Comparative analysis of the effect of two antihypertensive therapy regimens on maternal hemodynamic parameters in early- and late-onset preeclampsia
Objective: To investigate the effect of antihypertensive therapy on maternal hemodynamic profile and the relationship between hemodynamic parameters and endothelial glycocalyx (eGCX) status in patients with preeclampsia.Muminova K.T., Khodzhaeva Z.S., Gorina K.A., Shmakov R.G., Ziganshina M.M.
Materials and methods: The study comprised 82 patients, including women with uncomplicated pregnancy (n=30, comparison group) and patients with preeclampsia (n=52, study group) receiving mono- or combined antihypertensive therapy. Monotherapy consisted of Dopegit (mean dose 1500 mg/day) and two-component therapy included Dopegit (mean dosage 2000 mg/day) and Cordaflex (mean dosage 40 mg/day). All hypertensive patients underwent 24-hour ambulatory blood pressure monitoring (24h-ABPM) using a BPLab device (Peter Telegin, Nizhny Novgorod, Russia). Blood components of eGCX were measured by an enzyme immunoassay.
Results: Early-onset preeclampsia was associated with worse perinatal outcomes, such as earlier gestational age at delivery, lower birth weight, and a lower Apgar score. Patients with early-onset preeclampsia receiving two-component therapy had higher systolic and diastolic BP. In patients with early-onset preeclampsia,
(dP/dt)max values were significantly elevated, most markedly in patients on two-component antihypertensive therapy. This subgroup also had a higher augmentation index (AIx) and a higher RWTT index. Patients with late-onset preeclampsia on monotherapy showed a significant increase in (dP/dt)max and ED; they also had a borderline significant increase in AIx. All patients receiving Dopegit monotherapy had a significant increase in syndecan-1 blood levels. Patients with early-onset preeclampsia on two-component therapy showed a significant decrease in hyaluronan levels and a borderline significant increase in syndecan-1 levels compared to women with healthy pregnancy. No significant differences in eGCX status were detected in patients with late-onset preeclampsia.
Conclusion: The findings suggest a pathogenetic link between molecular and functional vascular changes, which is partially compensated by antihypertensive therapy. In early-onset preeclampsia, antihypertensive therapy is less effective because hemodynamic changes are not corrected by Dopegit and Cordaflex. At the same time, in late-onset preeclampsia the effectiveness of combined antihypertensive therapy is confirmed by compensation of hemodynamic parameters of the maternal cardiovascular system.
Authors’ contributions: Khodzhaeva Z.S., Ziganshina M.M., Shmakov R.G., Muminova K.T., Gorina K.A. – conception and design of the study, statistical analysis, manuscript drafting; Muminova K.T., Gorina K.A. – data collection and analysis, review and translation of relevant publications; Ziganshina M.M. – analysis of molecular, immunological studies; Khodzhaeva Z.S. – data analysis, structuring and editing the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the RF Ministry of Health State Assignment No. 121040600435-0 “Validation of Personalized Approaches to Antihypertensive Therapy for HDP and Preeclampsia”.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No: 5/May 27, 2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Muminova K.T., Khodzhaeva Z.S., Gorina K.A.,
Shmakov R.G., Ziganshina M.M. Comparative analysis of the effect of two antihypertensive therapy regimens on maternal hemodynamic parameters in early- and late-onset preeclampsia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; 1: 55-66 (in Russian)
https://dx.doi.org/10.18565/aig.2022.290
Keywords
An expanded understanding of the heterogeneous nature of preeclampsia has allowed the identification of two clinical phenotypes of the disease, including preeclampsia with early-onset disease (before 34 weeks of gestation) and late-onset disease (after 34 weeks of gestation). These phenotypes have different pathophysiology and disease severity and, therefore, different pregnancy outcomes [1]. Both clinical phenotypes are also characterized by different pathophysiological changes in the maternal cardiovascular system (CVS), because altered placental morphogenesis and placental ischemia cause changes in blood flow in the fetal-placental system, as well as impaired regulation of systemic blood pressure (BP). These consequences are realized in the maladaptation of maternal hemodynamics and hemodynamics of the fetal-placental system [2–4].
The maternal hemodynamic profile is currently assessed by 24h-ABPM using a limited set of parameters that reflect the 24h BP profile and arterial stiffness using the augmentation index (AIx) [5–7]. However, the analysis of the CVS of the functional state of the pregnant woman and the long-term prognosis of complications requires a comprehensive assessment that takes into account both basic and additional hemodynamic parameters. In particular, one of the variants of the 24-hour-ABPM technique performed with a portable recorder of the BPLab daily monitor, the data of which are analyzed using Vasotens technology, allows an estimation of an extended range of parameters not only of arterial stiffness but also of myocardial functioning [8]. These parameters may be informative for understanding and predicting changes in the condition of the pregnant woman, but they are not currently used in clinical practice.
An equally significant problem is the lack of effective antihypertensive therapy in preeclampsia. Treatment is symptomatic and does not provide a sustained clinical effect, especially in early-onset preeclampsia. Therapy including mono-component methyldopa (Dopegit) and two-component Dopegit combined with nifedipine (Cordaflex) is not always effective, and the impact of these antihypertensive drugs on maternal hemodynamic profile has not been adequately evaluated. Dopegit and Cordaflex are antihypertensive drugs with different mechanism of action [9]. Our earlier data indicate a different effect of both drugs on the destruction of the surface layer of endothelial cells that is referred to as the endothelial glycocalyx (eGCX). This mechanism is a key stage of endothelial dysfunction and one of the leading pathogenetic factors of preeclampsia of both clinical phenotypes [10–12]. However, the effect of these drugs on the maternal hemodynamic profile has not been established in view of the wide spectrum of parameters.
The present study aimed to investigate the effect of mono- and two-component antihypertensive therapy on the maternal hemodynamic profile and identify the relationship between hemodynamic parameters characterizing changes in maternal hemodynamics and the hemodynamic profile in the maternal-fetal-placental unit, as well as indicators of eGCX degradation in early- and late-onset preeclampsia.
Materials and methods
This interventional longitudinal study enrolled 82 second- and third-trimester pregnant women, of whom 30 women with uncomplicated pregnancy were included in the comparison group [Group 1A – second-trimester patients (n=15), Group 1B – third-trimester patients (n=15)]. The study group included 52 patients with preeclampsia [Group 2A – patients with early-onset preeclampsia (n=12) receiving monotherapy; Group 2B – patients with early-onset preeclampsia receiving two-component therapy (n=12); Group 3A – patients with late-onset preeclampsia on monotherapy (n=16); Group 3B – patients with late-onset preeclampsia receiving two-component therapy (n=12)]. The study was conducted at the V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia (hereafter referred to as the Center) from January 2021 to May 2022 and was organized according to the Declaration of Helsinki of the principles of the World Medical Association with modifications at the 64th WMA General Assembly (Fortaleza, Brazil, 2013). The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No: 5/May 27, 2021). Early-onset preeclampsia was registered at symptom manifestation before 34 weeks of gestation; late-onset preeclampsia was registered after 34 weeks. Inclusion criteria for all groups were spontaneous singleton pregnancy, age 18–42 years, and signed informed consent to participate in the study. Inclusion criteria for the study group were the diagnosis of preeclampsia established according to the criteria of the clinical guidelines of the Russian Ministry of Health [13]; for the comparison group – healthy pregnancy. Non-inclusion criteria were pregnancy resulting from the use of assisted reproductive technologies, severe nonobstetric comorbidities, organ transplants, malignancies, systemic rheumatological and autoimmune diseases. Exclusion criteria were HELLP syndrome, fetal chromosomal abnormalities, fetal malformations, and acute infectious and viral diseases. Patients were included in the study by matched pairs based on age, body mass index, and gestational age.
All patients in the study group underwent standard examination and treatment in hospital settings. Patients in the comparison group received no drug therapy. First-line basic therapy was represented by the antihypertensive drug Dopegit, the dose of which was determined by the severity of hypertension. The average daily dose of the drug was 1500 mg. In patients with persistent hypertension, despite taking Dopegit in the maximum daily dose (2000 mg), the patient was additionally administered an extended-release calcium channel blocker Cordaflex. Its average daily dose was 40 mg. The indication for starting antihypertensive therapy was the registration of a steady increase in BP≥140/90 mm Hg. The efficiency of the therapy was evaluated by the patient's medical records (BP was checked 4–6 times a day by the patient and the medical staff). The mean duration of therapy was 14.83 (3.5) days in Group 2A; 43.33 (15.7) days in Group 2B; 11.81 (8.62) days in Group 3A; 52.55 (8.46) days in Group 3B. Because the study group also included patients with chronic arterial hypertension, they usually received antihypertensive therapy before the manifestation of preeclampsia. However, their development of preeclampsia required optimization of therapy.
Antihypertensive therapy, according to clinical guidelines [13], was administered when BP≥140/90 mm Hg. To adjust therapy in all patients, 24h-ABPM was performed using the BPLab device (Petr Telegin, Nizhny Novgorod, Russia), which is recommended for use in pregnant women [14]. We used a BPLab MnSDP-2 blood pressure monitor [24h monitor with cuff pressure registration (oscillogram) to increase the reliability of blood pressure monitoring] with an additional auscultatory channel, but without ECG sensor. Measurement intervals were 30 min during the day and 60 min at night. Oscillograms were analyzed using Vasotens software (Russia) with determination of a number of hemodynamic parameters. The following parameters were used to characterize changes in central aortic pressure, assess arterial stiffness and intracardiac hemodynamics: maximal diastolic BP in aorta (max DIAao), maximal systolic BP in aorta (max SYSao), minimal diastolic BP in aorta (min DIAao), minimal systolic BP in aorta (min SYSao), mean BP in aorta (MBPao), mean systolic BP in aorta (mean SYSao), Reflected Wave Transit Time (RWTT), Aortic Pulse Wave Velocity (PWVao), Aix, Ambulatory Arterial Stiffness Index (АASI) calculated as AASI=1-(DAP slope-SAP); Maximal Rate of Rise of Arterial Pressure (dP/dt)max), Pulse Pressure Amplification (PPA), Ejection Duration (ED), and Subendocardial Viability Ratio (SEVR) [8].
The blood eGCX components were analyzed by enzyme immunoassay. There were used commercial test systems by Cloud-Clone Corp., USA: CEA182Ge (determination of hyaluronan – minimum detectable quantity 1.77 ng/ml); SEB966Hu (determination of syndecan-1 – minimum detectable quantity 0.61 ng/ml); SEC463Hu (determination of endocan-1 – minimum detectable quantity 0.065 ng/ml).
The endpoints of the study were parameters characterizing central BP in aorta, arterial stiffness, intracardiac hemodynamics, and blood content of structural components of eGCX; correlations between proteoglycan content and indices characterizing maternal hemodynamics, and hemodynamics of the fetal-placental system.
The evaluation criteria were differences between the mean values of the parameters in the groups with mono- and two-component antihypertensive therapy and those in women with healthy pregnancy; a correlation between the parameters.
Statistical analysis
Statistical analysis and graphical representation were performed using MedCalc version 16.4 software (MedCalc, Belgium). The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. Continuous variables were compared between two groups with a Student’s t test. Nonnormally distributed data were reported as the median (Me) and interquartile range (Q1; Q3) and compared with a nonparametric Mann–Whitney test. Correlation analysis was performed using the Spearman rank correlation coefficient. The null hypothesis was rejected if the p-value was less than 0.050.
Results
Since the study participants were pair-matched, they were comparable by age, weight and height, and gestational age at the time of blood sampling. The analysis of perinatal outcomes once again demonstrated that patients with early-onset preeclampsia had worse perinatal outcomes compared to those with late-onset preeclampsia. Thus, all patients in this group gave birth significantly earlier; accordingly, their newborn birthweight and Apgar score were significantly lower. Moreover, the type of antihypertensive therapy had no effect on perinatal outcomes (Table 1). The duration of delivery in patients with late-onset preeclampsia was comparable with that in the comparison group; at the same time, the mean birth weight of infants born to mothers with late-onset preeclampsia was significantly lower than that in the comparison group; however, the Apgar score did not differ significantly between these groups. It is important to note that these outcomes were independent of the antihypertensive therapy (Table 1).
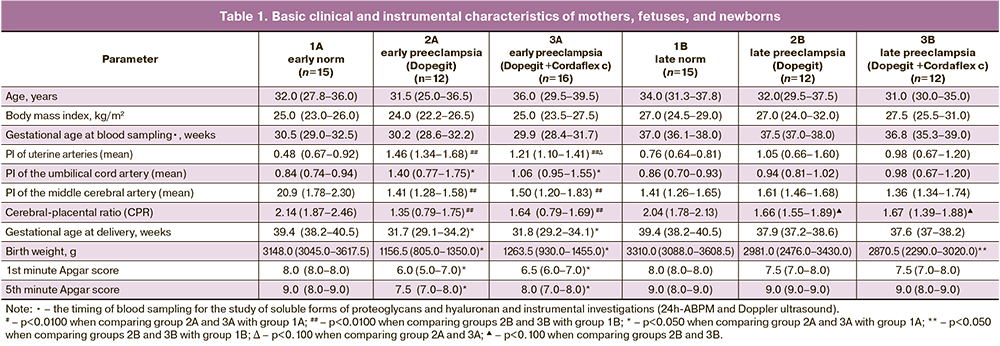
The results of 24h-ABPM in patients receiving mono and two-component antihypertensive therapy (Fig. 1) demonstrated that maximum, minimum and 24h central BP in patients with early- and late-onset preeclampsia are significantly higher than in the comparison groups at the corresponding gestational ages. No significant differences in BP were found in patients with preeclampsia taking mono- and two-component antihypertensive therapy. However, there was a trend indicating that median values of both SBP and DBP in early-onset preeclampsia were higher in patients taking two-component therapy in all comparisons. In patients with late-onset preeclampsia, median SBP and DBP are comparable in most comparisons for both therapy regimens (Fig. 1).
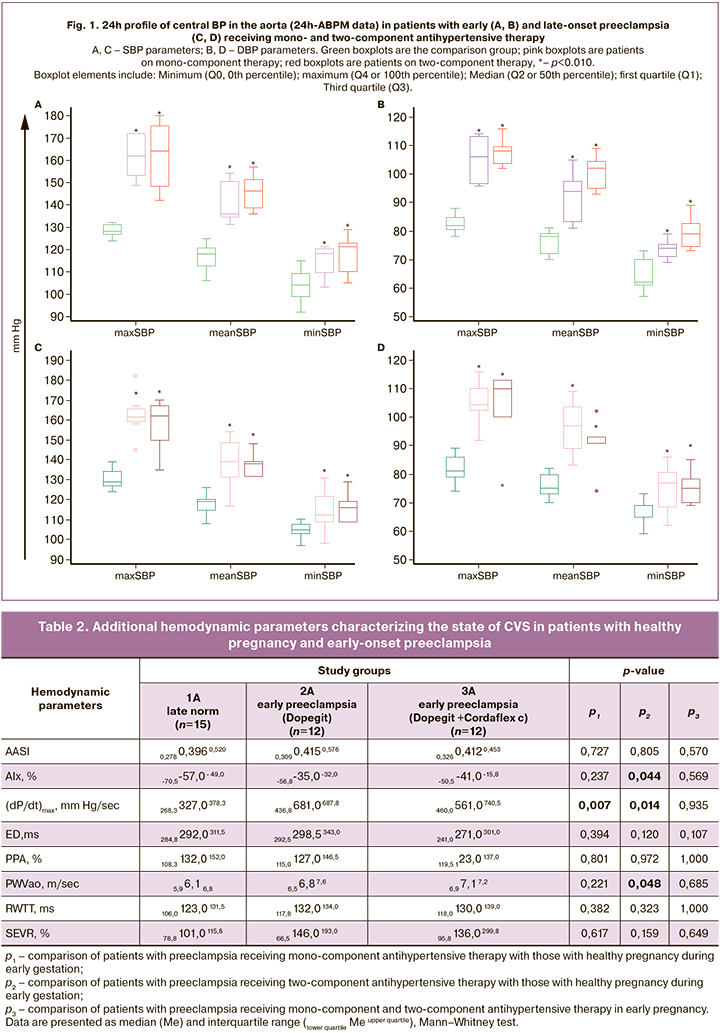
The combined use of 24h-ABPM with the BPLab monitor and Vasotens technology allowed the assessment not only of central aortic pressure but also of arterial stiffness and a number of myocardial function parameters, providing an extended functional diagnosis of maternal CVS (Tables 2 and 3). In patients with early-onset preeclampsia receiving monotherapy, it was found that there was compensation of the main hemodynamic parameters, indicating an effect of Dopegit on arterial stiffness. However, these patients had a significant increase in (dP/dt)max compared to patients with healthy pregnancy. This parameter shows the maximum arterial pressure over time and indirectly reflects myocardial contractility, total arterial stiffness, and dynamic load on the vessel walls during the pulse wave passage. The (dP/dt)max values also significantly increased in patients receiving two-component antihypertensive therapy. At the same time, the effect of the combination of Dopegit and Cordaflex on maternal arterial hypertension was less pronounced: higher AIx and RWTT values were found during its use (Table 2).
In patients with late-onset preeclampsia, when receiving combined two-component therapy, parameters assessing arterial stiffness and myocardial function were within reference values. On the contrary, a significant increase in (dP/dt)max and ED was detected with monotherapy. The ED index reflects the time interval from the closure of the onset of pulsation to the aortic valve and allows indirectly judging about systolic myocardial function. A borderline significant increase in AIx was also detected in these patients (Table 3).
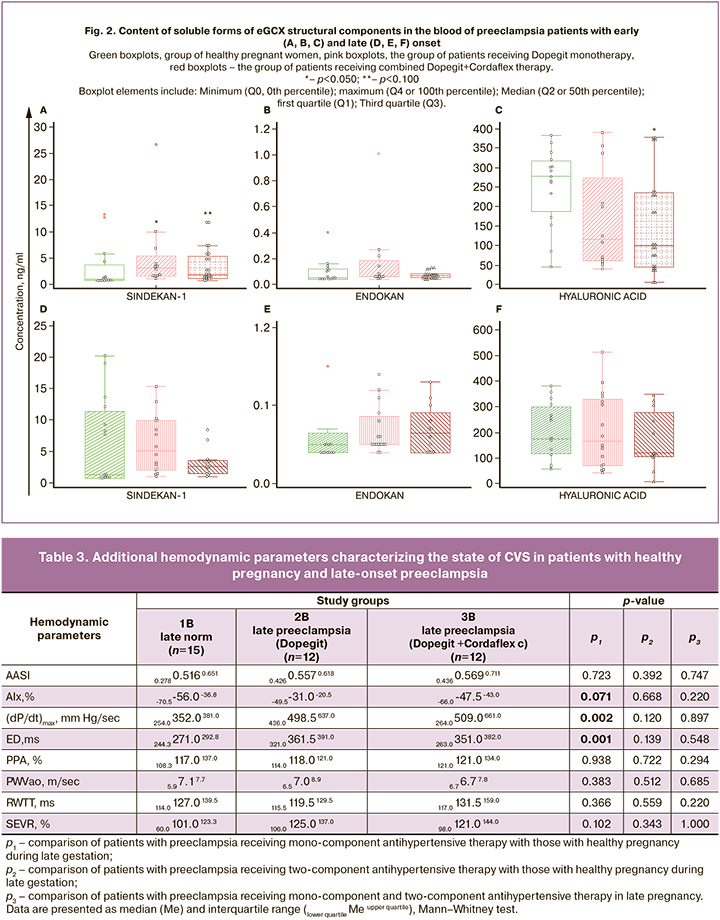
Analysis of the results of proteoglycan and hyaluronan blood levels in healthy patients and patients with preeclampsia on different antihypertensive regimens indicates that patients receiving Dopegit monotherapy showed a significant increase in syndecan-1 blood levels compared to normal pregnancies at comparable times (Fig. 2A). Two-component antihypertensive therapy revealed a significant decrease in hyaluronan levels and a borderline significant increase in syndecan-1 levels compared to healthy early pregnant women (Fig. 2A, B). No significant differences in the content of free circulating proteoglycans and hyaluronan were detected in patients with late-onset preeclampsia against the background of both antihypertensive therapy regimens, indicating the absence of manifestations of eGCX dysfunction. The blood content of endocan in all pregnant women was low. In some pregnant women, the detectable level of endocan was outside the sensitivity range of the kit, so the blood endocan content was not considered in the correlation analysis.
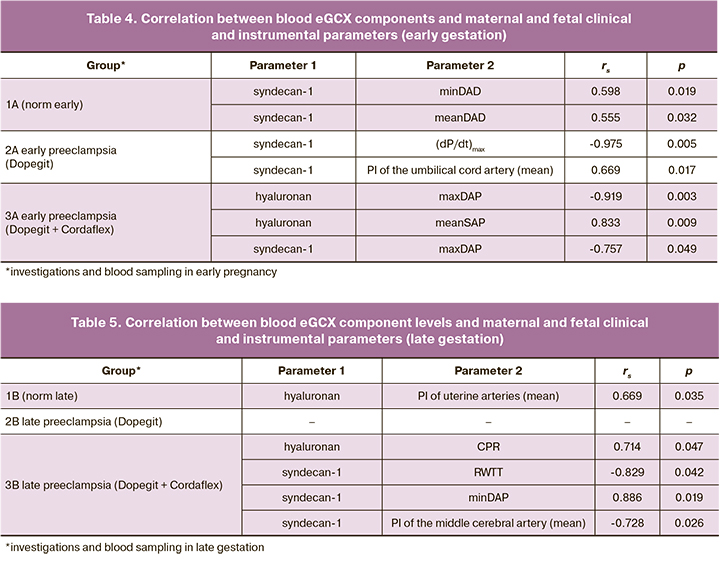
A correlation analysis was performed to assess associations between clinical and laboratory and instrumental parameters, including maternal blood proteoglycan and hyaluronan content, mean blood flow velocities in uterine, umbilical cord, and fetal cerebral artery vessels, and data from a comprehensive hemodynamic study of maternal arterial circulation. There were differences in the correlation profiles between the study groups, both in early and late pregnancy (Tables 4, 5).
Discussion
Blood pressure is mainly determined by cardiac output and systemic vascular resistance. The major pregnancy-related hemodynamic changes include increased cardiac output, increased blood volume, and reduced systemic vascular resistance and blood pressure. These changes contribute to optimal growth and development of the fetus and help to adapt maternal CVS to the load due to increased blood volume [15]. However, pregnancy superimposed on chronic hypertension or first developed during pregnancy is characterized by altered cardiovascular parameters [16]. In particular, it is known that the main hemodynamic parameters are different in early and late-onset preeclampsia. As a rule, the early-onset preeclampsia complicated by fetal growth restriction is associated with compromised placental blood flow. Late-onset preeclampsia, in contrast, is associated with maternal somatic pathology (arterial hypertension, diabetes mellitus, obesity, etc.) and, accordingly, initially altered cardiovascular parameters. Hemodynamic profiles in early preeclampsia have been found to be associated with high total peripheral vascular resistance and low cardiac output and decreased intravascular volume, and, as a rule, significant cardiovascular disorders develop in patients delayed after delivery. On the contrary, in late-onset preeclampsia, high cardiac output is combined with normal or low vascular resistance and hypervolemia [17]. Given the different pathophysiology of cardiovascular abnormalities and differently shaped hemodynamic profiles of patients with early- and late-onset preeclampsia, we analyzed the effect of two antihypertensive therapy regimens on maternal hemodynamics using an expanded spectrum of parameters.
According to the 24h-ABPM findings, despite significantly increased SBP and DBP levels in patients with preeclampsia undergoing antihypertensive therapy, they achieved target BP (for a short time in patients with early-onset preeclampsia). However, despite normalization of blood pressure, the minimum values of SBP and DBP in all patients were significantly higher than in women with healthy pregnancy (Fig. 1). Despite ongoing antihypertensive therapy in patients with early-onset preeclampsia, it was not possible to prolong pregnancy due to a progressive increase in the severity of hypertension and the deterioration of the fetal condition. This, in turn, results in preterm birth and low birth weight deliveries (Table 1). There were no significant differences in birthweight and Apgar scores, nor in the parameters of the 24-hour-ABPM profile of central aortic BP between the groups of patients with early preeclampsia receiving mono- and combined therapy (Table 1). This substantiates the feasibility of the evaluation of additional calculated indices obtained by pulse wave contour analysis, characterizing the state of systolic and diastolic heart function, pulse wave velocity, subendocardial blood flow index, etc., which play an essential role in the evaluation of CVS. Analysis of these parameters has allowed us to establish that early-onset preeclampsia, despite therapy, is associated with changes in systemic hemodynamics, which are more pronounced in patients receiving a combined two-component therapy. In particular, altered parameters that are not compensated by Dopegit and Cordaflex include the augmentation index (AIx), the most commonly used parameter in clinical practice, which reflects arterial stiffness; maximal rate of rise of arterial pressure (dP/dt)max characterizing not only myocardial contractile function, but also aortic status [18]; estimated aortic pulse wave velocity (PWVao), a parameter estimating the time of reflected wave propagation in aorta (Table. 2). It should be noted that the most important parameter PWV (pulse wave velocity), recommended as the main parameter of the assessment of arterial wall stiffness (class I, level of evidence A) [19], which reflects the vascular condition of the arterial bed, determines BP, and reflects blood viscosity, thus allowing a comprehensive characterization of the patient's CVS function [20], is not included in Vasotens software. However, the RWTT index and PWVao, which is interrelated with it, are alternative indices of CVS evaluation. An increase in all three of the above indices in patients receiving combined antihypertensive therapy indicates a lack of drug effect on systemic hemodynamics (Table 2). Dopegit monotherapy in patients with early-onset preeclampsia showed only a twofold increase in the parameter (dP/dt)max compared with that in patients with healthy pregnancy. According to the manufacturer (BPLab), this parameter indirectly reflects myocardial contractility, the total stiffness of the main arteries, as well as the "dynamic" load on the vessel walls during pulse wave transit [8] (Table 2).
Hemodynamic equilibrium in CVS was more effectively maintained when antihypertensive therapy was used for late-onset preeclampsia. The combined two-component therapy showed no differences in additional hemodynamic indices compared with their profile in healthy pregnancies (Table 3), so the therapy can be assessed as effective. This conclusion is confirmed by carrying the pregnancy to full term in the group of patients on combined antihypertensive therapy (Table 1). Dopegit monotherapy demonstrated less efficacy. The patients in this group showed significantly increased values of (dP/dt)max and the relative duration of left ventricular ejection (ED). AIx was of borderline significance in this group of patients (Table 3). Apparently, the identified characteristics of the hemodynamic profile against the background of late-onset preeclampsia indicate myocardial changes in these patients. This conclusion can be based on the increase in the index (dP/dt)max, which reflects the rate of pressure change in the left ventricle during isovolumic contraction, and characterizes the contractility of the left ventricle, which indicates an increase in the heart oxygen consumption and an increase in the load on the heart, as well as functional and compensatory changes of the myocardium. This conclusion is also confirmed by the changes in the ED index indicating the development of systolic myocardial dysfunction [21] and the detected upward trend of AIx in patients with preeclampsia receiving mono-component therapy in late pregnancy.
The hemodynamic profile of patients with early and late-onset preeclampsia before antihypertensive therapy has previously been studied previously [22]. In this study, similar changes in hemodynamic parameters were found in the absence of efficacy of the drugs used. Comparison of previously obtained data with those demonstrated in this study indicates that antihypertensive therapy affects CVS even in early-onset preeclampsia, since a number of parameters are compensated during Dopegit monotherapy. Similarly, the profile of hemodynamic parameters in late-onset preeclampsia was similar in patients without therapy [22] and with monocomponent therapy, indicating that monotherapy is not effective in late-onset preeclampsia.
The functional state of the CVS is determined not only by the state of the vessels, in particular their tone, elasticity, vascular resistance, but also by the adequate functioning of the endothelium. The initiating factor for endothelial dysfunction, the key pathogenetic element of preeclampsia, which determines the development of endotheliosis and multiple organ failure, is the surface protective layer associated with the endothelial cell membrane and facing the lumen of the vessel – eGCX [12]. The process of eGCX shedding with the release of its structural components into the bloodstream is established in various diseases, including preeclampsia, and is always associated with the loss of barrier, anti-adhesive functions of the endothelium, as well as with the loss of the ability to maintain physiological values of BP in the CPS [11]. There is experimental evidence of a direct dependence of vascular stiffness on the degree of destruction of eGCX [23, 24]. In our study, destruction of eGCX was found only in early-onset preeclampsia. Furthermore, pronounced destruction was observed with monocomponent antihypertensive therapy, indicating the absence of pharmacological protection of eGCX under the influence of the drugs used. With combined antihypertensive therapy, the signs of eGCX destruction were less pronounced, because in addition to a slight increase in blood syndecan-1 (borderline level of significance), a significantly lower hyaluronan content was detected compared with normal pregnancy (Fig. 2).
It should be noted that studies of the circulating structural components of eGCX in have been performed previously in preeclampsia, but the results of these studies are contradictory [13]. In particular, destruction of eGCX suggests an intense loss of proteoglycans and glycosaminoglycans from the eGCX layer, and their increased content in blood, which was revealed in our previous study, where different molecular and functional patterns of vessels in early and late-onset preeclampsia were characterized [22]. However, a number of studies have found decreased syndecan-1, and increased hyaluronan in preeclampsia, which is opposite to our findings [25]. In the studies cited above, there was no indication of the therapy used in patients with preeclampsia or of the comparability of the compared groups in terms of age and maternal body mass index. In our study, these confounders were taken into account (Table 1) and patients were included in the study by matched pairs, since it is known that vascular stiffness increases with age and signs of activation of the systemic inflammatory response, the main destabilizing factor of eGCX, are observed even in normal pregnancy and with increasing gestational age and, especially, in obesity [26]. The differences in the content of the circulating components we obtained may be due to the characteristic features of the cohort of patients, but we cannot rule out the presence of factors that mask these components in the blood of female patients. In particular, we have previously shown that preeclampsia has elevated levels of autoantibodies to the eGCX component hyaluronan, which may explain its decrease in blood of preeclampsia patients [27]. Antihypertensive therapy contributes to a decrease in the release of hyaluronan into the bloodstream, but this needs to be further proved.
Correlation analysis between maternal hemodynamic parameters, fetal placental unit, and eGCX components showed different correlation patterns in study groups. Syndecan-1, given the significant differences detected early in pregnancy, appears to have a special role (Fig. 2, Table 4), which is determined by its changing blood concentrations. Syndecan-1 is a multifunctional molecule that positively and negatively regulates the processes of angiogenesis, blood coagulation, inflammation, and lipid metabolism. It was established that it affects mechanosensitive endothelial cells [28]. Given the lack of efficacy of both mono- and combined antihypertensive therapy in early-onset preeclampsia, the effect of syndecan-1 should be investigated in more detail, with the study of intracellular signaling pathways in model experiments. On the contrary, the correlation patterns found in late-onset preeclampsia, given the clinical efficacy of antihypertensive therapy (especially combined therapy), indicate a positive effect of hyaluronan on the hemodynamics of the fetal placental unit (Table 5).
Conclusion
First, our findings suggest a pathogenetic link between molecular and functional vascular changes, partially compensated by antihypertensive therapy in early and late-onset preeclampsia. Secondly, we present the successful use of the BPLab device and Vasotens software that offer recording a wide range of maternal hemodynamic parameters to assess the effectiveness of antihypertensive therapy for both phenotypes of preeclampsia. The lack of efficacy of antihypertensive therapy in early-onset preeclampsia is confirmed by hemodynamic changes that are not corrected by antihypertensive drugs. Monotherapy for early-onset preeclampsia has a greater protective effect, but it does not result in the prolongation of pregnancy. However, this effect can be studied in the long-term follow-up of the patient’s CVS state. The efficacy of combined antihypertensive therapy for late-onset preeclampsia is confirmed by compensation of maternal hemodynamic parameters, which is an important conclusion of this study, since the additional administration of Cordaflex is made for indications when the patients' condition worsens and the Dopegit monotherapy is ineffective. Monotherapy appears to be less effective in late-onset preeclampsia, as altered parameters suggestive of myocardial dysfunction are detected.
References
- Ходжаева З.С., Холин А.М., Вихляева Е.М. Ранняя и поздняя преэклампсия: парадигмы патобиологии и клиническая практика. Акушерство и гинекология. 2013; 10: 4-11. [Khodzhaeva Z.S., Kholin A.M., Vikhlyaeva E.M. Early and late preeclampsia: Pathobiology paradigms and clinical practice. Obstetrics and Gynecology. 2013; 10: 4-11. (in Russian)].
- Di Pasquo E., Ghi T., Dall'Asta A., Angeli L., Fieni S., Pedrazzi G., Frusca T. Maternal cardiac parameters can help in differentiating the clinical profile of preeclampsia and in predicting progression from mild to severe forms. Am. J. Obstet. Gynecol. 2019; 221(6): 633.e1-633.e9. https://dx.doi.org/10.1016/j.ajog.2019.06.029.
- Vasapollo B., Novelli G.P., Valensise H. Total vascular resistance and left ventricular morphology as screening tools for complications in pregnancy. Hypertension. 2008; 51(4): 1020-6. https://dx.doi.org/10.1161/HYPERTENSIONAHA.107.105858.
- Ghossein-Doha C., Khalil A., Lees C.C. Maternal hemodynamics: a 2017 update. Ultrasound Obstet. Gynecol. 2017; 49(1): 10-4. https://dx.doi.org/10.1002/uog.17377.
- Ли О.А. Результаты суточного мониторирования артериального давления у женщин с метаболическим синдромом во II триместре беременности. Лечебное дело. 2011; 4: 77-84. [Lee O.A. 24-hour blood pressure monitoring in women with metabolic syndrome during the second trimester of pregnancy. Lechebnoe delo. 2011; 4: 77-84. (in Russian)].
- Чулков В.С., Вереина Н.К., Синицын С.П., Долгушина В.Ф. Оценка показателей центрального артериального давления и ригидности артерий у беременных с различными формами артериальной гипертонии. Терапевтический архив. 2014; 86(12): 15-9. https://dx.doi.org/10.17116/terarkh2014861215-19. [Chulkov V.S., Vereina N.K., Sinitsyn S.P., Dolgushina V.F. Estimation of central blood pressure and arterial stiffness in pregnant women with different forms of hypertension. Therapeutic Archive. 2014; 86(12): 15-9. (in Russian)]. https://dx.doi.org/10.17116/terarkh2014861215-19.
- Tan M.Y., Koutoulas L., Wright D., Nicolaides K.H., Poon L.C.Y. Protocol for the prospective validation study: 'Screening programme for pre-eclampsia' (SPREE). Ultrasound Obstet. Gynecol. 2017; 50(2): 175-9. https://dx.doi.org/10.1002/uog.17467.
- Руководство пользователя ПО BPLab® V.06.02.00 (редакция 01.2018): с. 28. [BPLab® User Manual V.06.02.00 (revision 01.2018): page 28. (in Russian)].
- Easterling T., Mundle S., Bracken H., Parvekar S., Mool S., Magee L.A. et al. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial. Lancet. 2019; 394(10203): 1011-21. https://dx.doi.org/10.1016/S0140-6736(19)31282-6.
- Муминова К.Т., Зиганшина М.М., Ходжаева З.С. Оценка влияния различных схем гипотензивной терапии на состояние эндотелиального гликокаликса системы кровообращения у пациенток с преэклампсией. Экспериментальная и клиническая фармакология. 2022; 85(10): 4-10. https://dx.doi.org/10.30906/0869-2092-2022-85-10-4-10. [Muminova K.T., Ziganshina M.M., Khodzhaeva Z.S. Evaluation of the effect of different strategies of antihypertensive treatment on endothelial glycocalyx in patients with preeclampsia. Experimental and clinical pharmacology. 2022; 85(10): 4-10. (in Russian)]. https://dx.doi.org/10.30906/0869-2092-2022-85-10-4-10.
- Ziganshina M.M., Yarotskaya E.L., Pavlovich S.V., Sukhikh G.T., Bovin N.V. Can endothelial glycocalyx be a major morphological substrate in pre-eclampsia? Int. J. Mol. Sci. 2020; 21(9): 3048. https://dx.doi.org/10.3390/ijms21093048.
- Зиганшина М.М., Зиганшин А.Р., Халтурина Е.О., Баранов И.И. Артериальная гипертензия как следствие дисфункции эндотелиального гликокаликса: современный взгляд на проблему сердечно-сосудистых заболеваний. Кардиоваскулярная терапия и профилактика. 2022; 21(9): 91-103. [Ziganshina M.M., Ziganshin A.R., Khalturina E.O., Baranov I.I. Arterial hypertension as a consequence of endothelial glycocalyx dysfunction: a modern view of the problem of cardiovascular diseases. Cardiovascular therapy and prevention. 2022; 21(9): 91-103. (in Russian)].
- Министерство здравоохранения Российской Федерации. Клинические рекомендации «Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде». 2021. [Ministry of Health of the Russian Federation. Clinical guidelines "Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, childbirth and the postpartum period." 2021. (in Russian)].
- Dorogova I.V., Panina E.S. Comparison of the BPLab®sphygmomanometer for ambulatory blood pressure monitoring with mercury sphygmomanometry in pregnant women: Validation study according to the British Hypertension Society protocol. Vasc. Health Risk Manag. 2015; 11: 245-9. https://dx.doi.org/10.2147/VHRM.S82381.
- Ling H.Z., Guy G.P., Bisquera A., Poon L.C., Nicolaides K.H., Kametas N.A. Maternal hemodynamics in screen-positive and screen-negative women of the ASPRE trial. Ultrasound Obstet. Gynecol. 2019; 54(1):51-7. https://dx.doi.org/10.1002/uog.20125.
- Ferrazzi E., Stampalija T., Monasta L., Di Martino D., Vonck S., Gyselaers W. Maternal hemodynamics: a method to classify hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2018; 218(1): 124.e1-124.e11. https://dx.doi.org/10.1016/j.ajog.2017.10.226. Повтор см. №2
- Ghossein-Doha C., Khalil A., Lees C.C. Maternal hemodynamics: a 2017 update. Ultrasound Obstet. Gynecol. 2017; 49(1): 10-14. https://dx.doi.org/10.1002/uog.17377.
- Евсевьева М.Е., Ерёмин М.В., Итальянцева Е.В., Кошель В.И., Карпов В.П. Сосудистая ригидность, центральное давление и некоторые показатели функционирования миокарда при декомпенсированном хроническом тонзиллите. Медицинский вестник Северного Кавказа. 2020; 15(2): 229-33. https://dx.doi.org/10.14300/mnnc.2020.15054. [Evsevyeva M.E., Eremin M.V., Italyantseva E.V., Koshel V.I., Karpov V.P. Vascular stiffness, central pressure and some indicators of myocardial function in the presence of decompensated chronic tonsillitis. Medical news of North Caucasus. 2020; 15(2): 229-33. (in Russian)]. https://dx.doi.org/10.14300/mnnc.2020.15054.
- Townsend R.R., Wilkinson I.B., Schiffrin E.L., Avolio A.P., Chirinos J.A., Cockcroft J.R. et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015; 66(3): 698-722. https://dx.doi.org/10.1161/HYP.0000000000000033.
- Глазков А.А., Лапитан Д.Г., Макаров В.В., Рогаткин Д.А. Оптический неинвазивный автоматизированный прибор для исследования центральной и периферической гемодинамики. Физические основы приборостроения. 2021; 10(4): 28-36. https://dx.doi.org/10.25210/jfop 2104-028036. [Glazkov A.A., Lapitan D.G., Makarov V.V., Rogatkin D.A. Optical non-invasive automated device for the study of central and peripheral hemodynamics. Physical fundamentals of instrumentation. 2021; 10(4): 28-36. (in Russian)]. https://dx.doi.org/10.25210/jfop 2104-028036.
- Васюк Ю.А., Иванова С.В., Школьник Е.Л., Котовская Ю.В., Милягин В.А., Олейников В.Э., Орлова Я.А., Сумин А.Н., Баранов А.А., Бойцов С.А., Галявич А.С., Кобалава Ж.Д., Кожевникова О.В., Конради А.О., Лопатин Ю.М., Мареев В.Ю., Новикова Д.С., Оганов Р.Г., Рогоза А.Н., Ротарь О.П., Сергацкая Н.В., Скибицкий В.В. Согласованное мнение российских экспертов по оценке артериальной жесткости в клинической практике. Кардиоваскулярная терапия и профилактика. 2016; 2: 4-19. [Vasyuk Yu.A., Ivanova S.V., Shkolnik E.L., Kotovskaya Yu.V., Milyagin V.A., Oleynikov V.E. et al. The agreed opinion of Russian experts on the assessment of arterial stiffness in clinical practice. Cardiovascular therapy and prevention. 2016; 2: 4-19. (in Russian)].
- Ziganshina M.M., Muminova K.T., Khasbiullina N.R., Khodzhaeva Z.S., Yarotskaya E.L., Sukhikh G.T. Characterization of vascular patterns associated with endothelial glycocalyx damage in early- and late-onset preeclampsia. Biomedicines. 2022; 10: 2790. https://dx.doi.org/10.3390/biomedicines10112790.
- Ikonomidis I., Voumvourakis A., Makavos G., Triantafyllidi H., Pavlidis G., Katogiannis K. et al. Association of impaired endothelial glycocalyx with arterial stiffness, coronary microcirculatory dysfunction, and abnormal myocardial deformation in untreated hypertensives. J. Clin. Hypertens. (Greenwich). 2018; 20(4): 672-9. https://dx.doi.org/10.1111/jch.13236.
- Mahmoud M., Mayer M., Cancel L.M., Bartosch A.M., Mathews R., Tarbell J.M. The glycocalyx core protein glypican 1 protects vessel wall endothelial cells from stiffness-mediated dysfunction and disease. Cardiovasc. Res. 2021; 117(6): 1592-605. https://dx.doi.org/10.1093/cvr/cvaa201.
- Davutoglu А.E., Firat А.A., Ozel A., Yılmaz N., Uzun I., Yuksel Т.I. et al. Evaluation of maternal serum hypoxia inducible factor-1α, progranulin and syndecan-1 levels in pregnancies with early- and late-onset preeclampsia. J. Matern. Fetal Neonatal Med. 2018; 31(15): 1976-82. https://dx.doi.org/10.1080/14767058.2017.1333098.
- Weinbaum S., Cancel L.M., Fu B.M., Tarbell J.M. The glycocalyx and its role in vascular physiology and vascular related diseases. Cardiovasc. Eng. Technol. 2021; 12(1): 37-71. https://dx.doi.org/10.1007/s13239-020-00485-9.
- Зиганшина М.М., Шилова Н.В., Хасбиуллина Н.Р., Новаковский М.Е., Николаева М.А., Кан Н.Е., Вавина О.В., Николаева А.В., Тютюнник Н.В., Сергунина О.А., Бот И., Тютюнник В.Л., Бовин Н.В., Сухих Г.Т. Аутоантитела к антигенам эндотелия при преэклампсии. Акушерство и гинекология. 2016; 3: 24-31. https://dx.doi.org/10.18565/aig.2016.3.24-31. [Ziganshina M.M., Shilova N.V., Khasbiullina N.R., Novakovsky M.E., Nikolaeva M.A., Kan N.E., Vavina O.V., Nikolaeva A.V., Tyutyunnik N.V., Sergunina O.A., Bot I., Tyutyunnik V.L., Bovin N.V., Sukhikh G.T. Autoantibodies against endothelial antigens in preeclampsia. Obstetrics and Gynecology. 2016; 3: 24-31. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.3.24-31.
- Gandley E., Althouse А., Jeyabalan А., Bregand-White J.M., McGonigal S., Myerski A.C. et al. Low soluble syndecan-1 precedes preeclampsia. PLoS One. 2016; 11(6): e0157608. https://dx.doi.org/10.1371/journal.pone.0157608.
Received 05.12.2022
Accepted 22.12.2022
About the Authors
Kamilla T. Muminova, PhD, Researcher at the High Risk Pregnancy Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-06-74, kamika91@mail.ru, https://orcid.org/0000-0003-2708-4366, 117997, Russia, Moscow, Akademika Oparina str., 4.Zulfiya S. Khodzhaeva, Dr. Med. Sci., Professor, Deputy Director of Obstetrics Institute, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-07-88, zkhodjaeva@mail.ru, https://orcid.org/0000-0001-8159-3714, 117997, Russia, Moscow, Akademika Oparina str., 4.
Kseniia A. Gorina, PhD, Researcher at the High-Risk Pregnancy Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-06-74, kseniiagorina@gmail.com, https://orcid.org/0000-0001-6266-2067, Russia, Moscow, Akademika Oparina str., 4.
Roman G. Shmakov, Dr. Med. Sci., Professor, Director of the Institute of Obstetrics, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(495)438-07-88, mdshmakov@mail.ru, https://orcid.org/0000-0002-2206-1002, 117997, Russia, Moscow, Akademika Oparina str., 4.
Marina M. Ziganshina, PhD (Bio), Leading Researcher at the Laboratory of Clinical Immunology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-11-83, mmz@mail.ru, https://orcid.org/0000-0003-1578-8403, 117997, Russia, Moscow, Akademika Oparina str., 4.



