Comparative analysis of the accuracy of ultrasonography and magnetic resonance imaging in estimating fetal weight
Syrkashev E.M., Nikolaeva A.V., Stoliarova E.V., Kholin A.M., Gorina K.A., Kesova M.I., Baev O.R., Kan N.E., Gus A.I.
Objective: To compare the accuracy of ultrasonography (USG) and magnetic resonance imaging (MRI) in determining estimated fetal weight (EFW).
Materials and methods: This prospective study included 103 pregnant women who underwent both MRI and USG before delivery. The EFW based on MRI data was calculated using the formula by Baker et al., while the EFW based on USG data was calculated using the Hadlock et al. formula. The EFW values were assessed using absolute measurements and on a percentile scale (INTERGROWTH-21st).
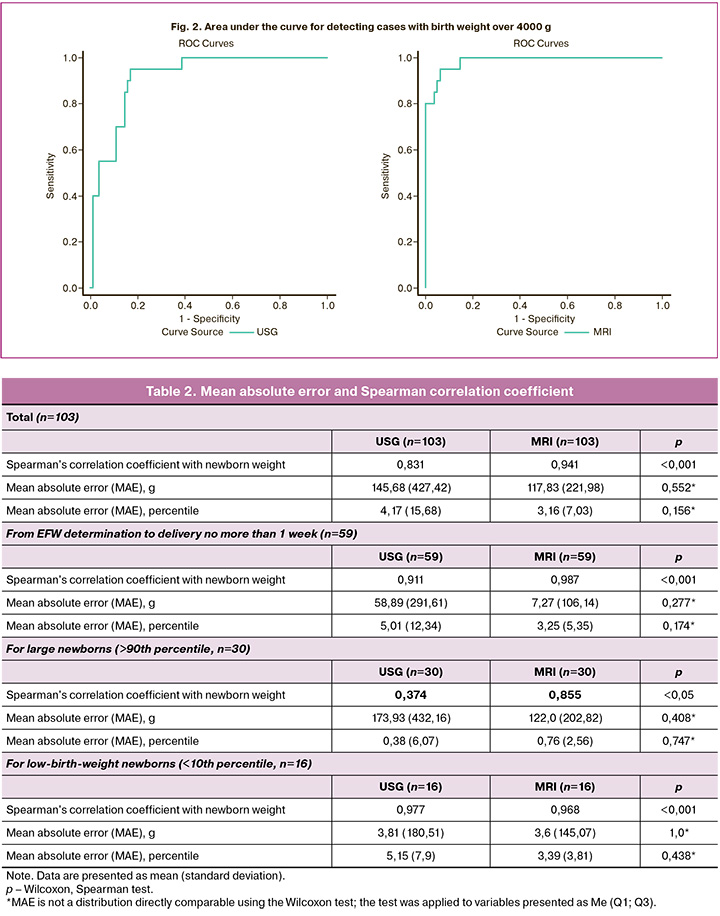
Results: The correlation coefficient between EFW based on USG data and the newborn's birth weight was 0.831 (p<0.001), while for MRI, it was 0.941 (p<0.001). The mean absolute error (MAE) of EFW in absolute values for USG was 145.68 (427.42) g, and for MRI, it was 117.83 (221.98) g, on a percentile scale, the MAE for USG was 4.17 (15.68), for MRI, it was 3.16 (7.03). The correlation coefficient between EFW above the 90th percentile was 0.374 (p=0.041) for USG and 0.855 (p<0.001) for MRI. The MAE for determining EFW (>90th percentile) was 173.93 (432.16) g for USG and 122.0 (202.82) g for MRI. On a percentile scale, the MAE was 0.38 (6.07) for USG and 0.76 (2.56) for the MRI. The area under the curve (ROC AUC) for identifying cases with birth weights > 4000 g was 0.916 (95% CI: 0.860–0.973) for USG and 0.986 (95% CI: 0.967–1.000) for MRI.
Conclusion: EFW determination based on MRI data is more accurate than that based on USG data, with the most significant differences noted in cases of fetal macrosomia. Developing machine learning algorithms is essential to reduce the time required for segmenting areas of interest, thereby enhancing the role of artificial intelligence in automating the EFW determination processes. Further research is necessary to establish the optimal timing and indications for using MRI as an additional method for determining the EFW.
Authors' contributions: Syrkashev E.M. – concept development, conducting research, text writing; Nikolaeva A.V. – recruitment of patients into the study, conducting research, text editing; Stoliarova E.V. – recruitment of patients into the study, collection and analysis of initial data; Kholin A.M. – concept development, conducting research, collection and analysis of initial data, editing; Gorina K.A., Kesova M.I. – recruitment of patients into the study; Baev O.R. – research management, recruitment of patients into the study, interpretation of the results obtained, text editing; Kan N.E. – research management, interpretation of the results obtained, text editing; Gus A.I. – research management, concept development, conducting research, text editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Syrkashev E.M., Nikolaeva A.V., Stoliarova E.V., Kholin A.M., Gorina K.A., Kesova M.I.,
Baev O.R., Kan N.E., Gus A.I. Comparative analysis of the accuracy of ultrasonography and
magnetic resonance imaging in estimating fetal weight.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (9): 82-88 (in Russian)
https://dx.doi.org/10.18565/aig.2025.152
Keywords
Determining the estimated fetal weight (EFW) is crucial for assessing fetal development and normal progression of pregnancy. In cases of intrauterine growth restriction and macrosomia, the risk of obstetric and neonatal complications is significantly increased. Therefore, EFW is a key factor influencing pregnancy management, including the timing and method of delivery [1, 2]. Over the past few decades, numerous methods for determining EFW have been developed, with ultrasonography (USG) and external obstetric examinations being the most accessible. However, accurately identifying the extreme conditions of macrosomia and fetal growth restriction before delivery remains challenging. Although the height of the uterine fundus is a relatively reliable indicator for suspecting fetal growth abnormalities when the weight falls below the 10th percentile or exceeds the 90th percentile, it lacks specificity. One of the most accurate methods is USG using the Hadlock et al. formula, which incorporates the head circumference, biparietal diameter, abdominal circumference, and femur length. This algorithm has demonstrated the highest accuracy rates for fetal weight assessment, particularly in identifying small and large fetuses for their gestational age [3–5]. However, these measurements can be influenced by various modifiable and non-modifiable factors, including gestational age, maternal body mass index, amniotic fluid volume, fetal presentation, and placental location.
Magnetic resonance imaging (MRI) is a modern diagnostic tool used to diagnose a wide range of obstetric diseases. It is essential for specifically characterizing changes detected by USG, describing anatomical features, and identifying combined fetal developmental anomalies. Given the advancements in this area and the potential of MRI for fetal biometry, this modality could become a vital additional component of a comprehensive antenatal examination.
This study aimed to compare the accuracy of USG and MRI in determining EFW.
Materials and methods
This prospective study included 103 pregnant women who underwent MRI and USG before delivery. All imaging studies were performed using a Toshiba Excelart Vantage tomograph with a magnetic field induction of 1.5 T. EFW from the MRI data was calculated using the formula by Baker et al.: EFW (kg) = 1.031 × FBV (L) + 0.12. Fetal body volume (FBV) was expressed in liters and determined by manually selecting regions of interest at the fetal level (excluding the umbilical cord) and summing the selected areas using third-party software (ITK-SNAP 3.0, Cognitica, Philadelphia, PA, USA) (Fig. 1). For manual segmentation, T2WI (single-shot fast spin-echo FSE) was used in the axial plane with the following scanning parameters: TR, 11520; TE, 130; FOV, 350 × 350; matrix, 256 × 256; and slice thickness, 6 mm. The EFW from the USG data was calculated using Hadlock et al.'s formula, which incorporates the head circumference, biparietal diameter, abdominal circumference, and femur length. The estimated fetal weight from the USG and MRI data was determined in absolute values and according to the INTERGROWTH-21st percentile scale (International Fetal Growth Standards – Estimated Fetal Weight, Version 2.0) [6].
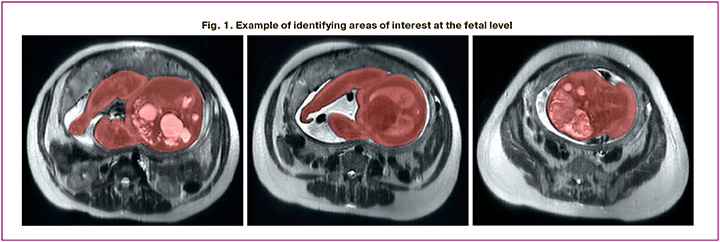
The inclusion criteria for the study were as follows: pregnant women aged over 18 years; singleton pregnancies; gestational age of >20 weeks with fetal growth restriction; signed informed consent to participate in the study; and delivery at the V.I. Kulakov NMRC for OG&P. Exclusion criteria included: multiple pregnancy; fetal developmental abnormalities; severe somatic pathology; fetal chromosomal abnormalities; and absolute contraindications to MRI.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 29.0.2.0 software. The following statistical methods were employed: a test for the normality of distribution in the studied sample, correlation analysis (calculation of the correlation coefficient r, Spearman's test) to describe the relationship between the expected and actual fetal weights, a nonparametric statistical test (Wilcoxon T-test) to test for differences in continuous variables, and an analysis of classifications using receiver operating characteristic (ROC) curves. ROC analysis was used to evaluate the diagnostic performance of EFW (in absolute values from USG and MRI) in detecting macrosomia, defined as a neonatal birth weight greater than 4000 g. The variable examined was EFW for each method, and the binary outcome was the presence or absence of macrosomia. The mean absolute error (MAE) was calculated as the arithmetic mean of the absolute differences between the EFW determined by USG or MRI and the actual birth weight. This metric quantified prediction accuracy but was not intended to analyze the interchangeability of methods. Statistical significance was set at p<0.05.
Results
The median gestational age at the time of USG was 38.4 (36.4; 39.2) weeks, and at the time of MRI, it was 38.2 (37.0; 39.4) weeks (p<0.001). However, no statistically significant difference was found in the number of days between USG and MRI and delivery (p>0.05): USG was 6 (3; 13) days and MRI was 5 (1; 12) days. Delivery occurred at 39.4 (38.7; 40.0) weeks of gestation. The age of the pregnant women was 32 (28; 36) years (Table 1).
The median EFW according to USG was 3270 (2600; 3901) g, for MRI – 3194 (2853; 3664) g, according to the percentile scale – 77.1 (17.3; 96.3), and 69.5 (29.1; 91.7), respectively. No statistically significant differences were found between the comparison groups for absolute values and values expressed in percentiles (p> 0.05). The body weight of newborns was 3360 (2875; 3920) g, which, according to the INTERGROWTH-21st scale, corresponded to 61.2 (22.0; 91.7) percentiles (Table 1). During the study, it was determined that the correlation coefficient between EFW according to USG data and the newborn's body weight was 0.831 (p<0.001), while for MRI the corresponding figure was 0.941 (p<0.001). The mean absolute error (MAE) in determining EFW in absolute values for USG was 145.68 (427.42) g, for MRI – 117.83 (221.98) g, in the percentile scale for USG – 4.17 (15.68), and for MRI – 3.16 (7.03) (Table 2). Moreover, if we exclude the cases in which more than one week passed from the EFW assessment (MRI/USG) to delivery (n=44), the correlation coefficient between EFW according to USG and the newborn's body weight was 0.911 (p<0.001), and the corresponding indicator for MRI was 0.987 (p<0.001). The mean absolute error (MAE) in determining EFW in absolute values for USG was 58.89 (291.61) g, for MRI – 7.27 (106.14) g, in the percentile scale for USG – 5.01 (12.34), and for MRI – 3.25 (5.35) (Table 2).
Determination of low- and high-weight fetuses by gestational age
Of the 103 newborns, body weights above the 90th percentile were observed in 30 cases. The correlation coefficient between EFW according to USG and birth weight in this group was 0.374 (p=0.041), and for MRI, it was 0.855 (p<0.001). The mean absolute error (MAE) of EFW determination was 173.93 (432.16) for USG and 122.0 (202.82) for MRI. On the percentile scale, the MAE was 0.38 (6.07) for USG and 0.76 (2.56) for MRI (Table 2). Actual birth weights below the 10th percentile were observed in 16 cases. The correlation coefficient between EFW according to USG and birth weight in this group was 0.977 (p<0.001), and for MRI, the corresponding figure was 0.968 (p<0.001). The mean absolute error (MAE) of EFW determination in absolute values for USG was 3.81 (180.51) g, for MRI – 3.6 (145.07) g, in percentile scale for USG 5.15 (7.9), and for MRI – 3.39 (3.81) (Table 2).
The area under the curve (ROC AUC) for detecting cases with birth weight over 4000 g for USG was 0.916 (95% CI: 0.860–0.973), and for MRI, it was 0.986 (95% CI: 0.967–1.000) (Fig. 2).
Discussion
MRI is an important supplementary diagnostic tool for various fetal pathologies because of its high soft tissue contrast, spatial resolution, and absence of ionizing radiation. In addition to facilitating high-quality analysis of two-dimensional data, MRI enables three-dimensional image reconstruction, allowing for a more detailed examination and calculation of various volumetric characteristics. The identification of areas of interest within the fetal body or specific structures, followed by volume calculation, has been extensively studied in previous publications [7–11].
Our study revealed that the volumetric assessment of EFW based on MRI data is more accurate than the linear measurements obtained through echography, which is consistent with the findings of earlier studies [10, 12–15]. Notably, for newborns with actual body weights above the 90th percentile, MRI was more accurate than USG was even greater. In contrast, for body weights below the 10th percentile, MRI and USG demonstrated comparable accuracy, likely due to the relatively small sample size (n=16). The highest accuracy for both MRI and USG was observed when measurements were taken within one week of delivery. However, our study has several limitations, including a small overall sample size, particularly within subgroups, and a lack of consideration of potentially influencing factors such as fetal position, amniotic fluid volume, maternal body mass index, and various anatomical and clinical features. It is important to note that the estimated fetal weight (EFW) based on the volumetric assessment of MRI data is not commonly performed in routine clinical practice, as the manual selection of areas of interest within the fetal body is time-consuming and relatively expensive. The active development of machine learning over the past decade has introduced numerous methods suitable for the automatic analysis of fetal images [16–18]. These algorithms can significantly reduce the time and cost associated with segmentation and diagnostic search, positioning artificial intelligence as a valuable tool for automating the EFW determination process.
Conclusion
This pilot study provides a comparative analysis of two diagnostic methods, USG and MRI, used to assess EFW. We focused on a practical comparison of the accuracy of these modalities, enabling us to interpret the results within the context of diagnostic accuracy rather than predictive modeling.
The data indicate that MRI offers higher accuracy than USG in assessing EFW, particularly in cases of fetal macrosomia. This is supported by a stronger correlation with the actual weight of the newborn, lower mean absolute error values, and higher diagnostic value (ROC AUC). These findings underscore the potential of MRI as an additional diagnostic method, particularly in complex clinical situations.
Due to the significant effort required by the current method of determining EFW from MRI data, implementing machine learning algorithms to automate the selection of areas of interest and fetal weight calculation appears promising. However, future multicenter studies with larger sample sizes and standardized methodologies are necessary to clarify the optimal timing and indications for using MRI as an additional method for assessing EFW.
References
- Щербакова Е.А., Баранов А.Н., Истомина Н.Г. Исходы ранней и поздней форм задержки роста плода в зависимости от критериев диагностики. Вопросы гинекологии, акушерства и перинатологии. 2024; 23(3): 23-9. [Shcherbakova E.A., Baranov A.N., Istomina N.G. Outcomes of early- and late-onset fetal growth restriction according to diagnostic criteria. Gynecology, Obstetrics and Perinatology. 2024; 23(3): 23-9 (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2024-3-23-29
- Тысячный О.В., Приходько А.М., Баев О.Р. Акушерские и неонатальные исходы самопроизвольных родов при крупном плоде в зависимости от срока гестации. Акушерство и гинекология. 2025; 4: 44-50. [Tysyachnyi O.V., Prikhodko A.M., Baev O.R. Obstetric and neonatal outcomes of spontaneous labor with a large fetus depending on the gestational age. Obstetrics and Gynecology. 2025; (4): 44-50 (in Russian)]. https://dx.doi.org/10.18565/aig.2025.36
- Hadlock F.P., Harrist R.B., Carpenter R.J., Deter R.L., Park S.K. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology. 1984; 150(2): 535-40. https://dx.doi.org/10.1148/radiology.150.2.6691115
- Hadlock F.P., Harrist R.B., Sharman R.S., Deter R.L., Park S.K. Estimation of fetal weight with the use of head, body, and femur measurements – a prospective study. Am. J. Obstet. Gynecol. 1985; 151(3): 333-7. https://dx.doi.org/10.1016/0002-9378(85)90298-4
- Hammami A., Mazer Zumaeta A., Syngelaki A., Akolekar R., Nicolaides K.H. Ultrasonographic estimation of fetal weight: development of new model and assessment of performance of previous models. Ultrasound Obstet. Gynecol. 2018; 52(1): 35-43. https://dx.doi.org/10.1002/uog.19066
- Stirnemann J., Villar J., Salomon L.J., Ohuma E., Ruyan P., Altman D.G. et al. International estimated fetal weight standards of the INTERGROWTH-21(st) Project. Ultrasound Obstet. Gynecol. 2017; 49(4): 478-86. https://dx.doi.org/10.1002/uog.17347
- Cordier A.G., Russo F.M., Deprest J., Benachi A. Prenatal diagnosis, imaging, and prognosis in congenital diaphragmatic hernia. Semin. Perinatol. 2020; 44(1): 51163. https://dx.doi.org/10.1053/j.semperi.2019.07.002
- Kulseng C.P.S., Hillestad V., Eskild A., Gjesdal K.I. Automatic placental and fetal volume estimation by a convolutional neural network. Placenta. 2023; 134: 23-9. https://dx.doi.org/10.1016/j.placenta.2023.02.009
- Watzenboeck M.L., Heidinger B.H., Rainer J., Schmidbauer V., Ulm B., Rubesova E. et al. Reproducibility of 2D versus 3D radiomics for quantitative assessment of fetal lung development: a retrospective fetal MRI study. Insights Imaging. 2023; 14(1): 31. https://dx.doi.org/10.1186/s13244-023-01376-y
- Kadji C., Cannie M.M., Kang X., Carlin A., Etchoua S.B., Resta S. et al. Fetal magnetic resonance imaging at 36 weeks predicts neonatal macrosomia: the PREMACRO study. Am. J. Obstet. Gynecol. 2022; 226(2): 238.e1-e12. https://dx.doi.org/10.1016/j.ajog.2021.08.001
- Dütemeyer V., Cordier A.-G., Cannie M.M., Bevilacqua E., Huynh V., Houfflin-Debarge V. et al. Prenatal prediction of postnatal survival in fetuses with congenital diaphragmatic hernia using MRI: lung volume measurement, signal intensity ratio, and effect of experience. J. Matern. Neonatal. Med. 2022; 35(6): 1036-44. https://dx.doi.org/10.1080/14767058.2020.1740982
- Zaretsky M.V., Reichel T.F., McIntire D.D., Twickler D.M. Comparison of magnetic resonance imaging to ultrasound in the estimation of birth weight at term. Am. J. Obstet. Gynecol. 2003; 189(4): 1017-20. https://dx.doi.org/10.1067/s0002-9378(03)00895-0
- Kadji C., Cannie M.M., Resta S., Guez D., Abi-Khalil F., De Angelis R. et al. Magnetic resonance imaging for prenatal estimation of birthweight in pregnancy: review of available data, techniques, and future perspectives. Am. J. Obstet. Gynecol. 2019; 220(5): 428-39. https://dx.doi.org/10.1016/j.ajog.2018.12.031
- Kadji C., Bevilacqua E., Hurtado I., Carlin A., Cannie M.M., Jani J.C. Comparison of conventional 2D ultrasound to magnetic resonance imaging for prenatal estimation of birthweight in twin pregnancy. Am. J. Obstet. Gynecol. 2018; 218(1): 128.e1-e11. https://dx.doi.org/10.1016/j.ajog.2017.10.009
- Kadji C., De Groof M., Camus M.F., De Angelis R., Fellas S., Klass M. et al. The use of a software-assisted method to estimate fetal weight at and near term using magnetic resonance imaging. Fetal. Diagn. Ther. 2017; 41(4): 307-13. https://dx.doi.org/10.1159/000448950
- Specktor-Fadida B., Link-Sourani D., Rabinowich A., Miller E., Levchakov A., Avisdris N. et al. Deep learning–based segmentation of whole-body fetal MRI and fetal weight estimation: assessing performance, repeatability, and reproducibility. Eur. Radiol. 2024; 34(3): 2072-83. https://dx.doi.org/10.1007/s00330-023-10038-y
- Lo J., Nithiyanantham S., Cardinell J., Young D., Cho S., Kirubarajan A. et al. Cross Attention Squeeze Excitation Network (CASE-Net) for whole body fetal MRI segmentation. Sensors (Basel). 2021; 21(13): 4490. https://dx.doi.org/10.3390/s21134490
- Zhang T., Matthew J., Lohezic M., Davidson A., Rutherford M., Rueckert D. et al. Graph-based whole body segmentation in fetal MR images. Proceedings of the MICCAI Work PIPPI, Athens, Greece, 21 October 2016.
Received 11.06.2025
Accepted 01.09.2025
About the Authors
Egor M. Syrkashev, PhD, Senior Researcher at the Radiology Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,117997, Russia, Moscow, Ac. Oparin str., 4, e_syrkashev@oparina4.ru, https://orcid.org/0000-0003-4043-907X
Anastasia V. Nikolaeva, PhD, Chief Physician, V V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
a_nikolaeva@oparina4.ru, https://orcid.org/0000-0002-0012-6688
Elizaveta V. Stoliarova, PhD student, 1st Obstetric Department of Pregnancy Pathology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, ev_stolyarova@oparina4.ru, https://orcid.org/0009-0001-2049-3119
Alexey M. Kholin, PhD, Head of the Department of Telemedicine, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, a_kholin@oparina4.ru, https://orcid.org/0000-0002-4068-9805
Ksenia A. Gorina, PhD, Junior Researcher at the 1 Department of Obstetric Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, k_gorina@oparina4.ru, https://orcid.org/0000-0001-6266-2067
Marina I. Kesova, Dr. Med. Sci., Senior Researcher at the Obstetric Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, m_kesova@oparina4.ru, https://orcid.org/0000-0001-7764-8073
Oleg R. Baev, Dr. Med. Sci., Professor, Head of the 1st Maternity Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4; Professor at the Department of Obstetrics, Gynecology, Perinatology, and Reproductology, I.M. Sechenov First MSMU,
Ministry of Health of Russia, 119991, Russia, Moscow, Trubetskaya str., 8-2, o_baev@oparina4.ru, https://orcid.org/0000-0001-8572-1971
Natalia E. Kan, Dr. Med. Sci., Professor, Deputy Director for Science, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, kan-med@mail.ru, https://orcid.org/0000-0001-5087-5946
Aleksandr I. Gus, Dr. Med. Sci., Chief Researcher at the Department of Ultrasound and Functional Diagnostics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Head of the Department of Ultrasound Diagnostics, Medical Institute, Patrice Lumumba Peoples’ Friendship University of Russia, 127015, Russia, Moscow, Pistsovaya str., 10, a_gus@oparina4.ru, https://orcid.org/0000-0003-1377-3128



