Comparative analysis of different regimens for the prevention of placenta-associated complications in high-risk women
Objective: To analyze the course and outcomes of pregnancy in patients with severe preeclampsia concurrent in some cases with a history of fetal growth restriction and/or Hemolysis, Elevated Liver enzymes and Low Platelets (HELLP) syndrome according to different regimens for the prevention of these complications (acetylsalicylic acid (ASA) or ASA and low-molecular-weight heparin (LMWH)) during subsequent pregnancy.Minaeva E.A., Shmakov R.G.
Materials and methods: An investigation conducted in the V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology in 2016 and 2022 included 48 pregnant women with a family obstetric history (severe preeclampsia, HELLP syndrome, fetal growth restriction), who were divided into 2 groups: 1) 20 pregnant women who took only ASA for prophylactic purposes; 2) 28 pregnant women who were treated with ASA and LMWH. Clinical, laboratory, and instrumental examinations were made.
Results: The incidence of preeclampsia in the ASA groups was 50%, that of fetal growth restriction was 30%; combination (ASA and LMWH) therapy caused a 1.75-fold decrease in the incidence of preeclampsia from 50 to 28.6% (p=0.113) and a 4.43-fold reduction in that of fetal growth restriction (p=0.045). The incidence of early-onset severe preeclampsia was slightly lower in Group 2; however, there were no statistically significant results. It was also noted that there was no case of recurrent antenatal fetal death in this pregnancy during anticoagulant therapy started no later than 16 weeks’ gestation in women with a history of antenatal fetal death.
Conclusion: The investigation revealed a positive trend in reducing the incidence of early-onset severe preeclampsia and fetal growth restriction and showed the effectiveness of preventing antenatal fetal death with the combined prophylactic use of ASA and LMWH. The findings may suggest that it is expedient to add LMWH at a prophylactic dose to ASA in the first trimester of pregnancy. Clearly, further studies are needed to recruit more patients.
Keywords
Placental pathology and related complications, such as preeclampsia, fetal growth restriction, and antenatal fetal death, are considered to result from improper uterine spiral artery remodeling, leading to impaired uteroplacental blood flow, followed by placental ischemia, oxidative stress, and an imbalance between angiogenic and antiangiogenic factors.
Large randomized trials have shown that the prescription of acetylsalicylic acid (ASA) before 16 weeks of gestation moderately reduces the birth rates of fetuses with weight small for gestational age in women at high risk for placenta-associated diseases (relative risk (RR) 0.90, 95% confidence interval (CI) 0.81–1.00)) [1–3].
However, other authors have observed no significant difference in the effects of treatment between the women who took aspirin before 16 weeks’ gestation (RR 0.90, 95% CI 0.79–1.03) and those who received at 16 weeks of gestation or later (RR 0.90, 95% CI 0.83–0.98) on the development of preeclampsia and other complications (premature birth occurring before 34 weeks: after 16 weeks (RR 0.90, 95% CI 0.77–1.04), before 16 weeks (RR 0.90, 95% CI 0.82–1.00; fetal weight small for gestational age: after 16 weeks (RR 0.76, 95% CI 0.61–0.94); before 16 weeks (RR 0.95, 95% CI 0.84–1.08) [4].
A number of recent studies have reported that low-molecular-weight heparins (LMWH) may be effective in preventing placenta-associated complications [5–7]. The main mechanisms of action of LMWH in preventing placenta-associated diseases are rather than anticoagulant properties and the ability to prevent placental thrombosis (since unfractionated heparins have failed to demonstrate their efficacy), whereas due to other biological properties, including anti-inflammatory, proangiogenic ones, and the effects on spiral artery remodeling, those on angiogenesis, etc. [8–12].
In their randomized controlled trial, Abheiden C. et al. (2015) revealed no differences between combination therapy with LMWH and ASA and ASA monotherapy in terms of fetal growth and blood flow velocity in the uterine artery and umbilical cord, as evidenced by Doppler velocimery [13].
However, the Lancet published a study by Rodger A. et al. in 2016, which showed the effect of LMWH on the development of placenta-associated complications. And, if the multicenter studies showed that LMWH slightly reduced the risk of recurrent placental-mediated pregnancy complications in 62/444 (14%) patients in the LMWH group and in 95/443 (22%) in the non-LMWH group; the absolute difference was 8% (95% CI 17.3–1.4, p=0.09) (RR 0.64, 95% CI 0.36–1.11, p=0.11), the single-center studies demonstrated a positive effect of LMWH use in a group of women at high risk for placenta-associated complications [5].
Mello G. et al. (2005) reported that LMWH had an effect on the development of early-onset preeclampsia. There were reductions in preeclampsia by 74.1%, fetal growth restriction by 77.5%, and severity of preeclampsia (early-onset preeclampsia by 88.3% and early-onset fetal growth restriction by 86.4%). In the treated women, RR for preeclampsia was 0.26 (p=0.02), and that for fetal growth restriction was 0.14 (p<0.001) [6].
Karadağ C. et al. (2019) also reported that there was a reduction in the risk of preeclampsia in women with recurrent miscarriage and a high risk for inherited thrombophilia (Factor V Leiden). The number of patients who developed preeclampsia was much larger in the ASA group than in the ASA+LMWH and LMWH groups (p=0.042). The rate of preterm delivery was significantly higher in the ASA group than in the other two groups (p=0.046) [7].
The aim of the study was to analyze the course and outcomes of pregnancy in patients with a history of severe preeclampsia, fetal growth restriction and/or HELLP syndrome according to different regimens to prevent these complications using ASA or ASA+LMWH.
Materials and methods
The study enrolled 48 pregnant women aged 18 to 45 years who were included in a group at high risk for placenta-associated complications. After an informed consent form was obtained, the patients were divided into 2 groups: Group 1 consisted of 20 patients with a history of severe preeclampsia, fetal growth restriction, and concomitant disease; 7 patients had chronic hypertension treated with only ASA during this pregnancy. Group 2 comprised 28 multigrvidas with severe preeclampsia, fetal growth restriction, and antenatal fetal death and 2 patients who developed HELLP syndrome in a previous pregnancy and used both ASA and LMWH. During this pregnancy, the patients received therapy with ASA 150 mg at 12 to 16 weeks of pregnancy until its 36th week, according to the clinical practice guidelines “Preeclampsia. Eclampsia. Edema, Proteinuria and Hypertensive Disorders during Pregnancy, Labor, and the Postpartum Period” [1]. LMWH was prescribed at the first outpatient visit, but before 16-week gestation. The patients continued to take LMWH until full term delivery and discontinued the drug 24 hours before planned delivery or at the onset of labor activity. These were nadroparin, enoxyparin, and dalteparin.
Inclusion criteria:
- an age of 18–45 years;
- singleton pregnancy;
- an inclusion gestational age of 7–13 weeks;
- a family obstetric history (severe preeclampsia, fetal growth restriction, and HELLP syndrome in a previous pregnancy);
- risk for preeclampsia and fetal growth restriction identified by the first prenatal screening.
Non-inclusion criteria:
- multiple pregnancy;
- transplanted organs;
- autoimmune diseases;
- cancers.
Exclusion criteria:
- fetal chromosomal abnormalities;
- fetal congenital malformations.
Preeclampsia and fetal growth restriction were diagnosed based on the criteria laid down in the Russian clinical practice guidelines [1, 14].
The patients of the two groups were followed up in an outpatient setting according to Order 1130H. Additionally, they underwent the following examinations: a biochemical blood test measuring the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and alkaline phosphatase (AP), the markers of preeclampsia: placental growth factor (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1), and sFlt-1/PlGF).
Statistical analysis
Statistical analysis was carried out using GraphPad Prism Software (USA). The generalized D’Agostino-Pearson normality test was used. Normally distributed data are represented as mean (standard deviation), in terms of the equality of variances; t-test was used to compare the means. Qualitative data are represented as absolute value (n) and % and were compared using one-sided Fisher’s exact test. All p<0.05 values were considered statistically significant.
Results
Comparison of the two groups of multigravidas revealed that their mean age was 36.0 (5.6) and 34.7 (4.5) years in Groups 1 and 2, respectively; the body mass index (BMI) in these groups was 24.7 (6.5) and 23.5 (3.9) kg/m2.
In Groups 1 and 2, 15/20 (75%) and 22/28 (78.6%) patients had a history of early-onset severe preeclampsia (p = 0.519). The history of severe preeclampsia was complicated by HELLP syndrome in 2/28 (7.2%) Group 2 patients (p = 0.481). In this group, 6/28 (21.4%) patients had pregnancy losses at different periods (antenatal fetal death and non-developing pregnancy) (p = 0.031) (Table 1).
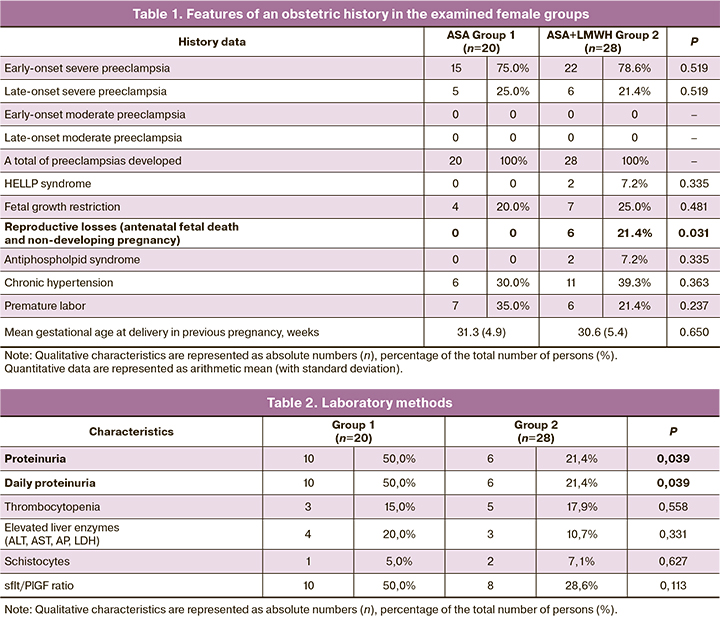
Medication-adjusted chronic hypertension was evident in 6/20 (30.0%) and 11/28 (39.3%) patients in Groups 1 and 2, respectively. Thus, Group 2 patients had a more compromised obstetric history (HELLP syndrome, reproductive losses), as well as antiphospholipid syndrome.
Laboratory tests showed that during the current pregnancy, proteinuria was significantly more common in 10/20 (50.0%) patients in Group 1 than in 6/28 (21.4%) in Group 2 (p=0.039). Thrombocytopenia was noted in 3/20 (15.0%) and 5/28 (17.9%) patients in Groups 1 and 2, respectively (p = 0.558). Biochemical blood test found an increase in liver enzymes (ALT, AST, ALP, and LDH) and creatinine in 4/20 (20.0%) and 3/28 (10.7%) patients in Groups 1 and 2 (p=0.331). The sFlt-1/PlGF ratio was also comparable (p=0.113) (Table 2).
Analyzing the Doppler parameters indicated that deviations of varying severity were much more frequently identified in the second trimester of pregnancy in Group 1 patients (p=0.009) (Table 3), while the frequency of deviations in the Doppler parameters in the third trimester of pregnancy did not differ in both groups (p=0.193).
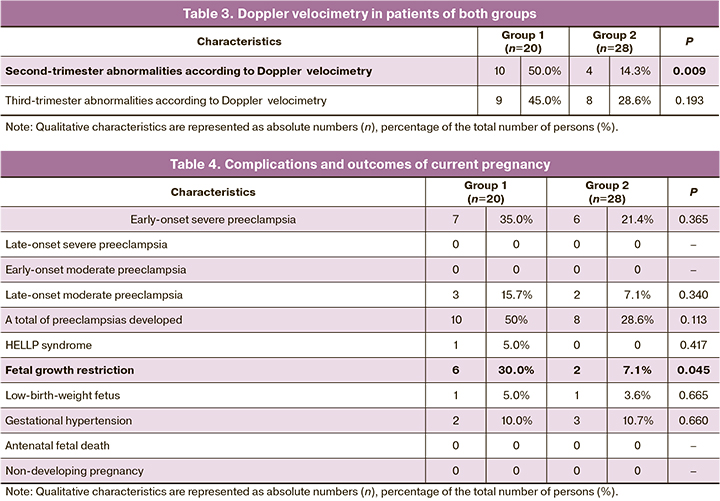
Placenta-associated complications developed in 10/20 (50.0%) and 8/28 (28.6%) patients of Groups 1 and 2, respectively (p=0.113). There was an increasing trend in both the frequency of early-onset severe preeclampsia in (7/20) (35.0%) and 6/28 (21.4%) patients of Groups 1 and 2, respectively (p=0.365), and the total number of preeclampsia cases in general (10/20 (50.0%) compared with 8/28 (28.6%) in Groups 1 and 2, respectively, p=0.113).
Of special note is that НЕLLР syndrome was present only in one patient in Group 1.
A comparative analysis of the two groups showed substantial differences in the development of fetal growth restriction. In Group 1, the latter was found in 5/20 (25.0%) patients with early-onset severe preeclampsia and in 1/20 (5.0%) with late-onset moderate preeclampsia. In Group 2, this complication developed in 2/28 (7.1%) patients with early-onset severe preeclampsia. The revealed differences were statistically significant (p=0.045).
There were no statistically significant differences between the two groups in the incidence of gestational hypertension (p=0.660) and in the detection rate of a low-birth-weight fetus (p=0.665) (Table 4).
Preterm delivery was performed in 7/20 (35.0%) patients (Group 1) due to an increase in the severity of preeclampsia, to HELLP syndrome, and/or the deterioration of maternal and fetal condition. Very early preterm birth occurred in 1/20 (5.0%) Group 1 pregnant woman at a gestational age of 26 weeks and 6 days. Early preterm birth occurred in 6/20 (30.0%) patients in this group. This occurred in 6/28 (21.4%) Group 2 patients (p=0.340). It should be noted that there was no statistical difference in the volume of blood loss in the patients of both groups during delivery (p=0.644). This may suggest that combination therapy with ASA and LMWH does not increase the risk of bleeding compared with aspirin alone (Table 5).
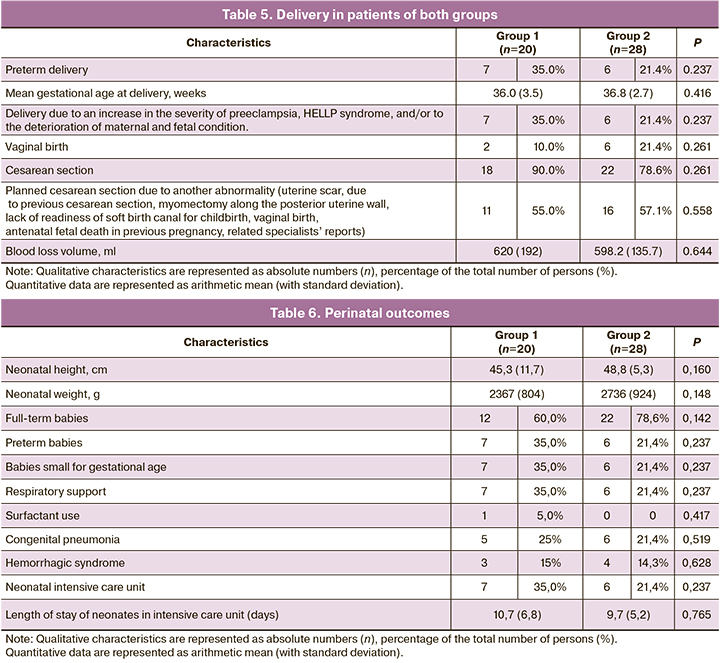
The perinatal outcomes did not differ significantly in the patients of both groups. The height of newborns was 45.3 (11.7) and 48.8 (5.3) cm in Groups 1 and 2, respectively (p=0.160). Their birth weight was 2367 (804) and 2736 (924) g in these groups (p=0.148). There were 13 (65.0%) and 22 (78.6%) full-term newborns in Groups 1 and 2, respectively. There were 7 (35.0%) and 6 (21.4%) premature newborns in these groups (p=0.237). All were hospitalized in the intensive care unit. The mean length of their stay there was 10.7 (6.8) bed days in Group 1 and 9.7 (5.2) bed days in Group 2. One newborn in Group 1 required the administration of a surfactant (p=0.417). 7/20 (35%) and 6/28 (21.4%) neonates were born small for gestational age in Groups 1 and 2, respectively (p=0.237). All the newborns required respiratory support. Congenital pneumonia developed in 5/20 (25%) and 6/28 (21.4%) newborns (p=0.519), hemorrhagic syndrome in 3/20 (15%) and 4 (14.3%) newborns (p=0.628) in Groups 1 and 2, respectively (Table 6).
Conclusion
Whether to prescribe LMWH for the prevention of preeclampsia and fetal growth restriction remains still debatable. This study has revealed a positive decreasing trend in the incidence of early-onset severe preeclampsia, fetal growth restriction and shown that the combined prophylactic use of ASA and LMWH is effective in preventing antenatal fetal death.
The findings may suggest that it is advisable to add LMWH at a prophylactic dosage to ASA in the first trimester of pregnancy. Obviously, further investigations are needed to include more patients.
We believe that additional anticoagulant therapy started no later than 16 weeks’ gestation in all Group 2 patients with antennal fetal death in previous pregnancies could prevent antenatal fetal death in the current pregnancy.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. М.; 2021. [Ministry of Health of the Russian Federation. Clinical guidelines. Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, labor and the postpartum period. М.; 2021. (in Russian)].
- ACOG. Committee Opinion No. 638: First-trimester risk assessment for early-onset preeclampsia. Obstet. Gynecol. 2015; 126(3): e25-7.https://dx.doi.org/10.1097/AOG.0000000000001049.
- Henderson J.T., Whitlock E.P., O’Connor E., Senger C.A., Thompson J.H., Rowland M.G. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2014; 160(10): 695-703. https://dx.doi.org/10.7326/M13-2844.
- Meher S., Duley L., Hunter K., Askie L. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: an individual participant data meta-analysis. Am. J. Obstet. Gynecol. 2017; 216(2): 121-8. e2.https://dx.doi.org/10.1016/j.ajog.2016.10.016.
- Rodger M.A., Gris J.-C., de Vries J.I.P., Martinelli I., Rey É., Schleussner E. et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: a meta-analysis of individual patient data from randomised controlled trials. Lancet. 2016; 388(10060): 2629-41. https://dx.doi.org/10.1016/S0140-6736(16)31139-4.
- Mello G., Parretti E., Fatini C., Riviello C., Gensini F., Marchionni M. et al. Low-molecular-weight heparin lowers the recurrence rate of preeclampsia and restores the physiological vascular changes in angiotensin-converting enzyme DD women. Hypertension. 2005; 45(1): 86-91. https://dx.doi.org/10.1161/01.HYP.0000149950.05182.a3.
- Karadağ C., Akar B., Gönenç G., Aslancan R., Yılmaz N., Çalışkan E. Aspirin, low molecular weight heparin, or both in preventing pregnancy complications in women with recurrent pregnancy loss and factor V Leiden mutation. J. Matern. Fetal Neonatal Med. 2020; 33(11): 1934-9. https://dx.doi.org/10.1080/14767058.2019.1671348.
- Greer I.A., Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. 2005; 106(2): 401-7.https://dx.doi.org/10.1182/blood-2005-02-0626.
- Omri A., Delaloye J.F., Andersen H., Bachmann F. Low molecular weight heparin Novo (LHN-1) does not cross the placenta during the second trimester of pregnancy. Thromb. Haemost. 1989; 61(1): 55-6.
- Mousavi S., Moradi M., Khorshidahmad T., Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv. Pharmacol. Sci. 2015; 2015: 507151. https://dx.doi.org/10.1155/2015/507151.
- Oberkersch R., Attorresi A.I., Calabrese G.C. Low-molecular-weight heparin inhibition in classical complement activation pathway during pregnancy. Thromb. Res. 2010; 125(5): e240-5. https://dx.doi.org/10.1016/j.thromres.2009.11.030.
- Mousa S.A., Petersen L.J. Anti-cancer properties of low-molecular-weight heparin: preclinical evidence. Thromb. Haemost. 2009; 102(2): 258-67.https://dx.doi.org/10.1160/TH08-12-0832.
- Abheiden C., Van Hoorn M.E., Hague W.M., Kostense P.J., van Pampus M.G., de Vries J. Does low-molecular-weight heparin influence fetal growth or uterine and umbilical arterial Doppler in women with a history of early-onset uteroplacental insufficiency and an inheritable thrombophilia? Secondary randomised controlled trial results. BJOG. 2016; 123(5): 797-805.https://dx.doi.org/10.1111/1471-0528.13421.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи (задержка роста плода). М.; 2020. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient fetal growth requiring medical care (fetal growth retardation). М.; 2020. (in Russian)].
Received 28.06.2022
Accepted 21.11.2022
About the Authors
Ekaterina A. Minaeva, Post-graduate student, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)464-16-67, ek_minaeva@oparina4.ru,https://orcid.org/0000-0001-8555-6670, 4 Academica Oparina str., 117997, Moscow, Russia.
Roman G. Shmakov, Dr. Med. Sci., Professor, Professor of the Russian Academy of Sciences, Director of the Institute of Obstetrics, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia; Main Specialist of the Ministry of Health of Russia on Obstetrics, r_shmakov@oparina4.ru, https://orcid.org/0000-0002-2206-1002,
4 Academica Oparina str., 117997, Moscow, Russia.
Authors’ contributions: Minaeva E.A. – patient selection and examination, writing the text of the manuscript, review of publications on the topic of the article; Shmakov R.G. – checking the critically important content, approval of the manuscript for publication.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The investigation has been conducted without additional funding.
Ethical Approval: The investigation has been approved by the Local Ethics Committee, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients have signed an informed consent form to the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Minaeva E.A., Shmakov R.G. Comparative analysis of different regimens for the prevention of placenta-associated complications in high-risk women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 12: 83-89 (in Russian)
http://dx.doi.org/10.18565/aig.2022.150



