Vasoactive endothelial factors in women with poor and suboptimal ovarian response during infertility treatment using assisted reproductive technologies
Perfilova V.N., Muzyko E.A., Tikhaeva K.Yu., Kustova M.V., Mukhina A.V., Fokina A.Yu., Zhuravleva E.I.
Objective: To investigate the levels of vasoactive endothelial factors, endothelin-1, and final metabolites of nitric oxide in the blood and follicular fluid, along with the expression levels of endothelial and inducible nitric oxide synthase (eNOS and iNOS) in cumulus cells of women with varying outcomes of hormonal ovarian stimulation during assisted reproductive technology (ART) for infertility treatment.
Materials and methods: In a simple open comparative clinical trial with parallel groups, markers of endothelial dysfunction were analyzed in 71 women from the Volgograd region, aged up to 42 years, undergoing ART for infertility treatment. The control group, with a normal and high response to ovarian stimulation using gonadotropins, included 31 women (≥10 oocytes retrieved). Two groups were formed with suboptimal (5–9 oocytes retrieved) and poor responses (4 or fewer oocytes retrieved), each containing 20 women. All patients had anti-Müllerian hormone levels greater than 1.2 ng/mL. Endothelin-1 levels in the blood and follicular fluid were determined using enzyme-linked immunosorbent assay, while the levels of eNOS and iNOS were assessed in cumulus cell lysates. The concentration of final nitric oxide metabolites (nitrite and nitrate ions) in the blood serum and follicular fluid was determined using the method described by Metelskaya V.A. and Gumanova N.G. (2005).
Results: Women with a poor response had significantly higher levels of endothelin-1 and end metabolites of nitric oxide in the blood serum than those with a high and normal response. The amount of iNOS in cumulus cell lysates from women with suboptimal and poor responses was greater than that in the control group. This, combined with the increased concentration of nitrite and nitrate ions, may indicate the formation of a potent oxidant, peroxynitrite, potentially causing irreversible damage in the cells of patients with a poor response.
Conclusion: Changes in vasoactive endothelial factor levels may contribute to a reduced response to ovarian stimulation with gonadotropins during ART for infertility treatment. Further research into the mechanisms that determine oocyte quality and quantity can enable personalized approaches to infertility treatment, potentially increasing pregnancy rates.
Authors’ contributions: Perfilova V.N., Tikhaeva K.Yu. – conception and design of the study, editing of the manuscript; Muzyko E.A. – collection and processing of material, drafting of the manuscript; Kustova M.V. – collection and processing of material, statistical analysis; Mukhina A.V., Fokina A.Yu., Zhuravleva E.I. – collection and processing of material.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was supported by the Russian Science Foundation under project No. 23-25-00067 "The role of endothelium in the regulation of the oocyte maturation in the treatment of infertility by assisted reproductive technologies methods".
Ethical Approval: The study was reviewed and approved by the Volgograd State Medical University, Ministry of Health of Russia (Ref. No: 049/13.02.2022).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Perfilova V.N., Muzyko E.A., Tikhaeva K.Yu., Kustova M.V., Mukhina A.V.,
Fokina A.Yu., Zhuravleva E.I. Vasoactive endothelial factors in women with poor and suboptimal ovarian response during infertility treatment using assisted reproductive technologies.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (3): 89-95 (in Russian)
https://dx.doi.org/10.18565/aig.2023.279
Keywords
Infertility is a major challenge for reproductive health. According to the World Health Organization, this condition affects 48 million couples to 186 million people worldwide, with female infertility accounting for 50–80% of cases [1]. In particular, the incidence of infertility in different regions of the Russian Federation ranges from 17.2% to 24%, and this prevalence shows no signs of decrease [2].
Assisted reproductive technology (ART) plays a critical role in female infertility treatment. The effectiveness of ART depends on several factors, including the patient's age, endometrial condition, and quality and quantity of oocytes retrieved. It should be emphasized that an inadequate ovarian response to gonadotropin stimulation can occur even in young women with a normal ovarian reserve. This unexpected and unfavorable treatment outcome often necessitates the discontinuation of the treatment cycle, leading to an increase in the number of ART attempts, thereby escalating medical and economic risks.
A key factor influencing the quality and quantity of oocytes obtained is the state of the endothelium, whose cells synthesize various biologically active substances involved in the regulation of reproductive organs. Endothelial dysfunction, often associated with certain reproductive pathologies, leads to an increase in vasoconstrictors (such as endothelin-1) and a decrease in vasodilators, including nitric oxide (NO), produced by NO synthases (NOS). Studies have shown a 2.2-fold increase in endothelin-1 mRNA expression in the granulosa-luteal cells of women with polycystic ovary syndrome (PCOS) compared to women with normal ovulation [3]. A meta-analysis by Meng C. (2019) also showed a decrease in blood serum nitrites in PCOS patients compared to controls [4].
In view of these considerations, the study of vasoregulatory factors produced by the endothelium in women undergoing infertility treatment with ART is imperative to improve the efficacy of ART and increase pregnancy rates in patients with poor and suboptimal responses.
Objective: To investigate the levels of vasoactive endothelial factors, specifically endothelin-1 and endogenous metabolites of nitric oxide, in blood and follicular fluid. An additional aim was to assess the levels of endothelial and inducible NO synthases (eNOS and iNOS) in follicular cells in women with different outcomes of ovarian hormonal stimulation during infertility treatment using ART.
Materials and methods
This single-center, open-label, parallel-group, comparative clinical trial involved 71 women from the Volgograd region undergoing infertility treatment with ART at the Volgograd State Medical University Clinic No. 1 of the Ministry of Health of Russia (Volgograd, Russia). The mean age was 34.79 (4.26) years.
Inclusion criteria were age from 18 to 42 years, anti-Müllerian hormone (AMH) level more than 1.2 ng/ml, written informed consent to participate in a clinical trial, and stimulation protocol with antagonists and gonadotropins at a dose of at least 100 IU per day.
Exclusion criteria were age less than 18 and >42 years, “soft” ovarian stimulation protocols, and refusal to participate in a clinical trial.
Study design
In patients undergoing infertility treatment with ART, data on ovarian reserve indicators, including assessment of AMH and follicle-stimulating hormone levels, were collected before stimulation.
Hormonal ovarian stimulation was performed by physicians at Clinic No. 1 of the Volgograd State Medical University of the Ministry of Health of Russia (Volgograd, Russia) in accordance with the clinical recommendations, standards, and interests of the patient. We included cases of ovarian stimulation performed according to a protocol with antagonists using gonadotropic drugs (follitropin alpha or follitropin beta) at a daily dose of at least 100 IU until the preovulatory follicles reached a diameter of 17 mm, followed by the introduction of a final maturation trigger in standard doses of human chorionic gonadotropin.
The results of hormonal stimulation were evaluated after the retrieval of cumulus-oocyte complexes. The cumulus-oocyte complexes were counted microscopically and the number of mature oocytes was determined. Depending on the clinical situation and patient's interest, the retrieved oocytes were used for standard in vitro fertilization or intracytoplasmic sperm injection.
Based on the results of the stimulation, the following groups were formed:
1) Women with high and normal ovarian response – 10 or more retrieved oocytes (control group);
2) Women with a suboptimal ovarian response: 5–9 retrieved oocytes.
3) Women with a poor response: 4 or fewer retrieved oocytes.
Single sampling of blood from the antecubital vein, follicular fluid, and cumulus cells was performed from 8:00 am to 11:00 am. After venipuncture, which was performed in preparation for standard anesthesia, blood was collected in sterile tubes without anticoagulants. The samples were then centrifuged at 1000 g for 20 min at room temperature. Follicular fluid and cumulus cells are not used in the fertilization procedure but are by-products that are usually discarded after retrieval of cumulus-oocyte complexes. The tube containing follicular fluid and cumulus cells was centrifuged at 1000 g for 20 min. Follicular fluid was collected, and the cumulus cells were washed three times in a chilled phosphate-buffered saline solution (pH = 7.0–7.2) (Immunotex, Russia). After this, cells were counted in a Goryaev chamber according to the standard method [5], and the cells were lysed using Lysis Buffer 1 Specific for ELISA (Cloud-Clone Corp., USA) and Lysis Buffer 2 Specific for ELISA (Cloud-Clone Corp., USA) to determine eNOS and iNOS levels, respectively.
Serum, follicular fluid, and lysates collected after centrifugation were placed in Eppendorf tubes and frozen at -20°C until analysis. To quantify endothelin-1 by enzyme immunoassay, as well as the levels of eNOS and iNOS, the ELISA Kit for Endothelin 1 (Cloud-Clone Corp., USA) and ELISA Kit for Endothelial NOS (eNOS) (Cloud-Clone Corp., USA) were used. and ELISA Kit for Nitric Oxide Synthase 2, inducible (NOS2) (Cloud-Clone Corp., USA). The optical density was determined using a SPECTROstar Nano plate spectrophotometer (BMG Labtech, Germany) at a wavelength of 450 nm.
To determine the concentration of the final metabolites of nitric oxide, nitrite and nitrate ions, in blood serum and follicular fluid, a screening method modified by V.A. Metelskaya and Gumanova N.G. was used [6], which is based on the simultaneous reduction of nitrates to nitrites using vanadium (III) chloride (Sigma, USA) and the diazotization reaction with sulfonamide nitrite, with the development of a colored solution and subsequent spectrophotometry at 540 nm.
The study design was reviewed and approved by the Volgograd State Medical University, Ministry of Health of Russia (ref. No: 049/13.02.2022). All the women who participated in the clinical trial provided written voluntary informed consent to participate.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 8 software (GraphPad Software, USA). Normality of distribution was tested using the Shapiro-Wilk test. Tukey’s test was used for normally distributed data; if the distribution was not normal, the Kruskal–Wallis test was used, followed by Dunn's multiple comparison test. The equality of variances was also assessed. If the variances were different, the Brown-Forsythe and Welch tests were used. Differences were considered statistically significant at p<0.05. Quantitative variables with normal distribution are expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) is reported. To assess the clinical effect, we used the mean difference with 95% CI for normally distributed data and the median difference with 95% CI (Hodges–Lehman estimate) for non-normally distributed continuous variables.
Results
Women in all groups undergoing infertility treatment with ART had AMH levels > 1.2 ng/ml. However, responses to ovulation stimulation with gonadotropins were variable. In patients with poor and suboptimal responses, AMH levels were significantly lower than those in the control group. Other parameters of ovarian reserve, including the number of follicles punctured, the number of cumulus-oocyte complexes, and the number of mature oocytes retrieved, were also significantly lower in groups 2 and 3 compared with the indicators of women with a high and normal response (Table 1).
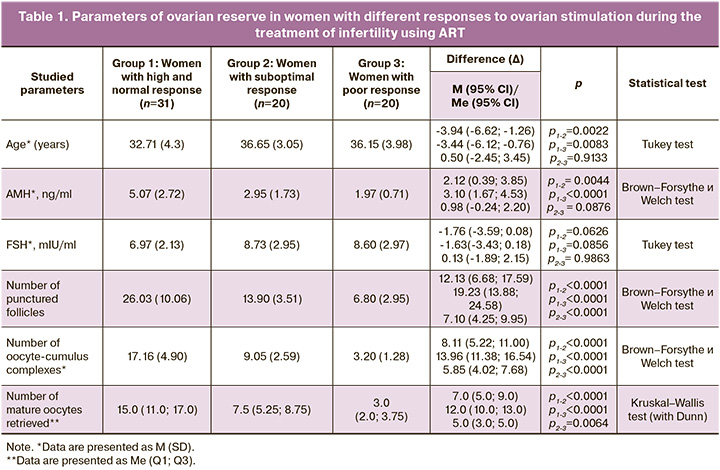
In women with a poor response, serum endothelin-1 levels were significantly higher than those in the control group. At the same time, the blood concentration of final NO metabolites in women in group 3 was also significantly higher than that in patients with a high and normal response.
There were no statistically significant differences in the levels of endothelin-1 and NO end metabolites in the follicular fluid between the groups (Table 2).
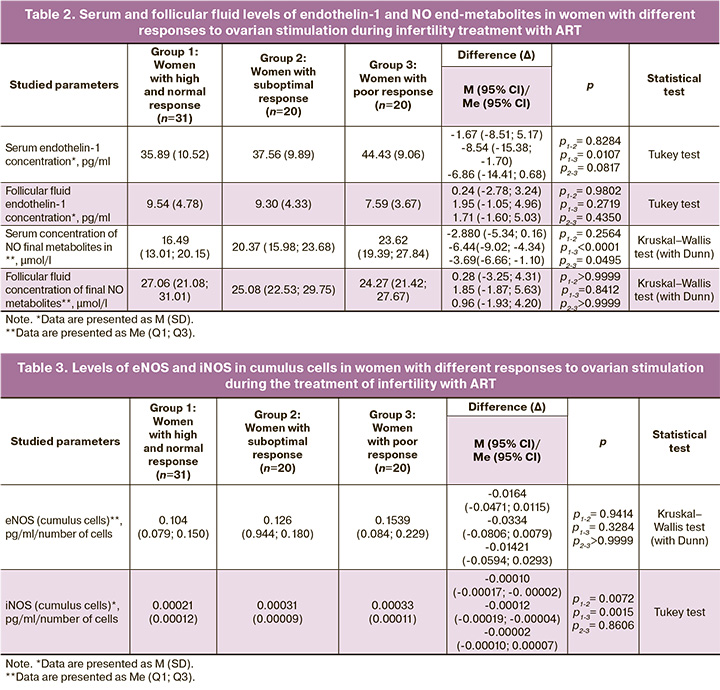
The level of iNOS in cumulus cells in women with suboptimal and poor response was significantly higher than that in the control group (Table 3), which, together with the increased concentration of final serum NO metabolites, may indicate the formation of a strong oxidant peroxynitrite, which can cause irreversible damage to cells in patients with poor response.
Discussion
Vascular endothelium plays a critical role in regulating the function of the female reproductive system. Studies have shown that co-culturing human endothelial progenitor cells with oocytes increases the rate of oocyte maturation and blastocyst formation by modulating the expression of genes involved in cumulus cell proliferation, oocyte formation, and apoptosis [7]. In a 2018 experimental study by Man et al., co-transplantation of exogenous endothelial cells (ExECs) with cryopreserved ovarian tissue in a mouse xenograft model increased the volume and improved the development of antral follicles. Furthermore, exogenous endothelial cells expressing AMH contribute to the maintenance of quiescent primordial follicles [8].
Endothelial dysfunction results in changes in the levels of vasoactive factors that regulate the female reproductive system. One such substance is endothelin-1, produced, among other things, by the female sex glands. Interaction with type A receptors leads to prolonged vasoconstriction, whereas activation of endothelin receptor type B is associated with vasodilation through the induction of NO signaling pathways [9]. Changes in endothelin-1 expression are associated with PCOS development. Granulosa-luteal cells in women with PCOS undergoing IVF exhibit a more than two-fold increase in endothelin-1 mRNA expression compared to the control group [3].
Our study found that serum concentrations of endothelin-1 were significantly higher in women with a poor response than in those with normal and high responses.
NO is involved in various physiological processes in the female reproductive system, including follicular development, control of ovarian steroidogenesis, and meiotic maturation of cumulus-oocyte complexes [10]. It plays a crucial role in maintaining oocyte quality [11]. According to our results, the concentration of the final NO metabolites in the blood of women with a poor response was higher than that of the control group.
Nitric oxide has a dual effect on oocyte maturation. An experimental study by Bu et al. (2003) demonstrated that treatment of cultured mouse oocytes enclosed in cumulus cells with low concentrations of the NO donor sodium nitroprusside (10-7, 10-6, 10-5 M) stimulated meiotic maturation of oocytes. However, high concentrations of sodium nitroprusside (0.1–4 mM) lead to a dose-dependent decrease in the number of oocytes at the stage of formation of the first polar body PB1 and a high percentage of atypical oocytes [12]. Excessive production of NO leads to peroxynitrite formation and oxidative stress, adversely affecting reproductive function [13]. High levels of reactive oxygen species in the culture medium during IVF/ICSI are associated with low rates of blastocyst development, fertilization, cleavage, and embryo fragmentation [14]. Moreover, excess serum NO is found in non-pregnant patients with tubal or peritoneal infertility, and elevated NO levels in follicular fluid are associated with implantation failure and decreased pregnancy rates [15]. Nitric oxide is involved in the pathogenesis of PCOS, a cause of infertility in women of reproductive age [16].
Our study also revealed that the amount of iNOS in cumulus cells in women with a suboptimal and poor response was significantly higher than that in women with a high and normal response.
NO, an essential regulator of vascular tone in vivo, is synthesized by three types of synthases: neuronal (nNOS), eNOS, and iNOS. It should be noted that iNOS is not typically expressed under physiological conditions, and its induction is associated with inflammation and immune response. However, the amount of NO produced by iNOS significantly exceeds that produced during the activation of eNOS and nNOS, contributing to peroxynitrite formation and subsequent cell damage [17].
Conclusion
Women with a poor response have increased levels of endothelin-1 and NO, as well as increased levels of iNOS in cumulus cells, compared to patients with a high and normal response. This may explain the reduced response to ovarian stimulation with gonadotropins in the treatment of infertility using assisted reproductive technology. Further research into the factors that influence the quantity and quality of oocytes retrieved during infertility treatment may lead to more personalized treatment plans and improved pregnancy rates.
References
- Rutstein S.O., Shah I.H. Infecundity, infertility, and childlessness in developing countries. DHS Comparative Reports No. 9. Calverton, Maryland, USA: ORC Macro and the World Health Organization; 2004.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Женское бесплодие. 2021. 81 c. [Ministry of Health of the Russian Federation. Clinical guidelines. Women's infertility. 2021. 81p. (in Russian)].
- Imbar T., Klipper E., Greenfield C., Hurwitz A., Haimov-Kochman R., Meidan R. Altered endothelin expression in granulosa-lutein cells of women with polycystic ovary syndrome. Life Sci. 2012; 91(13-14): 703-9. https://dx.doi.org/10.1016/j.lfs.2012.06.006.
- Meng C. Nitric oxide (NO) levels in patients with polycystic ovary syndrome (PCOS): a meta-analysis. J. Int. Med. Res. 2019; 47(9): 4083-94. https://dx.doi.org/10.1177/0300060519864493.
- Пескова Н.Н., Балалаева И.В., Брилкина А.А., Шилягина Н.Ю., Масленникова А.В., Мысягин С.А. Оценка жизнеспособности клеток in vitro. Учебно-методическое пособие. Нижний Новгород: Нижегородский госуниверситет; 2020. 25с. [Peskova N.N., Balalaeva I.V., Brilkina A.A., Shilyagina N.Yu., Maslennikova A.V., Mysyagin S.A. Evaluation of cell viability in vitro. A teaching and methodological manual. Nizhny Novgorod: Nizhny Novgorod State University; 2020. 25p. (in Russian)].
- Метельская В.А., Гуманова Н.Г. Скрининг-метод определения уровня метаболитов оксида азота в сыворотке крови. Клиническая лабораторная диагностика. 2005; 6: 15-8. [Metel’skaya V.A., Gumanova N.G. Screening as a method for determining the se¬rum level of nitric oxide metabolites. Russian Clinical Laboratory Diagnostics. 2005; (6): 15-8. (in Russian)].
- Lee S.H., Oh H.J., Kim M.J., Setyawan E.M.N., Choi Y.B., Lee B.C. Effect of co-culture human endothelial progenitor cells with porcine oocytes during maturation and subsequent embryo development of parthenotes in vitro. Mol. Reprod. Dev. 2018; 85(4): 336-47. https://dx.doi.org/10.1002/mrd.22969.
- Man L., Park L., Bodine R., Ginsberg M., Zaninovic N., Schattman G. et al. Co-transplantation of human ovarian tissue with engineered endothelial cells: a cell-based strategy combining accelerated perfusion with direct paracrine delivery. J. Vis. Exp. 2018; (135): e57472. https://dx.doi.org/10.3791/57472.
- Ko C., Meidan R., Bridges P.J. Why two endothelins and two receptors for ovulation and luteal regulation? Life Sci. 2012; 91(13-14): 501-6. https://dx.doi.org/10.1016/j.lfs.2012.05.010.
- Basini G., Grasselli F. Nitric oxide in follicle development and oocyte competence. Reproduction. 2015; 150(1): R1-9. https://dx/doi.org/10.1530/REP-14-0524.
- Goud P.T., Goud A.P., Najafi T., Gonik B., Diamond M.P., Saed G.M. et al. Direct real-time measurement of intra-oocyte nitric oxide concentration in vivo. PloS One. 2014; 9(6): e98720. https://dx.doi.org/10.1371/journal.pone.0098720.
- Bu S., Xia G., Tao Y., Lei L., Zhou B. Dual effects of nitric oxide on meiotic maturation of mouse cumulus cell-enclosed oocytes in vitro. Mol. Cell. Endocrinol. 2003; 207(1-2): 21-30. https://dx.doi.org/10.1016/s0303-7207(03)00213-2.
- Dutta S., Sengupta P. The role of nitric oxide on male and female reproduction. Malays. J. Med. Sci. 2022; 29(2): 18-30. https://dx.doi.org/10.21315/mjms2022.29.2.3.
- Bedaiwy M.A., Falcone T., Mohamed M.S., Aleem A.A., Sharma R.K., Worley S.E. et al. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil. Steril. 2004; 82(3): 593-600. https://dx.doi.org/10.1016/j.fertnstert.2004.02.121.
- Lee T.H., Wu M.Y., Chen M.J., Chao K.H., Ho H.N., Yang Y.S. Nitric oxide is associated with poor embryo quality and pregnancy outcome in in vitro fertilization cycles. Fertil. Steril. 2004; 82(1): 126-31. https://dx.doi.org/10.1016/j.fertnstert.2004.02.097.
- Hassani F., Karami M., 1 P.D., Jalali Nadoushan M.R., Yazdi P.E. Nitric oxide-induced polycystic ovaries in the wistar rat. Int. J. Fertil. Steril. 2012; 6(2): 111-6.
- Luo Y., Zhu Y., Basang W., Wang X., Li C., Zhou X. Roles of nitric oxide in the regulation of reproduction: a review. Front. Endocrinol. (Lausanne). 2021; 12: 752410. https://dx.doi.org/10.3389/fendo.2021.752410.
Received 30.11.2023
Accepted 06.03.2024
About the Authors
Valentina N. Perfilova, Dr. Sci. (Bio), Professor at the Department of Pharmacology and Pharmacy of ICMPE, Volgograd State Medical University, Ministry of Health of Russia, 400131, Russia, Volgograd, Pavshih Borcov sq., 1, +7(905)3945451, vnperfilova@mail.ru, https://orcid.org/0000-0002-2457-8486Elena A. Muzyko, PhD (Med), Associate Professor at the Department of Pathophysiology, Clinical Pathophysiology, Volgograd State Medical University, Ministry of Health of Russia, 400131, Russia, Volgograd, Pavshih Borcov sq., 1, +7(927)5302241, muzyko.elena@mail.ru, https://orcid.org/0000-0003-0535-9787
Ksenia Yu. Tikhaeva, PhD (Med), Obstetrician-Gynecologist-Reproductologist, Genom-Volga, 400078, Russia, Volgograd, Lenina Ave., 102A, +7(905)332-84-66,
tikhaeva34@gmail.com, https://orcid.org/0000-0002-1956-6448
Margarita V. Kustova, Assistant at the Department of Theoretical Biochemistry with a Course of Clinical Biochemistry, Volgograd State Medical University, Ministry of Health of Russia, 400131, Russia, Volgograd, Pavshih Borcov sq., 1, +7(904)4007615, kustova13@mail.com, https://orcid.org/0000-0002-6287-4120
Anna V. Mukhina, PhD (Med), Head of the Department of Assisted Reproductive Technologies, Clinic No. 1 of the Volgograd State Medical University, 400079, Russia, Volgograd, Nikitina str., 64, +7(902)382-55-99, https://orcid.org/0000-0003-1336-0543
Anna Yu. Fokina, Embryologist at the Department of Assisted Reproductive Technologies, Clinic No. 1 of the Volgograd State Medical University, 400079, Russia, Volgograd, Nikitina str., 64, +7(904)778-48-43.
Evgenija I. Zhuravleva, Embryologist at the Department of Assisted Reproductive Technologies, Clinic No. 1 of the Volgograd State Medical University, 400079, Russia, Volgograd, Nikitina str., 64, +7(904)778-48-43.
Corresponding author: Elena A. Muzyko, muzyko.elena@mail.ru



