Paradigm shift in pharmacotherapy for late-onset preeclampsia: advantages of two-component antihypertensive therapy compared to monotherapy based on analysis of vasoactive status data in pregnant women
Ziganshina M.M., Muminova K.T., Khodzhaeva Z.S., Oshkhunova M.S., Baranov I.I.
An important aspect of the effectiveness of antihypertensive therapy is its effect on the production of vasoactive factors that control blood pressure (BP). The most commonly used drugs, methyldopa (Dopegyt) and nifedipine (Cordaflex), have different mechanisms for reducing BP; however, their impact on the production of vasoactive factors in pregnant women with preeclampsia (PE) has not been studied.
Objective: To characterize the vasoactive status of pregnant women with late-onset PE who received different antihypertensive therapy regimens: monotherapy with Dopegyt and two-component therapy with Dopegyt and Cordaflex.
Materials and methods: The study included 47 pregnant women with a gestational age > 34 weeks. The control group comprised 18 patients with healthy pregnancies, while the study group included 29 pregnant women with PE; 15 received antihypertensive monotherapy (Dopegyt) (Group 1), and 14 received two-component therapy (Dopegyt+Cordaflex) (Group 2). Clinical assessments included the following: 1) hemodynamic status based on 24-hour ambulatory blood pressure monitoring, considering parameters characterizing changes in central aortic pressure, intracardiac hemodynamics, and arterial stiffness; 2) hemodynamic status in the fetoplacental system based on Doppler findings; and 3) factors regulating vascular tone, thrombosis, and markers of cardiovascular insufficiency in peripheral blood based on the results of the immunoassay analysis.
Results: A balance was established between vasoconstrictors and vasodilators in pregnant women with late-onset PE who received both antihypertensive regimens. Pregnant women receiving two-component therapy exhibited a more compensated hemodynamic status. An increase in antithrombin levels in the blood of pregnant women who received both regimens was noted, presumably owing to the effects of Dopegyt.
Conclusion: In late-onset PE, antihypertensive therapy provides control of the humoral regulation of vascular tone and promotes a balance between vasoconstrictors and vasodilators. The use of two antihypertensive drugs with different mechanisms of action favorably influences the cardiovascular system of pregnant women and adequately regulates the synthetic function of endothelial cells.
Authors’ contributions: Ziganshina M.M., Khodzhaeva Z.S., Baranov I.I. – conception and design of the study; Muminova K.T., Khodzhaeva Z.S., Oshkhunova M.S. – recruitment of patients into the study, obtaining hemodynamic profile data;
Ziganshina M.M. – ELISA, statistical analysis; Ziganshina M.M., Muminova K.T. – drafting of the manuscript;
Khodzhaeva Z.S., Baranov I.I. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the State Task of the Ministry of Health
of the Russian Federation № 121040600435-0 «Justification of personalized approaches to antihypertensive therapy in HDP and PE».
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC
for OG&P (Ref. No. 5 of May 27, 2021).
Patient Сonsent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Ziganshina M.M., Muminova K.T., Khodzhaeva Z.S.,
Oshkhunova M.S., Baranov I.I. Paradigm shift in pharmacotherapy for late-onset preeclampsia: advantages of two-component antihypertensive therapy compared to monotherapy based on analysis of vasoactive status data in pregnant women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (12): 104-113 (in Russian)
https://dx.doi.org/10.18565/aig.2023.240
Keywords
Antihypertensive therapy for patients with hypertensive disorders of pregnancy is limited to a narrow range of drugs that do not have teratogenic effects. The most frequently used antihypertensive drugs during pregnancy include a centrally acting alpha2-adrenomimetic drug with an inhibitory effect on sympathetic impulses, Dopegyt (active substance – methyldopa), and an extended-release selective calcium channel blocker, Cordaflex (active substance, nifedipine). Dopegyt serves as the first-line antihypertensive therapy, with Cordaflex added if Dopegyt proves ineffective [1].
The efficacy of these drugs in preeclampsia (PE) varies according to clinical phenotype. In early-onset PE (ePE), antihypertensive therapy demonstrates limited effectiveness, and prolongation of pregnancy is notably brief. Conversely, in late-onset PE (lPE), which arises from maternal factors associated with pre-existing endothelial dysfunction and is exacerbated by the development of sterile inflammation in placental tissues [2], antihypertensive therapy shows greater efficacy. Both drugs induce vasodilation by reducing the total peripheral vascular resistance through different mechanisms. However, the effect of these drugs on vasoactive mediators related to endothelial dysfunction in PE has not been comprehensively investigated. Existing evidence, primarily from in vitro studies, demonstrates the effects of methyldopa [3, 4] and nifedipine [5, 6] on endogenous nitric oxide (NO) production.
Our previous studies, aimed at broadening our understanding of the cardiovascular system's response to Dopegyt and Cordaflex during pregnancy, revealed a significant impact of these drugs on maternal hemodynamics [7]. These investigations revealed varying degrees of expression of the proinflammatory background and endothelial glycocalyx (eGC) destruction within the circulatory system in ePE and lPE, both in patients receiving Dopegyt alone and in combination with Cordaflex [8, 9]. Proinflammatory stimuli contribute to eGC destruction, a structure involved in mechanotransduction—an essential process leading to the activation of endothelial nitric oxide synthase (eNOS) and the synthesis of the endogenous vasodilator NO [10, 11]. Additionally, the eGC functions as a reservoir for vasoactive and vascular hemostasis-regulating factors. Based on the outcomes of our prior studies, which established a link between eGC destruction and hemodynamic disturbances [12], and the differential protective effects of the glycocalyx offered by antihypertensive therapies across various clinical phenotypes of PE [7-9], we postulated an imbalance in vasoconstrictors/vasodilators among PE patients. To validate this assumption, we investigated the vasoactive status of pregnant women with ePE receiving Dopegyt alone and in combination with Cordaflex. The term "vasoactive status" here refers to the characterization of the endothelium and blood vessels using quantitative and functional indices (including hemodynamic indices and indices reflecting the endothelium's capacity to produce vasoactive factors) determined by standard clinical and laboratory methods. These findings indicate the ineffectiveness of antihypertensive therapy in ePE for both treatment regimens [13].
This study aimed to investigate the vasoactive status of pregnant women with lPE receiving different antihypertensive therapy regimens, including monotherapy with Dopegyt and two-component therapy with Dopegyt+Cordaflex.
Materials and methods
Study design and selection criteria
This non-randomized controlled study was organized according to the principles of the Declaration of Helsinki of the World Medical Association and was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No. 5 of May 27, 2021) (hereinafter referred to as the Center) during the period 10.01.2021 to 31.06.2023. The study included 47 pregnant women with a gestational age > 34 weeks. The control group comprised 18 patients with healthy pregnancies, while the study group included 29 pregnant women with PE; 15 received antihypertensive monotherapy (Dopegyt) (Group 1), and 14 received two-component therapy (Dopegyt + Cordaflex) (Group 2). The inclusion criteria for both groups, as well as the criteria for non-inclusion, are described in [13], as this study was part of the study cited above. Pregnant women were selected for the study by pair matching based on age, body mass index (BMI), and gestational age.
Antihypertensive therapy regimens
All patients in the study group received antihypertensive therapy, which was prescribed when BP was ≥140/90 mmHg according to the clinical recommendations of the Ministry of Health of the Russian Federation [1]. Pregnant women underwent 24-hour blood pressure monitoring (24-h BPM) using a BPLab device (Peter Telegin, Nizhny Novgorod, Russia), according to which the dosage of drugs was adjusted. The main antihypertensive drug for antihypertensive monotherapy was methyldopa (Dopegyt); the initial dose was 750 mg/day, with an increase in the case of stable hypertension to 2000 mg/day (mean daily dose 1500 mg). Two-component antihypertensive therapy was administered in the event of a persistent increase in blood pressure that was not reduced by Dopegyt. In addition to Dopegyt, nifedipine (Cordaflex) was administered at a dose of 2000 mg/day from a starting dose of 20 mg/day, which was increased to 40 mg/day in the case of persistent hypertension.
Evaluation of maternal hemodynamic profile
The efficacy of antihypertensive therapy was evaluated, and antihypertensive therapy was adjusted according to the 24-hour BPM data. The oscillograms obtained from the device were analyzed using Vasotens software (Russia). The list of recorded hemodynamic parameters and analysis of oscillograms were similar to those previously presented [7]. We analyzed: maximal aortal diastolic BP (max DADao); maximal aortal systolic BP (max SADao); minimal aortal diastolic BP (min DADao); minimal aortal systolic BP (min SADao); mean aortal diastolic BP (med DADao); and mean aortal systolic BP (med SADao). The following parameters were evaluated: aortic Pulse Wave Transit Time (RWTT), Aortic Pulse Wave Velocity (PWVao), augmentation index (AIx), Ambulatory Arterial Stiffness Index (AASI) calculated as AASI=1-(slope of DAD-SAD), maximum rate of the arterial pressure rise (dP/dt)max), Pulse Pressure Amplification (PPA), Ejection Duration (ED), Subendocardial Viability Ratio (SEVR) [7, 12].
Assessment of uteroplacental and fetoplacental blood flows
Ultrasound Doppler study of uteroplacental and fetoplacental blood flow was performed on expert class devices (GE Voluson E8, USA), as previously described [13]. The following parameters were recorded: UtA-PI – pulsatility index (PI) in uterine (UtA-PI), umbilical (UmA-PI), middle cerebral (MCA-PI) arteries, venous duct, and calculation of cerebro-placental ratio (CPR).
Determination of blood factors for assessment of vasoactive status
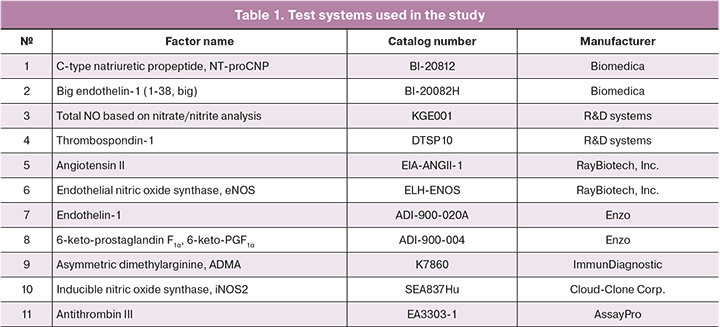
The peripheral blood serum of pregnant women processed and deposited in the Biobank of the Center served as material. The study was performed using an immunoenzymatic assay with the commercially available kits listed in Table 1. Determination of total NO by nitrate/nitrite assay was performed in accordance with the recommendations of R&D systems for the KGE001 kit with ultrafiltration of blood serum using tubes with Amicon Ultra 0.5ML 10 K 96PK filters (Millipore) and isolation of the low molecular weight fraction (less than 10,000 kDa), in which nitrate/nitrite determination was performed.
Statistical analysis
Statistical analysis and graphical presentation of the data were performed using MedCalc version 16.4 (MedCalc, Belgium). The normality of the distribution was tested using the Shapiro–Wilk test. Continuous variables are presented as median (Me) and maximum and minimum values (Q0 – 0th percentile; Q4 – 100th percentile). Intergroup differences when comparing three or more groups for continuous variables whose distribution differed from normal were calculated using the Kruskal–Wallis test with Bonferroni correction at p<0.025. For post-hoc comparisons, the Mann–Whitney U test was used at p<0.050. The null hypothesis was rejected when the p-value was less than 0.050. Correlation analysis was performed by calculating the Spearman's rank correlation coefficient; differences were considered significant at p<0.050.
Results
All patients were comparable in age, BMI, and gestational age at the time of blood sampling, and the main confounding factors were eliminated. The development of PE in patients in the study group resulted in significantly high BP values (Table 2). Newborns in the study group had lower birth weights. A significant difference from the control group was found only for newborns born to mothers receiving two-component antihypertensive therapy (Table 2). However, the 0th percentile of birth weight in Group 1 was lower than that in Group 2. The gestational age at delivery was significantly shorter in pregnant women with PE in both groups than in those without PE, but the median value was higher in patients receiving two-component therapy than in those receiving monotherapy (Table 2).
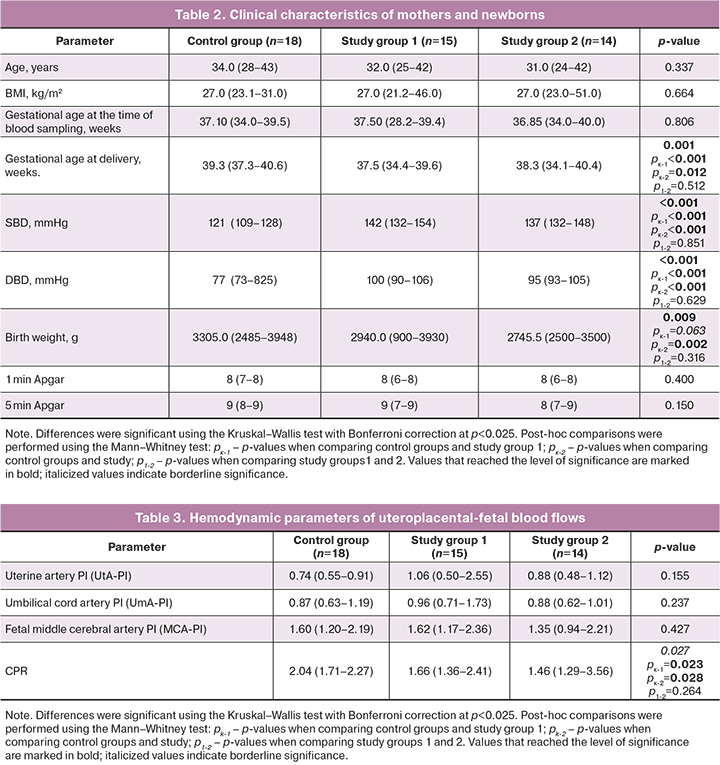
The analysis of hemodynamic data in the maternal-placental-fetal system indicated reduced CPR in patients with PE receiving both mono- and two-component antihypertensive therapy (Table 3).
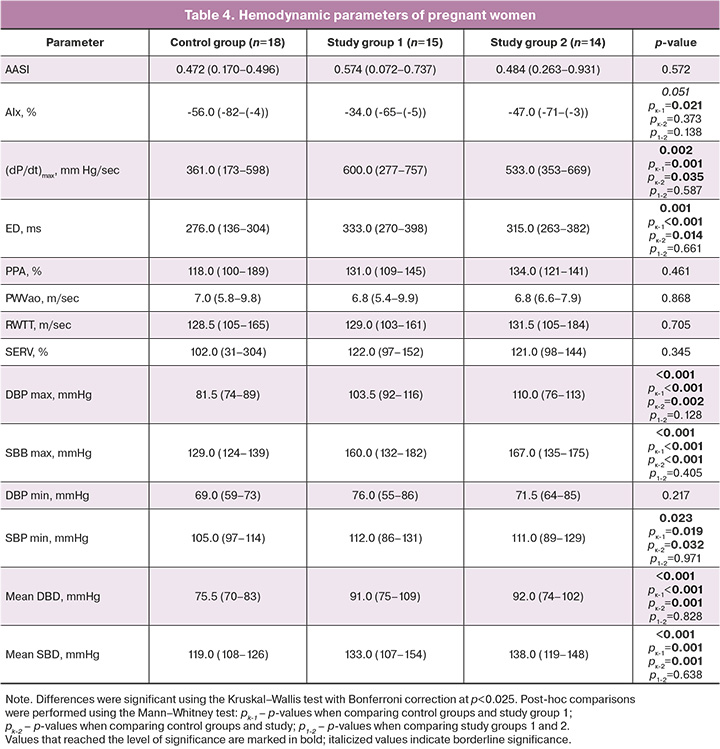
Analysis of the hemodynamic status of pregnant women showed that the study group had a borderline significant increase in the augmentation index (AIx), the main clinical parameter reflecting arterial stiffness. In post-hoc analysis, a significant increase in AIx was found only in patients receiving monotherapy. Pregnant women with PE receiving both antihypertensive therapy regimens showed a significant increase in (dP/dt)max, reflecting myocardial contractility, total stiffness of the main arteries, and dynamic load as well as in (ED), which characterizes the relative duration of the left ventricular ejection period. Higher values of this parameter were observed in pregnant women who received monotherapy. The 24-h BP trend showed significantly higher SBP and DBP in the study group (Table 4).
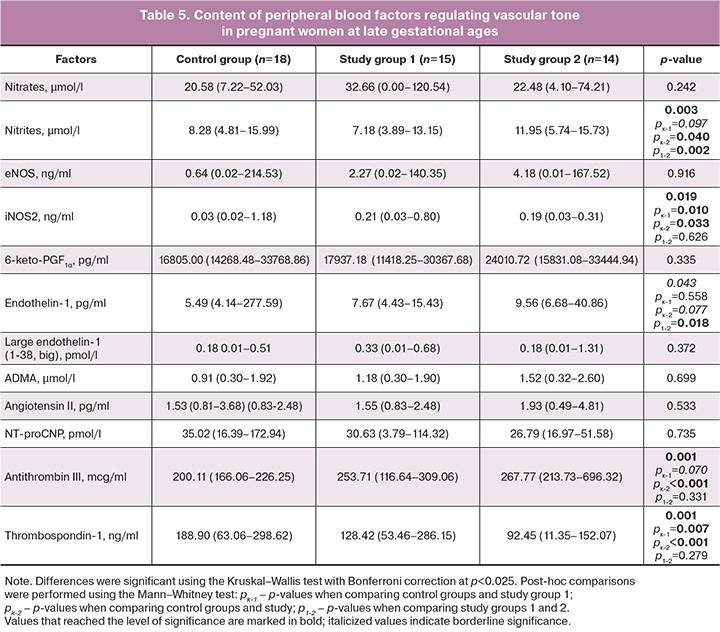
The profile of vasoactive factors identified in the blood of pregnant women indicated a relative compensation of the content of the main enzymes synthesizing vasoactive molecules and stable metabolites of NO and prostacyclin in pregnant women in the study group. Thus, the content of NO metabolite nitrate did not differ from the control group, while the content of nitrite, the main metabolite that more accurately reflects changes in endogenous NO [14], showed a tendency to decrease in pregnant women receiving monotherapy and, in contrast, was significantly higher in those receiving two-component therapy. The content of the key enzymes responsible for the production of endogenous NO and eNOS was not significantly different between the groups. In contrast, the level of inducible NO synthase (iNOS2) was significantly (6–7 times) higher in the blood of pregnant women with PE receiving both therapy regimens than in the control group (Table 5). The levels of 6-keto-PGF1α, a stable metabolite of prostacyclin, did not differ significantly between the study groups. Factors with vasoconstrictor effects (endothelin-1, large endothelin-1, ADMA, and angiotensin II) showed a slight increase in content in the study groups, but the significance level was not reached. A borderline level of significance was found for the increase in endothelin-1 in pregnant women with two-component therapy; however, pregnant women receiving monotherapy and two-component antihypertensive therapy differed significantly from each other in terms of the content of this factor (Table 5). Intergroup analysis revealed significant differences in antithrombin content; a post-hoc analysis showed a significant increase in antithrombin in pregnant women receiving two-component therapy. An increase was also observed in patients receiving monotherapy, but with a borderline level of significance. A significant decrease in blood thrombospondin-1 was found in both treatment regimens, which was more pronounced in Group 2, as confirmed by post-hoc analysis (Table 5).
Discussion
The effects resulting from increased circulating blood volume and consequently heightened cardiac output during physiological pregnancy are compensated by the induction of NO-induced systemic vascular dilatation [15]. This process ensures the adaptation of the pregnant woman's cardiovascular system to the load and facilitates blood pressure control [14]. As gestational age increases, NO synthesis [16, 17] leads to a decline in peripheral vascular resistance and an augmentation in uteroplacental blood flow [14, 17].
The vascular endothelium synthesizes NO through eNOS, which, in the presence of oxygen, triggers the conversion of L-arginine to L-citrulline, releasing NO. Transitioning from endothelial activation to dysfunction results in a gradual reduction in eNOS expression and activity, thereby decreasing NO production and bioavailability. Moreover, excessive ADMA (asymmetric dimethylarginine), a competitive inhibitor of L-arginine binding to eNOS, influences the decline in NO production [18, 19]. Pathological vessel processes, coupled with proinflammatory changes and reactive oxygen species generation, stimulate iNOS expression and mediate aberrant NO synthesis. Insufficient eNOS/NO production, impaired NO-related signaling pathways, substrate deficiency, excess competitive inhibitors, and increased vasoconstrictor factor production are pivotal in the pathogenesis of arterial hypertension, including pregnancy-induced hypertension [20, 21]. Addressing this issue is crucial for pharmacological intervention.
This study examined the key factors governing vascular tone regulation, focusing on stable metabolites of primary vasodilators such as prostacyclin and NO. Since NO rapidly oxidizes to nitrite and nitrate in the blood [22], and approximately 70% of blood nitrite stems from eNOS enzymatic activity [14], the levels of these metabolites in pregnant women's blood form the basis for interpreting the varying endogenous NO content among the study groups. Pregnant women receiving monotherapy exhibited blood levels of eNOS, endogenous NO, and 6-keto-PGF1α comparable to those in the control group. With two-component therapy, levels of 6-keto-PGF1α and eNOS mirrored those in the control group, while blood nitrite content was 1.5 times significantly higher than normal. Conversely, in pregnant women treated with both antihypertensive therapy regimens, iNOS content significantly surpassed that in the control group. Therapy likely contributes to restoring adequate cell biosynthetic functioning, since monotherapy maintained comparable NO/eNOS-related factors in the blood to normal levels. The observed NO hyperproduction in pregnant women under two-component therapy may be attributed to either eNOS expression stimulation, heightened iNOS production, or a combination of both. The study's power might not suffice to conclusively support the former because of the small number of pregnant women in the group. However, lower thrombospondin-1 levels in pregnant women in the main group, particularly those receiving two-component therapy, suggest this effect. As thrombospondin 1 antagonizes NO, reducing its relaxing effect on vascular smooth muscle cells [23], its suppressed production likely mediated by therapy positively affects NO production and vascular tone. The second factor was indirectly supported by a previous study [8] that detected proinflammatory activation, which was particularly pronounced in pregnant women receiving two-component therapy. Given that iNOS production is influenced by proinflammatory stimulation, elevated blood nitrite levels in pregnant women undergoing bicomponent therapy might result from iNOS-induced NO production.
The absence of significant differences in vasoconstriction-contributing factors –endothelin-1, its precursor, endothelin, angiotensin II, and ADMA (Table 5) – among the compared groups indicates control of vasoconstrictor production in the study group patients under both therapy regimens. Though initial vasoactive status pre-therapy in pregnant women wasn't determined, multiple studies have reported an imbalance favoring decreased vasodilator production and increased vasoconstrictor levels in PE [15, 20, 24]. Consequently, antihypertensive therapy appears to regulate humoral vascular tone and balance vasoconstrictors and vasodilators in patients with late-onset PE. Hemodynamic changes observed in mothers under both therapies (more pronounced in those under two-component therapy, Table 4) did not unfavorably impact perinatal outcomes and the mother-placenta-fetus system's hemodynamics (Tables 2, 3). Conversely, two-component antihypertensive therapy correlated with a higher median delivery term, a positive trend considering earlier delivery in severe PE [25] and the high frequency and severity of maternal hemodynamic complications [26]. In a similar study on patients with ePE [13], despite identical antihypertensive drug use and treatment regimens, the effects of therapy were not evident and were marked by altered humoral profiles of vascular tone-regulating factors, Doppler changes, maternal hemodynamic parameters, and early adverse perinatal outcomes in ePE patients under both therapy regimens. The study noted correlations suggesting NO's influence on maternal hemodynamics, fetoplacental system, and angiotensin II, iNOS, and thrombospondin-1 production in early pregnancy [13]. However, these relationships were not observed in the present study. Among the changes seen in pregnant women of the main groups against antihypertensive therapy, an increased antithrombin level stood out, known to enhance blood rheology, a vital aspect in PE, potentially predisposing to bleeding [13].
Conclusion
Investigation of the vasoactive status of pregnant women with lPE revealed a balance between vasoconstrictors and vasodilators in treated patients, reflecting the effectiveness and adequacy of antihypertensive therapy. Cordaflex proved beneficial in the case of ineffective Dopegyt monotherapy, suggesting a more severe status of pregnant women receiving two-component therapy. However, the data presented here suggest that the vasoactive status and hemodynamic profile of pregnant women with more severe PE who received two-component therapy were better compensated than those who received monotherapy. The use of two antihypertensive drugs with different mechanisms seems more favorable for pregnant women's cardiovascular system, regulating endothelial cell synthetic function. Significantly, there is a trend toward longer pregnancies in patients receiving two-component therapy, justifying its preferential use in lPE, and advocating a shift toward using drugs targeting the endothelium and blood vessels with varying mechanisms of action.
References
- Министерство здравоохранения Российской Федерации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Клинические рекомендации. 2021. [Ministry of Health of the Russian Federation. Preeclampsia. Eclampsia. Edema, proteinuria, and hypertensive disorders during pregnancy, childbirth, and postpartum. Clinical guidelines. 2021. (in Russian)].
- Brown M.A., Magee L.A., Kenny L.C., Karumanchi S.A., McCarthy F.P. et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018; 72(1): 24-43. https://dx.doi.org/10.1161/hypertensionaha.117.10803.
- Podjarny E., Benchetrit S., Katz B., Green J., Bernheim J. Effect of methyldopa on renal function in rats with L-NAME-induced hypertension in pregnancy. Nephron. 2001; 88(4): 354-9. https://dx.doi.org/10.1159/000046020.
- Xu B., Bobek G., Makris A., Hennessy A. Antihypertensive methyldopa, labetalol, hydralazine, and clonidine reversed tumour necrosis factor-α inhibited endothelial nitric oxide synthase expression in endothelial-trophoblast cellular networks. Clin. Exp. Pharmacol. Physiol. 2017; 44(3): 421-7.https://dx.doi.org/10.1111/1440-1681.12712.
- Chen Q., Guo F., Liu S., Xiao J., Wang C., Snowise S. et al. Calcium channel blockers prevent endothelial cell activation in response to necrotic trophoblast debris: possible relevance to pre-eclampsia. Cardiovasc. Res. 2012; 96(3): 484-93. https://dx.doi.org/10.1093/cvr/cvs279.
- Dijkhorst-Oei L.T., Rabelink T.J., Boer P., Koomans H.A. Nifedipine attenuates systemic and renal vasoconstriction during nitric oxide inhibition in humans. Hypertension. 1997; 29(5): 1192-8. https://dx.doi.org/10.1161/01.hyp.29.5.1192.
- Муминова К.Т., Ходжаева З.С., Горина К.А., Шмаков Р.Г., Зиганшина М.М. Сравнительный анализ влияния двух схем гипотензивной терапии на гемодинамические показатели матери при преэклампсии с ранним и поздним началом. Акушерство и гинекология. 2023; 1: 55-66. [Muminova K.T., Khodzhaeva Z.S., Gorina K.A., Shmakov R.G., Ziganshina M.M. Comparative analysis of the effect of two antihypertensive therapy regimens on maternal hemodynamic parameters in early-and late-onset preeclampsia. Obstetrics and Gynecology. 2023; (1): 55-66. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.290.
- Муминова К.Т., Ходжаева З.С., Яроцкая Е.Л., Зиганшина М.М. Анализ факторов, отражающих развитие стерильного воспаления, на фоне различных схем гипотензивной терапии у беременных с преэклампсией. Медицинская иммунология. 2023; 25(5): 1183-90. [Muminova K.Т., Khodzhaeva Z.S., Yarotskaya E.L., Ziganshina M.M. Analysis of factors associated with sterile inflammation in women with pe receiving different antihypertensive treatment strategies. Medical Immunology. 2023; 25(5): 1183-90. (in Russian)]. https://dx.doi.org/10.15789/1563-0625-AOF-2809.
- Муминова К.Т., Зиганшина М.М., Ходжаева З.С. Оценка влияния различных схем гипотензивной терапии на состояние эндотелиального гликокаликса системы кровообращения у пациенток с преэклампсией. Экспериментальная и клиническая фармакология. 2022; 85(10): 4-10. [Muminova K.T., Ziganshina M.M., Khodzhaeva Z.S. Evaluation of the effect of different strategies of antihypertensive treatment on endothelial glycocalyx in patients with preeclampsia. Experimental and Clinical Pharmacology. 2022; 85(10): 4-10. (in Russian)]. https://dx.doi.org/10.30906/0869-2092-2022-85-10-4-10.
- Pillinger N.L., Kam P. Endothelial glycocalyx: basic science and clinical implications. Anaesth. Intensive Care. 2017; 45(3): 295-307.https://dx.doi.org/10.1177/0310057X1704500305.
- Zeng Y., Zhang X. F., Fu B.M., Tarbell J.M. The role of endothelial surface gycocalyx in mechanosensing and transduction. Adv. Exp. Med. Biol. 2018; 1097: 1-27. https://dx.doi.org/10.1007/978-3-319-96445-4_1.
- Ziganshina M.M., Muminova K.T., Khasbiullina N.R., Khodzhaeva Z.S., Yarotskaya E.L., Sukhikh G.T. Characterization of vascular patterns associated with endothelial glycocalyx damage in early- and late-onset preeclampsia. Biomedicines. 2022; 10(11): 2790. https://dx.doi.org/10.3390/biomedicines10112790.
- Зиганшина М.М., Муминова К.Т., Ходжаева З.С., Баранов И.И., Сухих Г.Т. Патогенетическое обоснование неэффективности гипотензивной терапии на основании анализа «вазоактивного статуса» у пациенток с ранней преэклампсией. Акушерство и гинекология. 2023; 11: 60-70. [Ziganshina M.M., Muminova K.T., Khodzhaeva Z.S., Baranov I.I., Sukhikh G.T. Pathogenetic rationale for the ineffectiveness of antihypertensive therapy based on vasoactive status analysis in patients with early-onset preeclampsia. Obstetrics and Gynecology. 2023; (11): 60-70 (in Russian)]. https://doi.org/10.18565/aig.2023.231.
- Sandrim V.C., Palei A.C., Metzger I.F., Gomes V.A., Cavalli R.C., Tanus-Santos J.E. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension. 2008; 52(2): 402-7. https://dx.doi.org/10.1161/hypertensionaha.108.115006.
- Sutton E.F., Gemmel M., Powers R.W. Nitric oxide signaling in pregnancy and preeclampsia. Nitric Oxide. 2020; 95: 55-62. https://dx.doi.org/10.1016/j.niox.2019.11.006.
- Hodžić J., Izetbegović S., Muračević B., Iriškić R., Štimjanin Jović H. Nitric oxide biosynthesis during normal pregnancy and pregnancy complicated by preeclampsia. Med. Glas (Zenica). 2017; 14(2): 211-17. https://dx.doi.org/10.17392/915-17.
- Rezeck Nunes P., Cezar Pinheiro L., Zanetoni Martins L., Alan Dias-Junior C., Carolina Taveiros Palei A., Cristina Sandrim V. A new look at the role of nitric oxide in preeclampsia: Protein S-nitrosylation. Pregnancy Hypertens. 2022; 29: 14-20. https://dx.doi.org/10.1016/j.preghy.2022.05.008.
- Sankaralingam S., Xu H., Davidge S.T. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc. Res. 2010; 85(1): 194-203. https://dx.doi.org/10.1093/cvr/cvp277.
- Speer P.D., Powers R.W., Frank M.P., Harger G., Markovic N., Roberts J.M. Elevated asymmetric dimethylarginine concentrations precede clinical preeclampsia, but not pregnancies with small-for-gestational-age infants. Am. J. Obstet. Gynecol. 2008; 198(1): 112.e1-112.e1127. https://dx.doi.org/10.1016/j.ajog.2007.05.052.
- Osol G., Ko N.L., Mandalà M. Altered Endothelial Nitric oxide Signaling as a Paradigm for Maternal Vascular Maladaptation in Preeclampsia. Curr. Hypertens Rep. 2017; 19(10): 82. https://doi.org/10.1007/s11906-017-0774-6.
- Черний В.И., Костенко В.С., Кабанько Т.П., Бернадинер Е.А., Стецик В.Ю. Коррекция артериальной гипертензии у пациенток с преэклампсией тяжелой степени. Медицина неотложных состояний. 2014; 57(2): 13-6. [Cherniy V.I., Kostenko V.S., Kabanko T.P., Bernadiner Ye.A., Stetsik V.Yu. Correction of hypertension in patients with severe preeclampsia. Emergency Medicine. 2014; 57(2): 13-6. (in Russian)].
- Гуманова Н.Г. Оксид азота и его циркулирующие метаболиты NOx, их роль в функционировании человеческого организма и прогнозе риска сердечно-сосудистой смерти (Часть II). Профилактическая медицина. 2021; 24(10): 119-25. [Gumanova N.G. Nitrogen oxide and its circulating NOx metabolites, their role in human body functioning and cardiovascular death risk prediction (Part II). Profilakticheskaya meditsina. 2021; 24(10): 119-25.(in Russian)]. https://dx.doi.org/10.17116/profmed202124101119.
- Isenberg J.S., Wink D.A., Roberts D.D. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc. Res. 2006; 71(4): 785-93. https://dx.doi.org/10.1016/j.cardiores.2006.05.024.
- Tashie W., Fondjo L.A., Owiredu W.K.B.A., Ephraim R.K.D., Asare L., Adu-Gyamfi E.A., Seidu L. Altered bioavailability of nitric oxide and L-Arginine is a key determinant of endothelial dysfunction in preeclampsia. Biomed. Res. Int. 2020; 3251956. https://dx.doi.org/10.1155/2020/3251956.
- Хлестова Г.В., Карапетян А.О., Шакая М.Н., Романов А.Ю., Баев О.Р. Материнские и перинатальные исходы при ранней и поздней преэклампсии. Акушерство и гинекология. 2017; 6: 41-7. [Khlestova G.V., Karapetyan A.O., ShakayaM.N., Romanov A.Yu., Baev O.R. Maternal and perinatal outcomes in early and late preeclampsia. Obstetrics and Gynecology. 2017; (6): 41-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.6.41-7.
- Ахмадеев Н.Р., Фаткуллин И.Ф., Фаткуллина Л.С. Сердечно-сосудистые последствия больших акушерских синдромов. Акушерство и гинекология. 2023; 4: 5-11. [Akhmadeev N.R., Fatkullin I.F., Fatkullina L.S. Cardiovascular consequences of great obstetrical syndromes.Obstetrics and Gynecology. 2023; (4): 5-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.287.
Received 17.10.2023
Accepted 14.11.2023
About the Authors
Marina M. Ziganshina, PhD (Bio), Leading Researcher at the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-11-83, mmz@mail.ru, https://orcid.org/0000-0003-1578-8403,117997, Russia, Moscow, Ac. Oparina str., 4.
Kamilla T. Muminova, PhD, Researcher at the High-Risk Pregnancy Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-06-74, kamika91@mail.ru, https://orcid.org/0000-0003-2708-4366,
117997, Russia, Moscow, Aс. Oparina str., 4.
Zulfiya S. Khodzhaeva, Dr. Med. Sci., Professor, Deputy Director of Obstetrics Institute, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-07-88, zkhodjaeva@mail.ru, https://orcid.org/0000-0001-8159-3714,
117997, Russia, Moscow, Ac. Oparina str., 4.
Madina S. Оshkhunova, PhD Student at the High-risk Pregnancy Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-06-74, madina.oshkhunova@mail.ru, https://orcid.org/0000-0002-7044-7962,
117997, Russia, Moscow, Ac. Oparina str., 4.
Igor I. Baranov, Dr. Med. Sci., Professor, Head of the Department of Scientific and Educational Programs, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, i_baranov@oparina4.ru, https://orcid.org/0000-0002-9813-2823,
117997, Russia, 117997, Russia, Moscow, Ac. Oparina str., 4.
Corresponding author: Marina M. Ziganshina, mmz@mail.ru



