Comparative evaluation of different methods in the diagnosis of LSIL and HSIL
Objective. To conduct a comparative assessment of the informative value of routine cytology, liquid-based cytology, colposcopy, HPV testing after self-sampling and sampling by a health worker in the detection of LSIL, HSIL and cervical cancer.Artymuk N.V., Marochko K.V.
Materials and methods. The research enrolled 300 patients (average age 36.7±8.2 years). All women underwent testing including: routine cytology, liquid-based cytology, colposcopy, HPV testing (after self-sampling with Qvintip and sampling of the materials by a gynecologist). If cytology revealed precancerous lesions of the cervix, abnormal colposcopic patterns were detected, or high risk HPV DNA (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 genotypes) was found, women were offered targeted biopsy of the cervix.
Results. Pathological changes were detected by any method in 39% of the patients (117/300). After a targeted biopsy of the cervix (n=82) LSIL, HSIL, CC were confirmed in 51.2 % (LSIL – 45.2%; HSIL – 50%; CC – 4.7%). Sensitivity of methods in detection of LSIL were as follows: routine cytology and liquid-based cytology – 21%; HPV test after self-sampling or sampling by a medical worker – 57.9%; colposcopy – 57.9%. For detection of HSIL and CC sensitivity were 43.5, 34.8, 87, 91.3 and 78.3% respectively.
Conclusion. The highest sensitivity for HSIL detection was found for hr-HPV testing (87% in case of sampling by a medical worker, 91.3% using the Qvintip device), the highest specificity was noted for cytological methods: 87.5% for routine cytology and 100% for liquid-based cytology.
Keywords
About 570 thousand new cases of cervical cancer are diagnosed in the world annually. This type of cancer has been holding leading positions among cancers in women for the longest time (ranks IV in the world among women malignancy) [1]. For the past 10 лет the prevalence of cervical cancer in Russia (2009–2019) increased from 111.6 to 126.8 per 100,000. Besides, the rate of actively dignosed cases patients boosted from 28.2% to 41.1%, and the detection of intraepithelial cervical cancer grew from 19.7 to 28.4 per 100 patients. The rate of patients with early stage cervical cancer also increased. At the same time the number of patients with advanced stage cervical cancer decreased [2]. Despite some positive statistical changes cervical cancer in Russia is considered one of the main causes of mortality among young women, as the age of most of them varies between 35–50 years [3]. Development of cervical cancer is a long-term process which can last for 10–15 years. Therefore there is plenty of time for diagnosis and timely treatment of cervical precancers. This type is one of the few visually detectable forms of cancer that should be well diagnosed using secondary prevention – screening. Timely diagnosis is exactly the way that allows to decrease morbidity and mortality rates among women with cervical cancer [4].
РАР-test is a widely used cytological screening test to detect cervical cancer. It was implemented in the mid-twentieth century by a Greek scientist Georgios Papanikolaou, who was a pioneer in the field of early malignancy detection. This method improved the rate of precancerous conditions of the cervix, but unfortunately its sensitivity is not high. Non-compliance of screening time intervals, low program coverage, insufficient efficiency of cytology are the main reasons of high morbidity rate in cervical cancer [5]. The studies demonstrated that 20–40% of new cases are diagnosed in women, who regularly undergo cytological screening for cervical cancer [6].
Alternatively, liquid-based cytology may also be used as a method of diagnosis of cervical cancer, but some studies report no difference in its sensitivity compared to routine cytology [7]. WHO recommends (2010) to use acetic acid for cervical examination in developing countries [8, 9]. Extended colposcopy is applied to specify the condition in differential diagnosis of cervical disorders and to visualize the most transformed area in case of targeted biopsy. This method has low sensitivity and specificity in detecting precancerous conditions. It is also relatively expensive that is why it cannot be used as a screening test [10].
Given that high-risk human papillomavirus (hrHPV) persistence is the major and proven etiologic factor of cervical cancer, it was decided to apply hrHPV testing as a part of screening program [11, 12]. HRHPV testing is considered a very effective screening instrument, recognized by large international randomized studies: Swedescreen, POBASCAM, ARTISTIC, NTCC, ATHENA [3]. HRHPV testing in women aged 30 and older in the USA is carried out simultaneously with cytology (co-testing) [13]. In order to provide maximum coverage by the screening program it is possible to use Qvintip, the self-test device [14]. However specificity and sensitivity of High Grade Squamous Intraepithelial Lesion (HSIL) detection using self-sampling are comparable to the sampling done by a health worker, and the compliance of the study is higher than in the standard screening test [15, 16].
Materials and methods
Study design: one-stage cross-sectional study. Study design was approved by the Ethical committee of Kemerovo State Medical University (Kemerovo State Medical University, Ministry of Health of Russia). The study was conducted on the outpatient basis in “Semilia”, a general practitioner’s office.
The research enrolled 300 patients (average age 36.7±8.2 years). Inclusion criteria: age (25–59 years), outpatient admission, signed informed consent to participate in the study and to fulfill all the requirements for participation. Non-inclusion criteria: pregnant women, breastfeeding, vaginal bleeding, intravaginal medication or spermicides use less than 7 days prior to the study, patients with confirmed cervical cancer. Exclusion criteria: refusal to carry out targeted cervical biopsy.
All women underwent a survey set: routine cytology, liquid-based cytology, colposcopy, HPV testing. Collection of the material for the detection of hrHPV DNA was conducted in two ways: self-sampling with Qvintiр and routine sampling with an A-type urogenital probe by a health worker. DNA of the twelve hrHPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 и 59) was detected using Real-Time PCR (Real Best HPV DNAHCR screen).
If CIN or abnormal colposcopy patterns were detected or in case of positive HPV test, the patients were offered to undergo multifocal targeted biopsy (5 lesions) using electrosurgical unit FOTEK.
Statistical analysis
For statistical processing we used Statistica for Windows 6.0. Quantitative data is presented as arithmetic averages (М) and their standard deviations (SD). Comparing of the relative frequencies in two groups was conducted by means of 95% confidence interval comparison (95% CI). Significance level (р) for interpretation of the statistical analysis results was p<0.05.study were determined using standard formulas for sensitivity, specificity, positive and negative predictive values (PPV and NPV).
Results
Among 300 patients examined, pathological changes were detected in 117 of women (39%). According to the results of the routine cytology, CIN was found in 21 women (7%), according to liquid-based cytology – in 14 women (4.7%); hrHPV was detected in 31.3 % of women (94/300). HPV was diagnosed using both sampling methods in 71 women (23.7%), using Qvintip (self-sampling) – in 83 women (27.7%), with sampling by a health worker – in 82 women (27.3%). In 12 patients hrHPV was detected using only Qvintip; in 11 patients – by sampling performed by a medical practitioner. 1st grade abnormal colposcopy patterns (suspicious of LSIL) were diagnosed in 28 women (9.3%), 2nd grade (suspicious of HSIL) – in 31 patients (10.3%).
The results of the research are presented in the Figure below.
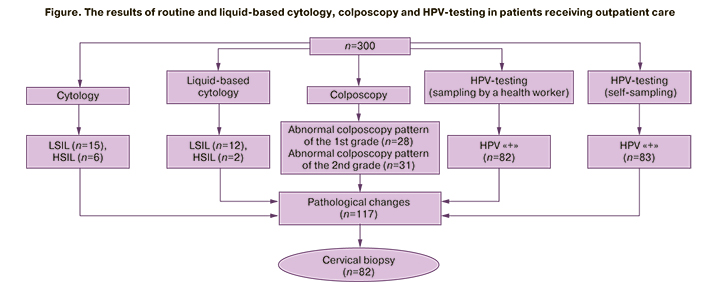
As a result, pathological changes were detected in 117 women. 70.1% of women signed the informed consent for targeted biopsy (82/117).
The results of histological study in these patients confirmed cervical lesions (LSIL, HSIL и РШМ) in 51.2% (42/82) of women. In 45.2% of the patients (19 women) LSIL was detected; in 50% of women (n=21) HSIL was found (in 21.4% of women – CIN II/III, in 7.1% of the cases – intraepithelial cervical cancer; squamous cell carcinoma was confirmed in 2 women (4.7%)). Multifocal cervical biopsy was conducted in 9 women due to hrHPV (the rest of the studies did not reveal any pathology); in total CIN of various grades was found in 100% of the patients. The age of women with LSIL was 36.8±9.1 years, with HSIL – 35.2±8.7 years (р=0.367). Cervical cancer was detected in 31 of 36 women. The results on the sensitivity, specificity, PPV and NPV are presented in Table 1.
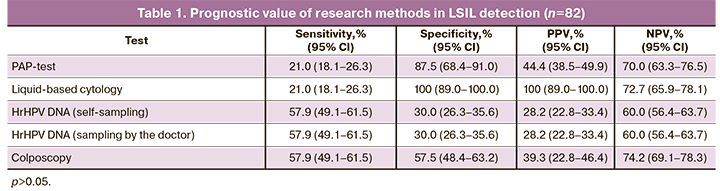
All the methods demonstrated their low sensitivity for LSIL detection. The highest sensitivity was registered in hrHPV testing in the samples obtained regardless of how the material was collected (57.9%). Routine as well as liquid-based cytology was associated with low sensitivity – only 21%. Besides, specificity of liquid-based cytology in LSIL diagnosis was 100%, of routine cytology – 87.5% .
Prognostic value of various methods in HSIL and cervical cancer detection is presented in Table 2.
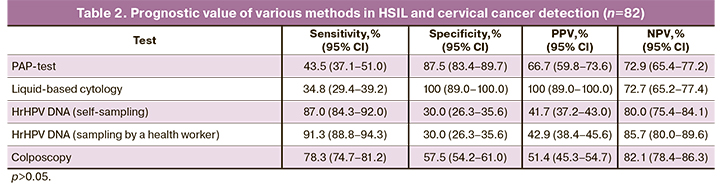
HRHPV DNA detection showed the highest sensitivity: 91.3% – using urogenital probe for sampling; 87% – using Qvintip. However specificity of HPV-testing was only 30%. Cytological methods proved to be less sensitive, though more specific: sensitivity of routine cytology was 43.5% and for liquid-based cytology it was 34.8%; specificity was 87.5% and 100%, respectively).
Apart from this, the sensitivity of a combination of several tests was analyzed. Summarized data is shown in Table 3.
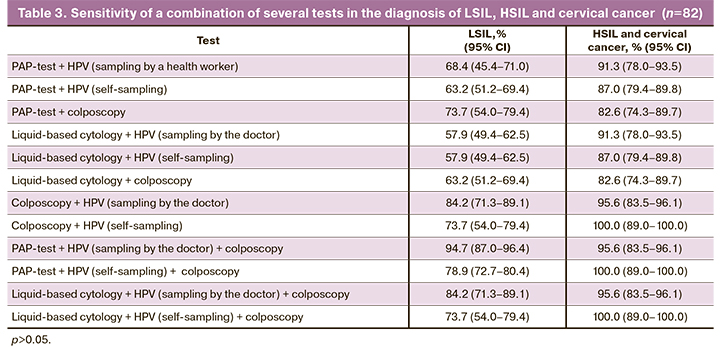
A combination of several tests allowed to increase sensitivity of the screening program up to 94.7% in LSIL detection, and up to 100% – in HSIL detection.
When providing colposcopy in combination with hrHPV DNA detection (sampling by a health worker) its sensitivity decreased to 84.2%. Colposcopy together with hrHPV DNA detection turned out to be the most sensitive research method (when sampling by the doctor – 95.6%, when using Qvintip – 100%).
Discussion
The results of the study demonstrated low sensitivity both in using routine (РАР-test) and liquid-based cytology, which can be compared to the results obtained in other studies: cytology sensitivity did not exceed 60%. However, liquid-based cytology allowed to decrease the number of inadequate results, while the rate of false-negative results in the diagnosis of precancer and cervical cancer conditions using cytology raised up to 50% [17, 18]. Systematic study review revealed that only 4 of 56 reports contained the data that could allow to conclude on sensitivity and specificity of each method and compare them [19]. One of the studies in Japan described comparable sensitivity of these methods: 71.3% – for routine cytology and 77.4% – for liquid-based cytology with 99% and 98.9% of specificity, respectively [20].
The results of our study indicated a sufficiently high sensitivity of hrHPV testing ВПЧ-ВР for HSIL and cervical cancer detection, which is consistent with the data obtained from foreign studies [12, 21]. The results of HPV-testing using Qvintip or sampling by a health worker were comparable [22–25]. The results of a large-scale ATHENA study proved the efficiency of using both hrHPV testing and cytology [26]. Our study recognized the sensitivity of this combined method as 100% when using hrHPV DNA testing and colposcopy together. Although, when combining HPV-testing and cytology, sensitivity remained the same. Prognostic value of colposcopy depends on the quality of instrument and a gynecologist’s experience; sensitivity for HSIL detection in this study was 78.3%, specificity – 57.5%. In systematic review by Mustafa R.А. (2016) sensitivity of this method varied between 29% and 100%, specificity − from 12% to 88% [27].
HPV-testing or co-testing (HPV+cytology) are the most efficient methods among screening programs. Colposcopy cannot be applied as a screening method due to its high cost and subjectivity, as the result depends on the quality of instrument and the specialist’s expertise. However it can be used for visual verification and differential diagnosis, as well as for choosing the area for targeted cervical biopsy.
Conclusion
Thus, the results of this research showed that hrHPV DNA testing has the highest sensitivity for HSIL detection, which is comparable to that when using Qvintip for self-sampling – 87% and when sampling by a health worker – 91.3%. Routine and liquid-based cytology demonstrated low sensitivity – 43.5% and 34.8%, though high specificity – 87.5% and 100%, respectively. Combination of tests enables to increase sensitivity for precancerous conditions and cervical cancer.
References
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6): 394-424. https://dx.doi.org/10.3322/caac.21492.
- Каприн А.Д., Старинский В.В., Петрова Г.В., ред. Состояние онкологической помощи населению России в 2019 году. М.: МНИОИ им. П.А. Герцена – филиал ФГБУ «НМИРЦ» Минздрава России; 2019. 236 с. [Kaprin A.D., Starinski V.V., Petrova G.V. Healthcare provision in oncology in the Russian in 2019. М.: MSROI named after P.A. Herzen, branch of FSBI “NMRRC” of the Russian Ministry of Health; 2019. 236 p. (in Russian)].
- Sasaki Y., Iwanari O., Arakawa I., Moriya T., Mikami Y., Iihara K., Konno R. Cervical сancer screening with human papillomavirus DNA and cytology in Japan. Int. J. Gynecol. Cancer. 2017; 27(3): 523-9. https://dx.doi.org/10.1097/IGC.0000000000000898.
- Sankaranarayanan R. Screening for cancer in low- and middle-income countries. Ann. Glob. Health. 2014; 80(5): 412-7. https://dx.doi.org/10.1016/j.aogh.2014.09.014.
- Artymuk N.V., Marochko K.V. Predictive value of different diagnostic methods for detection of cervical intraepithelial neoplasia and cervical cancer. Lietuvos akušerija ir ginekologija. 2017; 20(3): 222-7.
- Subramaniam A., Fauci J.M., Schneider K.E., Whitworth J.M., Erickson B.K., Kim K. et al. Invasive cervical cancer and screening: what are the rates of unscreened and underscreened women in the modern era? J. Low. Genit. Tract Dis. 2011; 15(2): 110-3. https://dx.doi.org/10.1097/LGT.0b013e3181f515a2.
- Pankaj S., Nazneen S., Kumari S., Kumari A., Kumari A., Kumari J. et al. Comparison of conventional Pap smear and liquid-based cytology: A study of cervical cancer screening at a tertiary care center in Bihar. Indian J. Cancer. 2018; 55(1): 80-3. https://dx.doi.org/10.4103/ijc.IJC_352_17.
- Naz U., Hanif S. Agreement between visual inspection with acetic acid and Papanicolaou's smear as screening methods for cervical cancer. J. Coll. Physicians Surg. Pak. 2014; 24(4): 228-31.
- Khan M., Sultana S.S., Jabeen N., Arain U., Khans S. Visual inspection of cervix with acetic acid: a good alternative to pap smear for cervical cancer screening in resource-limited setting. J. Pak. Med. Assoc. 2015; 65(2): 192-5.
- Роговская С.И., Липова Е.В., ред. Шейка матки, влагалище, вульва: физиология, патология, кольпоскопия, эстетическая коррекция. Руководство для практикующих врачей. М.: StatusPreasens; 2014. 832 c. [Rogovskaya S.I., Lipova E.V., ed. The cervix, vagina, vulva: physiology, pathology, colposcopy, and aesthetic correction. A guide for clinicians. Moscow. StatusPraesens; 2014. 832 p. (in Russian)].
- Curry S.J., Krist A.H., Owens D.K., Barry M.J., Caughey A.B., Davidson K.W. et al. Screening for cervical cancer: US preventive services task force recommendation statement. JAMA. 2018; 320(7): 674-86. https://dx.doi.org/10.1001/jama.2018.10897.
- Ogilvie G.S., van Niekerk D., Krajden M., Smith L.W., Cook D., Gondara L. et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV focal randomized clinical trial. JAMA. 2018; 320(1): 43-52. https://dx.doi.org/10.1001/jama.2018.7464.
- Castle P.E., Sanjose S., Qiao Y.L., Belinson J.L., Lazcano-Ponce E., Kinney W. Introduction of human papillomavirus DNA screening in the world: 15 years of experience. Vaccine. 2012; 30(Suppl. 5): F117-22. https://dx.doi.org/10.1016/j.vaccine.2012.05.071.
- Arrossi S., Thouyaret L., Herrero R., Campanera A., Magdaleno A., Cuberli M. et al. Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Glob. Health. 2015; 3(2): e85-94. https://dx.doi.org/10.1016/S2214-109X(14)70354-7.
- Nelson E.J., Maynard B.R., Loux T., Fatla J., Gordon R., Arnold L.D. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex. Transm. Infect. 2017; 93(1): 56-61. https://dx.doi.org/10.1136/sextrans-2016-052609.
- El-Zein M., Bouten S., Louvanto K., Gilbert L., Gotlieb W., Hemmings R. et al. Validation of a new HPV self-sampling device for cervical cancer screening: the cervical and self-sample in screening (CASSIS) study. Gynecol. Oncol. 2018; 149(3): 491-7. https://dx.doi.org/10.1016/j.ygyno.2018.04.004.
- Jeong H., Hong S.R., Chae S.W., Jin S.Y., Yoon H.K., Lee J. et al. Comparison of unsatisfactory samples from conventional smear versus liquid-based cytology in uterine cervical cancer screening test. J. Pathol. Transl. Med. 2017; 51(3): 314-9. https://dx.doi.org/10.4132/jptm.2017.03.17.
- Минкина Г.Н. Комбинированное тестирование в алгоритме цервикального скрининга. StatusPraesens. 2013; 15(4): 55-9. [Minkina G.N. Combined testing in cervical screening algorithm. StatusPraesens. 2013; 15(4): 55-9. (in Russian)].
- Davey E., Barratt A., Irwig L., Chan S.F., Macaskill P., Mannes P. et al. Effect of study design and quality on unsatisfactory rates, cytology classifications and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006; 367(9505): 122-32. https://dx.doi.org/10.1016/S0140-6736(06)67961-0.
- Hirai Y. Prospective study to evaluate the accuracy and usefulness of ThinPrep liquid-based cytology (LBC) for detecting uterine cervical lesions. J. Jpn. Soc. Clin. Cytol. 2010; 49(4):237-41.
- Ogilvie G.S., Niekerk D., Krajden M., Smith L.W., Cook D., Gondara L. et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months. The HPV FOCAL Randomized Clinical Trial. JAMA. 2018; 320(1): 43-52. https://dx.doi.org/10.1001/jama.2018.7464.
- Марочко К.В., Артымук Н.В., Белокриницкая Т.Е., Фролова Н.И. Возможность применения устройств для самозабора в выявлении вируса папилломы человека высокого риска. Фундаментальная и клиническая медицина. 2018; 3(3): 78-83. [Marochko K.V., Artymuk N.V., Belokrinitskaya T.E., Frolova N.I. Using vaginal self-sampling devices for detection of high-risk human papllomavirus. Fundamental and clinical medicine. 2018; 3(3): 78-83. (in Russian)].
- Аполихина И.А., Баширова Л.К., Летникова Л.И., Худякова О.В., Иванов С.В., Горбунова Е.А., Долгушина Н.В. Оценка инфицированности вирусом папилломы человека женщин Липецкой области с использованием диагностического теста самозабора материала. Акушерство и гинекология. 2018; 11: 98-104. [Apolikhina I.A., Bashirova, L.I. KLetnikova, Khudyakova O.V., Ivanov S.V., Gorbunova E.A., Dolgushina N.V. Evaluation of human papillomavirus infection in women in the Lipetsk Region, by using a diagnostic self-sampling test. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; 11: 98-104. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.11.98-104.
- Белокриницкая Т.Е., Фролова Н.И., Туранова О.В., Шемякина К.Н., Плетнева В.А., Самбуева Н.Б., Мальцева Е.Е. Результативность и приемлемость обследования на вирус папилломы человека при самостоятельном и врачебном заборе вагинального отделяемого. Акушерство и гинекология. 2017; 2: 97-105. [Belokrinitskaya T.E., Frolova N.I., Turanova O.V. et al. Effectiveness and acceptability of examination for the human papillomavirus virus in an independent and medical fence of the vaginal discharge. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2017; 2: 97-105 (in Russian)]. https://dx.doi.org/10.18565/aig.2017.2.97-105.
- Кира Е.Ф., Семенова К.Е., Боброва М.В., Белякова А.А., Гасилова Н.А. Оптимизация скрининга инфекций влагалища, ассоциированных с вирусами папилломы человека. Акушерство и гинекология. 2018; 8: 167-73. [Kira E.F., Semenova K.E., Bobrova M.V., Belyakova A.A., Gasilova N.A. Optimization of screening for vaginal infections associated with human papillomaviruses. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; 8: 167-73. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.167-173.
- Wright T.C., Stoler M.H., Behrens C.M., Sharma A., Zhang G., Wright T.L. Primary cervical cancer screening with human papillomavirus: End of study results from the ATHENA study using HPV as the first-line screening test. Gynecol. Oncol. 2015; 136(2): 189-97. https://dx.doi.org/10.1016/j.ygyno.2014.11.076.
- Mustafa R.A., Santesso N., Khatib R. Mustafa A.A., Wiercioch W., Kehar R. et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int. J. Gynaecol. Obstet. 2016; 132(3): 259-65. https://dx.doi.org/10.1016/j.ijgo.2015.07.024.
Received 21.01.2021
Accepted 26.03.2021
About the Authors
Natalia V. Artymuk, MD, PhD, Professor, the Head of Obstetrics and Gynecology Department named after Professor G.A. Ushakova, Kemerovo State Medical University, Ministry of Health of Russia. Тel.: +7(960)923-33-55. E-mail: artymuk@gmail.com. ORCID: 0000-0001-7014-6492. 650029, Russia, Kemerovo, Voroshilova str., 22a.Kristina V. Marochko, PhD, assistant of Obstetrics and Gynecology Department named after Professor G.A. Ushakova, Kemerovo State Medical University, Ministry of Health of Russia. Tel.: +7(923)612-48-92. E-mail: marochkokv@mail.ru. ORCID: 0000-0003-2832-6638. 650029, Russia, Kemerovo, Voroshilova str., 22a.
For citation: Artymuk N.V., Marochko K.V. Comparative evaluation of different methods in the diagnosis of LSIL and HSIL.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2021; 4: 98-103 (in Russian)
https://dx.doi.org/10.18565/aig.2021.4.98-103



