Гормональная поддержка препаратами половых стероидных гормонов – неотъемлемая составляющая программ вспомогательных репродуктивных технологий (ВРТ). Обязательным минимумом гормональной поддержки в тех циклах, где проводится перенос эмбрионов (ПЭ), являются препараты прогестерона, обеспечивающие сохранение функции желтого тела и секреторную трансформацию эндометрия и тем самым создающие условия для имплантации эмбриона [1]. Однако залогом успешной секреторной трансформации эндометрия является его адекватная пролиферация в фолликулиновую фазу цикла под действием эстрогенов [2].
На сегодняшний день именно стимуляция пролиферации эндометрия является наиболее частой целью применения эстрогенов в программах ВРТ. Экзогенная поддержка эстрогенами используется в циклах с переносом донорских ооцитов или эмбрионов, переноса криоконсервированных эмбрионов у пациенток с ановуляцией или истощением яичников и при «тонком» эндометрии [3]. Целью данного мини-обзора является описание современной доказательной базы по применению трансдермальных эстрогенов в программах ВРТ с акцентом на трансдермальный гель с концентрацией 17β-эстрадиола 0,06%*.
Пути введения эстрогенов: преимущества и недостатки
Препараты эстрадиола, зарегистрированные для применения в программах ВРТ, выпускаются в двух основных формах: пероральной и трансдермальной. Опыт применения пероральных эстрогенов в гинекологии составляет более 60 лет [4, 5]: эти препараты имеют хорошо изученный профиль эффективности и безопасности и, как любая пероральная форма, удобны для приема. Однако биодоступность эстрадиола при пероральном введении крайне невелика. Так, при приеме эстрадиола валерата до 97% действующего вещества при первичном пассаже через печень подвергается экстенсивному метаболизму до эстрона, эстриола и эстрона сульфата [6]. Низкая биодоступность перорального эстрадиола и его неблагоприятное влияние на липидный и углеводный обмен и коагуляционное звено гемостаза послужили предпосылкой для разработки новых путей введения [7].
Трансдермальные препараты эстрадиола выпускаются в форме трансдермальных систем доставки (пластырей) и водно-спиртовых гелей [8]. При контакте фазы-носителя с кожей эстрадиол быстро проникает в роговой слой эпидермиса и образует депо, из которого он постепенно поступает в системный кровоток [9, 10]. Ввиду того, что эпидермис играет роль барьера, всасывание эстрадиола из рогового слоя протекает согласно кинетике нулевого порядка: за единицу времени в кровоток поступает одно и то же количество действующего вещества, независимо от его концентрации [7].
Таким образом, преимуществами трансдермальных форм являются постоянная скорость высвобождения и всасывания действующего вещества, обеспечивающие минимальную вариабельность его концентраций в системном кровотоке, большая биодоступность, а также отсутствие влияния на желудочно-кишечный тракт и метаболический профиль [7, 9] (табл. 1).
Фармакокинетика и фармакодинамика эстрадиола при доставке в системный кровоток из трансдермальной системы или водно-спиртового геля существенно не различаются [11–13]. Таким образом, при выборе трансдермальной формы эстрадиола на первое место выходит индивидуальная переносимость и удобство лечения. В отличие от трансдермальных систем доставки, гели должны наноситься ежедневно, однако они реже вызывают местные реакции, особенно при длительном применении (табл. 2).
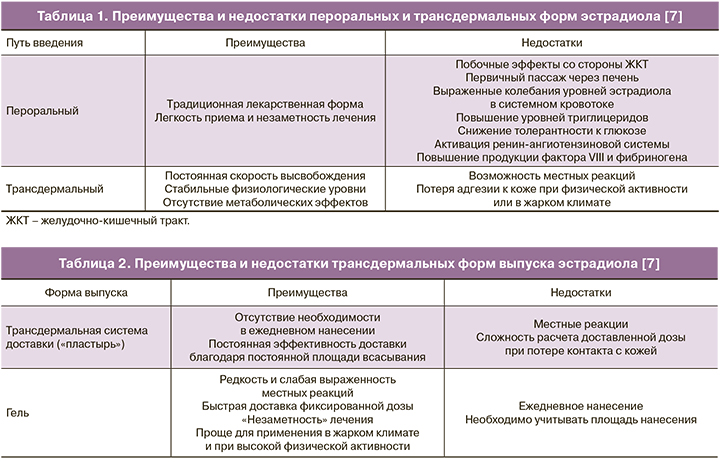
Основным источником прямых сравнительных данных по переносимости длительного применения различных трансдермальных форм эстрогенов являются исследования заместительной гормональной терапии у женщин в менопаузе. Так, в исследовании Hirvonen и соавт. (1997), местные реакции у пациенток, получавших эстрадиол в форме геля в течение одного года, наблюдались лишь в 3,3% случаев, по сравнению с 46,7% в группе использовавших трансдермальную систему доставки (пластырь). Единственной местной реакцией при использовании геля являлся слабый преходящий кожный зуд, в то время как применение пластыря сопровождалось стойким зудом и покраснением, а в редких случаях – отеком и шелушением кожи [14]. Сходные результаты были получены в исследовании Travassos de Figueiredo и соавт. (2000), сравнивавшем эффективность и переносимость трансдермального эстрадиола в форме геля и пластыря в течение не менее чем трех месяцев [15]. В данном исследовании кожный зуд и местные реакции (33,3 и 54,2%, соответственно) отмечались только у женщин, использовавших пластырь. Практически у каждой второй пациентки (54,2%) возникали сложности с фиксированием пластыря на коже. Авторы также отметили сезонные колебания переносимости: все типы местных реакций на трансдермальный эстрадиол чаще наблюдались летом, что соответствовало более ранним наблюдениям худшей переносимости трансдермальных систем доставки эстрадиола (пластырь), по сравнению с гелем, в регионах с жарким климатом [16].
Эстрогенная поддержка в программах ВРТ: клинические сценарии
Перенос криоконсервированных эмбрионов
Криоконсервация эмбрионов используется для длительного хранения эмбрионов, полученных в цикле стимуляции, с целью последующего переноса в полость матки в следующих основных клинических ситуациях: (1) отсутствие наступления беременности после ПЭ в цикле стимуляции; (2) отмена ПЭ для профилактики синдрома гиперстимуляции яичников (англ. «freeze all»); (3) циклы преимплантационного генетического скрининга (PGS), если получение результатов генетического анализа до конца 5-х суток культивирования эмбриона невозможно и клиника не располагает возможностями для культивирования эмбрионов до 6-х суток; (4) сохранение генетического материала у пациенток, которым предстоит гонадотоксичная терапия. Согласно статистике Российской ассоциации репродукции человека (РАРЧ) и Европейского общества репродукции и эмбриологии человека (ESHRE), в 2014–2015 гг. циклы переноса криоконсервированных эмбрионов в структуре программ ВРТ на территории РФ и Европы составляли около 25% [17, 18].
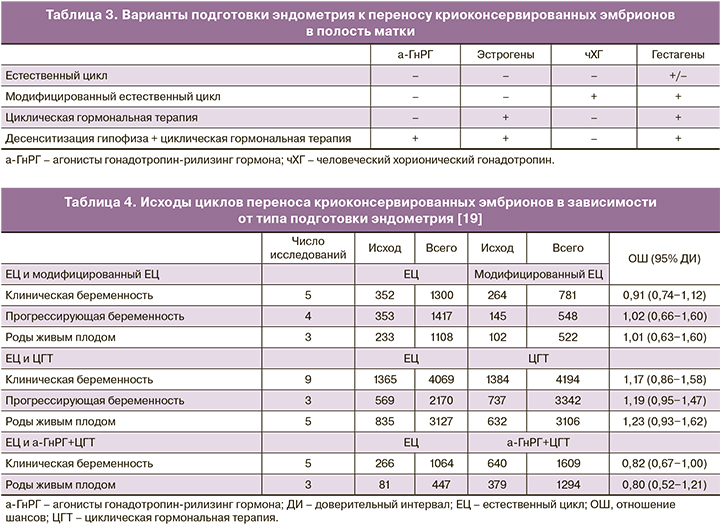
У пациенток с сохранной овуляцией перенос криоконсервированных эмбрионов может производиться в естественном цикле с введением человеческого хорионического гонадотропина (чХГ) или без него, как правило, на фоне гестагенной поддержки лютеиновой фазы [3]. У пациенток с ановуляцией или сниженным овариальным резервом стандартом подготовки эндометрия к ПЭ является циклическая эстрогенно-гестагенная терапия с десенситизации гипофиза агонистами гонадотропин-рилизинг гормона (а-ГнРГ) или без нее [3] (табл. 3).
Эффективность существующих схем подготовки эндометрия в циклах криопереноса была оценена в мета-анализе Groenewoud и соавт. (2013), включавшем 20 проспективных и ретроспективных исследований [19]. Авторы не выявили статистически значимых различий в ключевых показателях эффективности программ ВРТ: частоте клинической и прогрессирующей беременности и родов живым плодом – в зависимости от типа подготовки эндометрия и сделали вывод о равной эффективности всех используемых методик (табл. 4).
Мета-анализ Groenewoud и соавт. (2013) не ставил задачей оценку эффективности лечения при использовании различных путей введения эстрогенового компонента циклической гормональной терапии (ЦГТ). Однако сравнение эффективности и переносимости трансдермального геля с 17β-эстрадиолом и перорального эстрадиола валерата проводилось как минимум в трех исследованиях, опубликованных позднее [20, 21].
Рандомизированное контролируемое исследование Sun и соавт. (2014) [20] сравнивало результативность циклов переноса криоконсервированных эмбрионов при подготовке эндометрия эстрадиола валератом (с пошаговым увеличением дозы 3–6–8–10–12–15 мг/день перорально; n=124) и 17β эстрадиолом в форме трансдермального геля (0,06%, 1,5–3–4,5–6 мг/день; n=120). Исследование показало, что в день начала поддержки прогестероном толщина эндометрия в группе, получавшей трансдермальный и пероральный эстрадиол, статистически значимо не различалась (10,52±1,86 мм и 10,76±1,99 соответственно). Уровень эстрадиола в крови у пациенток, получавших трансдермальный эстрадиол, был статистически значимо выше, чем в группе с пероральным эстрадиолом (2362±118,7 и 1920,1±78,5 пмоль/л соответственно; P=0,04), а концентрация эстрона, наоборот, ниже, (556,8±44,6 и 1850,9±59,4 пмоль/л соответственно; P=0,03). Частота клинической беременности в исследуемой группе была выше, чем в контрольной группе (45,0 и 41,1% соответственно), а частота самопроизвольных выкидышей – ниже (1,67 и 4,84% соответственно), однако данные различия не достигали статистической значимости. Пероральный эстрадиол отличался худшей переносимостью, по сравнению с трансдермальным: 12 пациенток, получавших эстрадиол перорально, отметили нагрубание молочных желез, 5 – тошноту и рвоту.
Ретроспективное исследование, опубликованное Song и соавт. в 2015 году [22], сравнивало эффективность и переносимость циклов переноса криоконсервированных эмбрионов при подготовке эндометрия эстрадиола валератом перорально в дозе 4–6 мг/день (n=68) и гелем 17β эстрадиола (0,06%) трансдермально в дозе 4,5 мг 2 раза в день (n=70). Подготовку эстрогенами проводили до достижения эндометрием толщины ≥9 мм. Данный ретроспективный анализ подтвердил результаты рандомизированного исследования Sun и соавт. 2014 года [20]: толщина эндометрия в день начала приема прогестерона по группам статистически не различалась (10,42±1,16 и 10,79±1,29 мм в группе с пероральным и трансдермальным эстрадиолом соответственно, P>0,05). Уровень эстрадиола в день начала приема прогестерона на фоне применения эстрадиола валерата был значительно ниже, чем при использовании геля (741,34±585,36 и 1750,22±1390,56 пмоль/л соответственно; P<0,05), а длительность лечения – выше (12,60±3,02 и 11,13±0,57 дня соответственно; P<0,05). Кроме того, в данной работе частота клинической беременности при использовании трансдермального геля статистически значимо превосходила таковую при использовании перорального эстрадиола (46,8 и 69,1%, P<0,05). При сравнении частоты побочных эффектов статистически значимых различий между группами выявлено не было (P>0,05).
Рандомизированное контролируемое исследование Shahrokh Tehraninejad и соавт. (2018) сравнивало результативность циклов переноса криоконсервированных эмбрионов при подготовке эндометрия эстрадиола валератом (8 мг/день перорально; n=50) и 17β эстрадиолом в форме трансдермального геля (0,06%; 6 мг/день; n=50). В данном исследовании эстрогены назначали с 1-го дня цикла до достижения толщины эндометрия 8 мм на фоне десенситизации гипофиза агонистами гонадотропин-рилизинг гормона (а-ГнРГ). Исследование продемонстрировало, что, несмотря на отсутствие статистически значимых различий в средней толщине эндометрия между группой с пероральными и трансдермальными эстрогенами (8,63±1,21 и 8,52±1,46 мм соответственно; P=0,701), а также сопоставимой частоте биохимической и клинической беременности (16 и 24% соответственно (для обоих показателей); P=0,384), частота прогрессирующей беременности и родов живым плодом была статистически значимо выше при применении трансдермального эстрадиола (50 и 91,7% соответственно (для обоих показателей); P=0,035). Самопроизвольное прерывание беременности у пациенток, получавших трансдермальный эстрадиол, происходило статистически значимо реже, чем в группе с пероральными эстрогенами (8,3 и 50% соответственно). Оба препарата характеризовались хорошей переносимостью и сопровождались высоким уровнем удовлетворенности лечением. Так, приблизительно каждая третья пациентка в исследовании была полностью удовлетворена терапией (40 и 30% в группе с пероральными и трансдермальными эстрогенами соответственно) [21].
Таким образом, эффективность и переносимость циклов переноса криоконсервированных эмбрионов при подготовке эндометрия с использованием пероральных эстрогенов и геля с 17β эстрадиолом как минимум сопоставима, а в ряде случаев при трансдермальном пути доставки может превосходить таковую для «классического» перорального пути.
Донорство ооцитов и эмбрионов
Использование донорских ооцитов и эмбрионов позволяет добиться наступления беременности у пациенток со снижением овариального резерва в старшем репродуктивном возрасте или при преждевременном истощении функции яичников, а также репродуктивными потерями, вызванными высокой частотой анеуплоидий ооцитов. Доля донорских программ в практике клиник ВРТ на территории РФ и Европы в 2014−2015 гг. составляла приблизительно 7% [17, 18].
Главной задачей применения эстрогенной поддержки в данных программах, как и при переносе криоконсервированных эмбрионов, является создание условий для адекватной пролиферации эндометрия. Однако, учитывая, что пациентки в программах донации ооцитов и эмбрионов, как правило, имеют сниженный овариальный резерв, методом выбора для подготовки эндометрия является ЦГТ.
Как и при переносе криоконсервированных эмбрионов, главный вопрос практикующих врачей – какую схему подготовки эндометрия предпочесть? Исследование Madero и соавт. (2016) [23] ставило целью ответить на этот вопрос, сравнив эффективность циклов донации ооцитов с ПЭ в цикле стимуляции при подготовке эндометрия пероральным и трансдермальным эстрадиолом в возрастающей (n=5593) или постоянной (n=2769) дозе. Схема с возрастающей дозой подразумевала назначение перорального эстрадиола в дозе 2 мг/день с 1-го по 7-й день, 4 мг/день с 8-го по 12-й день и 6 мг/день с 13-го дня до дня ПЭ; трансдермальный эстрадиол в форме пластыря назначался в дозе 75 мг/3 дня с 1-го по 6-й день, 150 мг/3 дня с 7-го дня до дня ПЭ. Схема с фиксированной дозой подразумевала назначение перорального эстрадиола в дозе 2 мг/день или трансдермального эстрадиола в дозе 150 мг/3 дня с 1-го дня до дня ПЭ. Исследование продемонстрировало, что частота родов живым плодом не зависела от схемы поддержки эстрогенами, достигая 33,0 и 32,5% для фиксированной и возрастающей дозы перорального эстрадиола соответственно (P=0,81) и 35,3 и 33,5% для фиксированной и возрастающей дозы трансдермального эстрадиола соответственно (Р=0,33).
Таким образом, в настоящее время убедительные данные о преимуществах перорального или трансдермального пути доставки, а также фиксированной или возрастающей дозы эстрогенов, отсутствуют. Учитывая, что пациентки в программе донации ооцитов или эмбрионов чаще всего относятся к категории старшего репродуктивного возраста, у этих женщин выше вероятность сопутствующих соматических заболеваний и метаболических нарушений. В этих условиях применение трансдермальных эстрогенов может быть предпочтительнее ввиду их более благоприятного метаболического профиля и того факта, что при успешном наступлении беременности эти пациентки будут продолжать эстрогенно-гестагенную поддержку в течение всего первого триместра беременности [3].
«Тонкий» эндометрий
Тонкий эндометрий – термин, описывающий группу патологических состояний, при которых толщина эндометрия (М-эхо) в конце пролиферативной/начале секреторной фазы по данным трансвагинального ультразвукового исследования не превышает 8 мм. Причинами тонкого эндометрия могут быть синдром Ашермана, длительный прием оральных контрацептивов или кломифена цитрата, синдром Тернера, перенесенная лучевая терапия, повторные внутриматочные манипуляции и хронический эндометрит [24].
Частота тонкого эндометрия в программах ВРТ достигает 2,4%, и в некоторых случаях его явный этиологический фактор отсутствует [25]. Тем не менее, следует отметить, что для выявления этиологии тонкого эндометрия стандартного обследования перед программой ВРТ зачастую недостаточно: при детальном патоморфологическом и иммуногистохимическом исследовании приблизительно у 7 из 10 пациенток с тонким эндометрием в программах ВРТ выявляется хронический эндометрит [26]. Тонкий эндометрий в программе ВРТ снижает вероятность успеха лечения: показано, что шансы на успешный исход у пациенток с толщиной эндометрия ≤7 мм практически на 60% ниже, чем у пациенток с толщиной эндометрия >7 мм (отношение шансов 0,42, 95% доверительный интервал 0,27−0,67) [25].
Эстрогенная поддержка фолликулиновой фазы цикла – один из методов лечения тонкого эндометрия. Возможность применения трансдермальных эстрогенов для стимуляции пролиферации эндометрия у данного контингента больных подтверждена в исследовании Chi и соавт. (2018) [27]. В данном исследовании назначение трансдермального геля с 17β эстрадиолом (0,06%) в дозе 5 г/день (соответствует дозе эстрадиола 3 мг/день) и в течение 2 месяцев после гистерорезектоскопии по поводу внутриматочных синехий (n=18) статистически значимо увеличивало толщину эндометрия с 4,25±0,72 до 7,64±1,54 мм (P<0,05). При сочетании эстрогенной поддержки с приемом аспирина в дозе 100 мг/день (n=20) толщина эндометрия возрастала с 4,18±0,91 до 9,12±1,78 мм (P<0,05). Исследование также продемонстрировало, что обе схемы лечения повышали экспрессию маркеров рецептивности эндометрия αvβ3 интегрина и ламинина, улучшали васкуляризацию эндометрия и препятствовали его послеоперационному фиброзированию. Следует отметить, что это исследование оценивало естественную фертильность и не включало пациенток в программах ВРТ – результативность у данного контингента больных продолжает оставаться предметом изучения.
Заключение
Экзогенная поддержка эстрогенами – неотъемлемый компонент подготовки эндометрия к переносу криоконсервированных эмбрионов у пациенток с отсутствием спонтанной овуляции и в программах донорства ооцитов и эмбрионов. Существующая доказательная база показывает, что эффективность и переносимость пероральных и трансдермальных эстрогенов в программах ВРТ как минимум сопоставима. При этом трансдермальные эстрогены обеспечивают постоянную скорость высвобождения и всасывания действующего вещества и минимальную вариабельность его концентраций в системном кровотоке, при отсутствии влияния на желудочно-кишечный тракт и метаболический профиль, что дополнительно укрепляет позиции трансдермальных препаратов как терапии выбора в данной клинической ситуации.



