woman or overweight (BMI 19–30.0 kg/m2). The women with III/IV grade of external genital endometriosis, malformations of internal reproductive organs, acute pelvic inflammatory diseases, cyst/tumor-like formations of the ovaries, uterine myoma more than 4 cm or submucous location of myoma; oncologic diseases and the patients, who were planned to undergo donor egg IVF were not included in the study. The married couples/partners were examined according to “About the use of assisted reproductive technologies”.
The protocol of the study was approved by the Ethical Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
In the group of women with OS, recombinant FSH(rFSH)/GnRH antagonist protocols, or (rFSH) and recombinant Luteinizing Hormone (rLH) or Human menopausal gonadotropins (HMGs) treatment protocols were used; administration of gonadotropins started on the 2nd–4th days of menstrual cycle. For OS, the drugs dosage was prescribed considering the following parameters of each woman: age, anti-Mullerian hormone (AMH) level, the number of antral follicles visualized on pelvic ultrasound (US). Dosage correction of rFSH/rFSH+rLH/HMGs was implemented in accordance with ovarian response to ovarian stimulation. To prevent endogenous LH peaks, daily GnRH antagonist administration began when the diameter of the dominant follicle was more than 14 mm. The dose of 10 000 IU chorionic gonadotropin (hCG) or the dose of 0.2 mg GnRH agonist at the risk ovarian hyperstimulation syndrome (OHSS) and the final dose of antGnRH were prescribed when the diameter of the dominant follicle was more than 17–17.5 mm, when visualized on pelvic US.
Natural cycle IVF was chosen for patients in group 2 due to diminished ovarian reserve. The dose of 5000 IU hCG was prescribed, when the dominant follicle reached a diameter of 17–17.5 mm.
The women under the study underwent clinical interviews, the diagnosis was verified in accordance with ICD-10. Female sexual function was assessed using the FSFI questionnaire in domains: “desire”, “arousal”, “lubrication”, “orgasm”, “satisfaction”, “pain” and FSFI total score. The boundary score between clinical and preclinical cutoff scores was 26.55 points [21].
Sampling of material for analysis in both groups was carried out on the day of transvaginal ovarian puncture. After treatment with saline using urogenital probe type D (cervical cytobrush), the epithelium was collected from anterior wall of the outer third of the vagina.
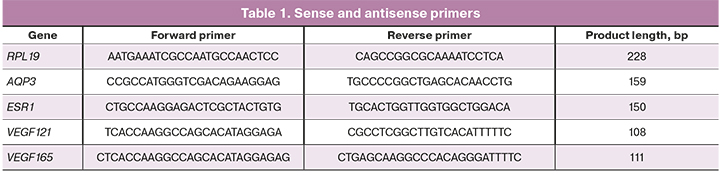
The samples of vaginal epithelium were placed in lysis buffer. Total RNA isolation from epithelial scrapings prelabeled on magnetic particles was performed using Silex kit (Russia) according to the manufacturer’s protocol. Concentration and purity of total RNA isolation was assessed using DeNovix spectrophotometer (USA). A reverse transcription reaction was performed using OT-1 kit (“Syntol”, Russia). The synthesized cDNA was used for quantitative polymerase chain reaction (PCR). The PCR amplification consisted of denaturation for 5 min at 95ºC, annealing and extention for 10 sec at 95ºС, for 20 sec at 60ºС and 35 amplification cycles. RPL19 gene was used as a reference gene (Table 1).
Statistical analysis
Statistical analysis was carried out using Attastat (USA) and Origin (USA) software packages. The data were presented by median (Me) and interquartile range (Q1, Q3). Analysis of continuous variables was performed using the Mann-Whitney U-test, and categorical variables were evaluated using Fisher's exact test. The diagrams were presented in the form of a swing chart (5%, Q1, Me, Q3, 95%). The differences were statistically significant at p<0,05.
Results
The analysis found the differences in socio-demographic, clinical-anamnestic data and laboratory results in patients included in the study (Table 2).
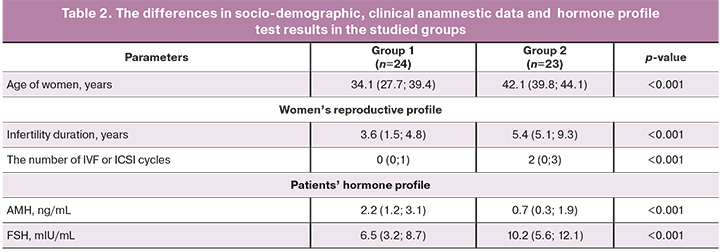
The analysis of clinical anamnestic data of the participants of the study found that the patients in group 1 were significantly younger compared to group 2. (р<0,001). The studied groups were comparable in terms of menstrual function (age of menarche, duration of the menstrual cycle and menstrual bleeding), as well as the age of sexual debut.
The patients had the following somatic diseases: chronic tonsillitis, pharyngitis, gastritis, enterocolitis, cystitis, urethritis; atopic dermatitis, bronchial asthma, autoimmune hypothyroidism and hyperprolactinemia. All somatic diseases remained in stable remission or compensation at the time when the patients underwent ART programs. The analysis of somatic morbidity did not find the differences between the groups of patients.
According to the medical history of women, gynecological morbidity of the patients under the study was comparable in both groups; the women had chronic salpingo-oophoritis and subsequent tubectomy, external genital endometriosis and adenomyosis stage I–II, intramural uterine myoma of small size and subsequent myomectomy, endometrial polyps and sexually transmitted diseases (chlamydia, gonorrhea). In patients who underwent NC-IVF (group 2), infertility duration was longer (p<0.001), averaging for 5 years, compared to women who underwent standard OS for IVF (group 1).
Assessment the obstetric history (the number of pregnancies, timely and preterm births, spontaneous and induced abortions) were also comparable in the groups. It is important to note, that in general, there was low gravidity and parity among the patients under the study. The number of IVF or ICSI attempts in history was higher in the group of patients, who underwent treatment for NC-IVF (р<0,001). The number of pregnancies that occurred due to IVF was comparable in both groups.
Assessment of hormone profile in patients in both groups revealed different AMH and FSH values (higher AMH levels were in group 1 and higher FSH levels were in group 2) (p <0.001). In patients in both groups, at the start pf their IVF cycle, no differences were found in the levels of LH, prolactin, STH, TSH, thyroxine, estradiol, testosterone, DHEA-S. It should be noted, that all patients during the study did not take hormonal drugs for contraception.
The study included the patients with decline in sexual function according to the FSFI (Table 3).
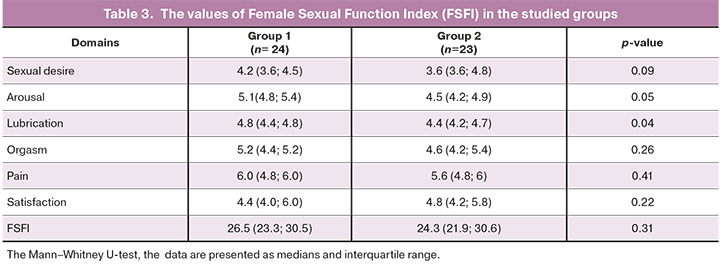
The assessement of FSFI values in patients in groups 1 demonstrated that higher values were in group 1 compared to the patients in group 2. The difference in medians between the groups of patients was found for domains “arousal” (p=0.05), “lubrication” (p=0.04), and there was a tendency for difference in the medians for domain “desire” (p=0.09).
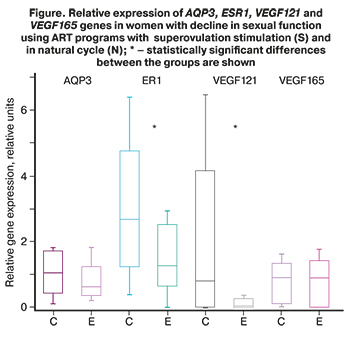 To assess the effect of ovulation stimulation on the molecular mechanisms of vaginal secretion associated with sexual response, gene expression analysis of AQP3, ESR1, VEGF121, and VEGF165 using PCR was performed in epithelial tissue scrapings from women with decline in sexual function (Figure.)
To assess the effect of ovulation stimulation on the molecular mechanisms of vaginal secretion associated with sexual response, gene expression analysis of AQP3, ESR1, VEGF121, and VEGF165 using PCR was performed in epithelial tissue scrapings from women with decline in sexual function (Figure.)
PCR test in patients with OS (group 1) found that ESR1 expression levels were 2 times (p=0.012) higher in patients with OS (group 1) compared to the patients, who underwent NC-IVF (group 2).
Also it was established that VEGF121 expression levels were 13 times (p=0.01) higher in group 1 compared to group 2; and along with this, there were no differences between VEGF165 expression levels in the groups.
AQP3 expression levels in vaginal epithelium were 1.64 times higher in patients with OS (group 1) compared to patients, who underwent NC-IVF (group 2). The differences were at the level of tendency (p=0.09).
Discussion
Decline in sexual function was one of inclusion criteria in this study. Despite the fact that the total score of the FSFI was < 26.55 in both groups, the assessment of the results found the differences: the patients in group 1 had higher median values on domains “arousal” (p=0.05), lubrication (p=0.04) and a tendency to difference on domain “desire” (p=0.09) compared to group 2.
Davari Tanha F. et al. also reported low sexual function in patients with primary and secondary infertility [22]. The structural analysis of sexual dysfunction showed, that although there was no significant difference in the overall FSFI score between the groups, the average score on domain “desire” was significantly lower in the group of women with secondary infertility. The authors stated that the age of patients was an independent predictor of sexual dysfunction in women with primary infertility compared to the patients with secondary infertility.
According to the results of their study, Keskin U. et al. reported that the age and the income of patients were independent predictors of low FSFI values; and in women with secondary infertility the risk of sexual dysfunction was 9.5 times higher, that in women with primary infertility, and in this group of patients the values of arousal, orgasm and satisfaction were significantly lower [23].
Sexual arousal in women is a neurovascular phenomenon associated with neuroregulated vascular responses [24]. Lubrication is an estrogen-dependent physiological process that is one of the indicators of sexual arousal. The data obtained from in vivo animal models strongly demonstrate that estrogens versus androgens, regulate genital blood flow, lubrication, and structural integrity of the vaginal tissue [25].
The effects of estradiol in the female reproductive tract are mainly mediated by ER1 receptor. In this study, PCR analysis in patients with OS showed a high level of ESR1 expression (p=0.012) compared to patients, who underwent natural cycle IVF.
Li S. et al. reported that the absence of epithelial ESR1 contributes to the lack of keratinization, decreased cell integrity, excessive glycoproteins production, vaginal leukocytosis, which will lead to excessive activity of matrix metalloproteinase and subsequent modification of the extracellular matrix [26]. Moreover, epithelial cells are not able to produce keratins (Krt6a and Krt10), leading to the formation of an undifferentiated epithelial layer. These aberrant extracellular matrix modifications and an undifferentiated epithelial layer result in a loss of epithelial tissue integrity, and the cells are readily detached from the stroma during mating.
Thus, low ESR1 expression level in the vaginal epithelium may be one of the pathogenetic mechanisms of impaired vaginal secretion, genital sexual response (arousal, lubrication), as well as dyspareunia and vulvodynia.
Islamov R.R. et al. reported the neuroprotective effect of estrogens associated with cross-interactions of estrogens with intracellular signaling cascades of many other biologically active compounds, including the activation of estrogen receptors ERα and ERβ, as well as VEGF [27].
VEGF is a potent angiogenic mitogen, and neurotrophic factor that has a stimulating, anti-apoptotic effect on all types of muscle cells and neurons [28–31]. Using a model of human umbilical vein endothelial cell (HUVEC), Zhang Y. et al. have shown that isoforms VEGF 121 and VEGF 165 activate different signaling pathways that facilitate different specialized biological functions. VEGF 165 is a more potent inducer of proliferation via activation of mitogen-activated protein kinase kinase (MEK)/ extracellular signal-regulated kinase (ERK) inhibitors (MEK /ERK) versus VEGF121 [17]. In contrast, VEGF121 induces hyperpermeability mediated by Src family kinases.
The assessment of VEGF121 and VEGF165 expression levels in PCR tests in both studied groups showed significant differences: VEGF165 expression level was comparable in the groups, but the level of VEGF121 expression differed 13 times (p=0.01). Thus, it was found, that in patients who received medications to stimulate ovarian function according to GnRH-ant protocol (rFSH, rFSH and LH or hMG), as well 8,000-10,000 IU of hCG in contrast to patients without ovarian stimulation, who received 5,000 IU of hCG, VEGF121 expression was significantly higher, while VEGF165 expression was comparable, indicating the induction of hyperpermeability in patients in group 1, rather than angiogenesis. In our opinion, high FSFI values in domains “arousal” (p=0.05), «lubrication” (p=0.04) in patients in group 1 are due to high VEGF121 expression. Decreased expression of VEGF121, as well as its signaling pathways, may be one of the pathogenetic mechanisms of impaired vaginal secretion, genital sexual response (arousal, lubrication).
Currently, the expression of VEGF is well studied in some of eye diseases and oncological diseases; medications for anti-VEGF therapy have been developed. Promising therapeutic strategies for treatment of skin diseases, including psoriasis and atopic dermatitis, are associated with a new class of agents based on blocking VEGF signaling pathway [17]. Dyspareunia occurring in various diseases of the vulva, including lichen sclerosus, atopic dermatitis, neurodermatitis, etc., is also a possible therapeutic target for future drugs that regulate VEGF function.
AQPs are associated with the pathogenesis of a significant number of human diseases, and they are of much interest due to their therapeutic potential. In this regard, clinical trials of AQP modulators and inhibitors are being developed [32, 33]. Ribeiro J.C. et al. consider differences between AQP expression patterns as potential biomarkers in both male and female reproductive health [34]. In their opinion, AQP expression in gametes and the male and female reproductive tract can be regulated to ensure the best results of infertility treatment in ART programs.
The study assessed the level of AQP3 expression in the vaginal epithelium in women with decline in sexual function and found the absence of overexpression in both groups, as well as a tendency (p=0.09) of higher values in patients with OS compared to patients who underwent natural cycle IVF. Thus, the level of AQP3 expression in vaginal epithelial cells in patients had no significant difference, despite different hormone therapy and age.
AQP3 allows to transport not only water, but also glycerol and hydrogen peroxide, promoting the signaling of the universal transcription factor (NF-κB), which controls the expression of genes for the immune response, apoptosis and the cell cycle [35]. Additionally, AQP3 plays a role in the normal function of bacterial phagocytosis by macrophages and transportation of T cells, i.e. in innate immunity [36, 37]. These data suggest that AQP3 is a novel therapeutic target for modulating the immune response in a variety of infectious and inflammatory conditions.
Conclusion
The study showed that the patients with OS for IVF in group 1 were younger (p<0.001) and duration of their infertility was shorter (p<0.001), the level of AMH was higher (p<0.001) and the level of FSH was lower (p<0.001) compared to the patients with natural cycle IVF in group 2.
In the group of patients with OS for IVF in the programs of ART, median values were higher for the FSFI in domains “erousal” (p=0.05), “lubrication” (p=0.04), and “desire” (p=0.09)compared to the patients with decline in sexual function, who underwent natural cycle IVF.
PCR analysis in patients with OS for IVF found a high level of ESR1 expression (p=0.012), VEGF121 (p=0.01), a tendency for high AQP3 values (p=0.09) compared to the patients, who underwent natural cycle IVF; the level of VEGF165 expression was comparable in both groups. High levels of ESR1, VEGF 121, AQP3 expression in patients with OS were associated with higher FSFI scores in domains “erousal”, “lubrication”, “desire”.
The studied specific features of gene expression of vaginal transcellular secretion factors (AQP3, ESR1, VEGF121, and VEGF165) in ovarian stimulation for IVF and in natural cycle IFV (NC-IVF) in women with decline in sexual function allowed to determine some pathogenetic mechanisms of disorders of vaginal secretion, genital sexual response (arousal, lubrication) , as well as the formation of dyspareunia. The results obtained reveal the therapeutic potential of secretory molecular components in various diseases of the vulva, including lichen sclerosus, atopic dermatitis, neurodermatitis, vulvodynia.
References
- Wischmann T., Schilling K., Toth B., Rösner S., Strowitzki T., Wohlfarth K., Kentenich H. Sexuality, self-esteem and partnership quality in infertile women and men. Geburtshilfe Frauenheilkd. 2014; 74(8): 759-63. https://dx.doi.org/10.1055/s-0034-1368461.
- Galhardo A., Moura-Ramos M., Cunha M., Pinto-Gouveia J. Infertility is a trap: how defeat and trapping are affected by depressive symptoms. Hum. Reprod. 2016; 31(2): 419-26. https://dx.doi.org/10.1093/humrep/dev311.
- Peterson B.D., Sejbaek C.S., Pirritano M., Schmidt L. Are there severe depressive symptoms associated with infertility of distress in individuals about related and their partners? Hum. Reprod. 2014; 29(1): 76-82. https://dx.doi.org/10.1093/humrep/det412.
- Cizmeli C., Lobel M., Franasiak J., Pastore L.M. Levels and associations among self-esteem, fertility distress, coping, and reaction to potentially being a genetic carrier in women with diminished ovarian reserve. Fertil. Steril. 2013; 99(7): 2037-44. https://dx.doi.org/10.1016/j.fertnstert.2013.02.033.
- Keramat A., Masoomi S.Z., Mousavi S.A., Poorolajal J., Shobeiri F., Hazavhei S.M. Quality of life and its related factors in infertile couples. J. Res. Health Sci. 2014; 14(1): 57-63.
- Васильченко Г.С., ред. Общая сексопатология. Руководство для врачей. М.: Медицина; 1977: 75-87, 168-75. [Vasilchenko G.S., ed. General sexopathology. M.: Medicine, 1977: 75-87, 168-175 (in Russian)].
- Васильченко Г.С., ред. Общая сексопатология. Руководство для врачей. 2-е изд. М.: Медицина; 2005. 510с. [Vasilchenko G.S., eds. General sexopathology. A guide for doctors. M.: Medicine, 2005, 512 p. (in Russian)].
- Kazakov D.V., Stewart C.J., Kacerovska D., Leake R., Kreuzberg B., Chudacek Z., Hora M., Michal M. Prostatic-type tissue in the lower female genital tract: a morphologic spectrum, including vaginal tubulosquamous polyp, adenomyomatous hyperplasia of paraurethral Skene glands (female prostate), and ectopic lesion in the vulva. Am. J. Surg. Pathol. 2010; 34(7): 950-5. https://dx.doi.org/10.1097/PAS.0b013e3181e0f371.
- Koduri S., Goldhar A., Vonderhaar B. Activation of vascular endothelial growth factor (VEGF) by the ER-α variant, ERΔ3. Breast Cancer Res. Treat. 2006; 95(1): 37-43. https://dx.doi.org/10.1007/s10549-005-9028-4.
- Huang Y.T., Zhou J., Shi S., Xu H.Y., Qu F., Zhang D. et al. Identification of estrogen response element in aquaporin-3 gene that mediates estrogen-induced cell migration and invasion in estrogen receptor-positive breast cancer. Sci. Rep. 2015; 5: 12484. https://dx.doi.org/10.1038/srep12484.
- Nephew K.P., Long X., Osborne E., Burke K.A., Ahluwalia A., Bigsby R.M. Effect of estradiol on estrogen receptor expression in rat uterine cell types. Biol. Reprod. 2000; 62(1): 168-77.
- Kim S.O., Oh K.J., Lee H.S., Ahn K., Kim S.W., Park K. Expression of aquaporin water channels in the vagina in premenopausal women. J. Sex. Med. 2011; 8(7): 1925-30. https://dx.doi.org/10.1111/j.1743-6109.2011.02284.x.
- Lee H.S., Kim S.O., Ahn K., Park K. All-trans retinoic acid increases aquaporin 3 expression in human vaginal epithelial cells. Sex. Med. 2016; 4(4): e249-54. https://dx.doi.org/10.1016/j.esxm.2016.07.001.
- Dvorak H.F., Brown L.F., Detmar M., Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995; 146(5): 1029-39.
- Sondell M., Sundler F., Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur. J. Neurosci. 2000; 12(12): 4243-54. https://dx.doi.org/10.1046/j.0953-816X.2000.01326.x.
- Storkebaum E., Lambrechts D., Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004; 26(9): 943-54. https://dx.doi.org/10.1002/bies.20092.
- Zhang Y., Furumura M., Morita E. Distinct signaling pathways confer different vascular responses to VEGF 121 and VEGF 165. Growth Factors. 2008; 26(3): 125-31. https://dx.doi.org/10.1080/08977190802105909.
- Reed B.G., Carr B.R. The normal menstrual cycle and the control of ovulation. [Updated 2018 Aug 5]. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dhatariya K. et al., eds. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. Available at: https://www.ncbi.nlm.nih.gov/books/NBK279054/
- Ullah K., Rahman T.U., Pan H.T., Guo M.X., Dong X.Y., Liu J. et al. Serum estradiol levels in controlled ovarian stimulation directly affect the endometrium. J. Mol. Endocrinol. 2017; 59(2): 105-19. https://dx.doi.org/10.1530/JME-17-0036.
- Carosso A., Revelli A., Gennarelli G., Canosa S., Cosma S., Borella F. et al. Controlled ovarian stimulation and progesterone supplementation affect vaginal and endometrial microbiota in IVF cycles: a pilot study. J. Assist. Reprod. Genet. 2020; 37(9): 2315-26. https://dx.doi.org/10.1007/s10815-020-01878-4.
- Rosen R., Brown C., Heiman J., Leiblum S., Meston C., Shabsigh R. et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital Ther. 2000; 26(2): 191-208. https://dx.doi.org/10.1080/009262300278597.
- Tanha F.D., Mohseni M., Ghajarzadeh M. Sexual function in women with primary and secondary infertility in comparison with controls. Int. J. Impot. Res. 2014; 26(4): 132-4. https://dx.doi.org/10.1038/ijir.2013.51.
- Keskin U., Coksuer H., Gungor S., Ercan C.M., Karasahin K.E., Baser I. Differences in prevalence of sexual dysfunction between primary and secondary infertile women. Fertil. Steril. 2011; 96(5): 1213-7. https://dx.doi.org/10.1016/j.fertnstert.2011.08.007.
- Azadzoi K.M., Siroky M.B. Neurologic factors in female sexual function and dysfunction. Korean J. Urol. 2010; 51(7): 443-9. https://dx.doi.org/10.4111/kju.2010.51.7.443.
- Woodard T.L., Diamond M.P. Physiologic measures of sexual function in women: a review. Fertil. Steril. 2009; 92(1): 19-34. https://dx.doi.org/10.1016/j.fertnstert.2008.04.041.
- Li S., Herrera G.G., Tam K.K., Lizarraga J.S., Beedle M.T., Winuthayanon W. Estrogen action in the epithelial cells of the mouse vagina regulates neutrophil infiltration and vaginal tissue integrity. Sci. Rep. 2018; 8(1): 11247. https://dx.doi.org/10.1038/s41598-018-29423-5.
- Исламов Р.Р., Валиуллин В.В., Мурашов А.К. Механизмы нейропротекторного действия эстрогенов, связанные с экспрессией фактора роста эндотелия сосудов. Биологический бюллетень РАН. 2007; 34: 110-9. [Islamov R.R., Valiullin V.V., Murashov A.K. Mechanisms of the neuroprotective action of estrogens associated with the expression of vascular endothelial growth factor. Biology Bulletin. 2007; 34: 110-9]. https://dx.doi.org/10.1134/S1062359007020021.
- Apte R.S., Chen D.S., Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019; 176(6): 1248-64.
- Giacca M., Zacchigna S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 2012; 19: 622-9. https://dx.doi.org/10.1038/gt.2012.17.
- Theis V., Theiss C. VEGF – A stimulus for neuronal development and regeneration in the CNS and PNS. Curr. Protein Pept. Sci. 2018; 19(6): 589-97. https://dx.doi.org/10.2174/1389203719666180104113937.
- Abir-Awan M., Kitchen P., Salman M.M., Conner M.T., Conner A.C., Bill R.M. Inhibitors of mammalian aquaporin water channels. Int. J. Mol. Sci. 2019; 20(7): 1589. https://dx.doi.org/10.3390/ijms20071589.
- Fiorentini D., Zambonin L., Dalla Sega F.V., Hrelia S. Polyphenols as modulators of aquaporin family in health and disease. Oxid. Med. Cell. Longev. 2015; 2015: 196914. https://dx.doi.org/10.1155/2015/196914.
- Ribeiro J.C., Alves M.G., Yeste M., Cho Y.S., Calamita G., Oliveira P.F. Aquaporins and (in)fertility: More than just water transport. Biochim. Biophys. Acta Mol. Basis Dis. 2020; 1867(3): 166039. https://dx.doi.org/10.1016/j.bbadis.2020.166039.
- Hara-Chikuma M., Satooka H., Watanabe S., Honda T., Miyachi Y., Watanabe T., Verkman A.S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-κB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015; 6: 7454. https://dx.doi.org/10.1038/ncomms8454.
- Zhu N., Feng X., He C., Gao H., Yang L., Ma Q. et al. Defective macrophage function in aquaporin-3 deficiency. FASEB J. 2011; 25(12): 4233-9. https://dx.doi.org/10.1096/fj.11-182808.
- Hara-Chikuma M., Chikuma S., Sugiyama Y., Kabashima K., Verkman A.S., Inoue S., Miyachi Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012; 209(10): 1743-52. https://dx.doi.org/10.1084/jem.20112398.
Received 25.02.2021
Accepted 03.06.2021
About the Authors
Natalia N. Stenyaeva, MD, PhD, Senior Researcher of the Department of Andrology and Urology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.E-mail: nataliasten@mail.ru. ORCID: 0000-0002-6495-3367. 4, Oparina str., Moscow, 117997, Russia.
Alexey M. Krasnyi, PhD, Head of the Cytology Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-22-72.
E-mail: alexred@list.ru. ORCID: 0000-0001-7883-2702; Researcher ID AAE-8138-2019. 4, Oparina str., Moscow, 117997, Russia.
Dmitry F. Khritinin, Corresponding Member of the RAS, Dr. Med. Sci., Professor of the Department of Psychiatry and Narcology, Faculty of General Medicine,
I.M. Sechenov FSMU, Ministry of Health of the Russian Federation. ORCID: 0000-0001-9107-2357. 8-2 Trubetskaya str., Moscow, 119991, Russia.
Anna G. Burduli, MD, PhD, Senior Researcher of IVF Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: burdulianna@gmail.com. 4, Oparina str., Moscow, 117997, Russia.
Alsu A. Sadekova, PhD (Bio), researcher of the Cytology Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-22-72.
E-mail: sialsad@gmail.com. ORCID: 0000-0003-4726-7477; Researcher ID AAI-3542-2020. 4, Oparina str., Moscow, 117997, Russia.
Marina N. Kostava, PhD, obstetrician-gynecologist, doctor of the highest category, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. 4, Oparina str., Moscow, 117997, Russia.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of IVF Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: e_kalinina@oparina4.ru. 4, Oparina str., Moscow, 117997, Russia.
For citation: Stenyaeva N.N., Krasnyi A.M., Khritinin D.F., Burduli A.G., Sadekova A.A., Kostava M.N., Kalinina E.A. Specific features of molecular mechanisms of vaginal secretion in women with decline in sexual function in assisted reproductive technology programs.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 7: 165-173 (in Russian)
https://dx.doi.org/10.18565/aig.2021.7.165-173



