1) Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of the Russian Federation, Moscow, Russia;
2) A.S. Loginov Moscow Clinical Research and Practical Center, Moscow Healthcare Department, Moscow, Russia;
3) Р.A. Herzen Moscow Oncology Research Institute, Branch, National Medical Radiology Research Center, Ministry of Health of the Russian Federation, Moscow, Russia
The paper presents the European Society for Urogenital Radiology (ESUR) guidelines for the radiodiagnosis
of cervical cancer (CC) and current concepts in its primary staging, by taking into account the main characteristics of the tumor and the possibilities of treating CC. Objective of this review – to systematize relevant scientific data on the possibilities and prospects for developing medical imaging techniques in CC. The ESUR guidelines standardize and promote the higher efficiency of using radiodiagnostic techniques for CC. The updated ESUR guidelines consider into account all the changes given in the FIGO classification in accordance of the 2009/2018 revisions and the 8th edition of the Timor-Node-Metastasis (TNM) staging system. In accordance with the updated FIGO system, the paper shows a significant role and place of MRI in CC before, during, and after antitumor treatment. Clinical assessment of the neoplasm is the basis of the FIGO classification as before. Modern instrumental diagnosis makes it possible to increase the objectivity of estimating the prevalence of the tumor process in the preoperative stage. This enables radiodiagnostic techniques to be used as an additional tool in clinical staging. The ESUR clarifies the criteria for primary staging and for planning anticancer therapy. The paper considers clinical cases with an emphasis on MRI staging criteria, evaluation of the efficiency of treatment and prognosis, diagnosis of recurrent CC. The review highlights the possibilities for the development of medical imaging, which are aimed at using hybrid methods for imaging and radiomics in the staging of advanced CC.
Conclusion: The current scientific data on urogenital radiology are of particular interest for radiation diagnosis and gynecologic oncology and serve as the basis for clinical application.
cervical cancer
magnetic resonance imaging
MRI
MRI assessment system
diffusion-weighted imaging
neoplasm staging
neoplasm recurrence
По оценкам GLOBOCAN, за 2020 г. в мире зарегистрировано около 604 тыс. новых случаев и 342 тыс. смертей от рака шейки матки (РШМ). В России в 2020 г. заболеваемость РШМ составила 128,1 случаев на 100 тыс. населения [1]. Неуклонный рост заболеваемости РШМ и большой процент впервые выявленных опухолей на поздних стадиях объясняет необходимость разработки новых алгоритмов обследования и ведения пациенток при РШМ.
По рекомендациям Российского общества клинической онкологии (RUSSCO) стадирование РШМ проводится по классификациям Международной федерации гинекологии и акушерства (FIGO) (2018) и Международной классификации стадий злокачественных новообразований (TNM) (8-е издание, 2016). Современная инструментальная диагностика дает возможность объективно оценивать распространенность опухолевого процесса на дооперационном этапе. Это позволяет использовать методы лучевой диагностики в качестве инструмента для клинического стадированиия, что подтверждают международные исследования, повлиявшие на пересмотр классификаций РШМ.
По-прежнему, клиническая оценка новообразования – основа классификации FIGO. Прежняя редакция не учитывала состояние лимфатических узлов (ЛУ). В классификации FIGO 2018 лучевая диагностика приобретает значимость наряду с гистологическим исследованием [2]. Магнитно-резонансная томография (МРТ), обладая высокой пространственной и тканевой визуализацией, позволяет с высокой точностью определять изменения, выявляемые при патоморфологическом исследовании. Понимание корреляции визуализационных и патоморфологических критериев способствовало изменению рекомендаций Европейского общества урогенитальной радиологии (ESUR) [2]. ESUR определяет МРТ, как метод выбора для неинвазивной оценки местной распространенности РШМ [3].
Обновление классификации FIGO и рекомендаций ESUR способствуют повышению клинической эффективности диагностического алгоритма, что в свою очередь создает основу реализации персонифицированного подхода при РШМ.
Часть I: Обзор систем стадирования рака шейки матки
Обзор классификаций FIGO 2009/2018, TNM 2016 обобщен в таблице 1.
Стадия I
Стадия I (IA и IB) характеризует опухоль в пределах шейки матки; стадия IA – микроинвазия; IB – образование, видимое при клиническом и лучевом обследовании.
Нововведение системы FIGO 2018 – дополнительное разделение стадии IB на 3 подгруппы по признаку максимального размера первичной опухоли: IB1 (≤ 2 см), IB2 (> 2 см и ≤ 4 см) и IB3 (> 4 см) [4]. При IB1 возможно применение органосохраняющего лечения, IB2 и IB3 подразумевают неоадъювантную химиотерапию и радикальное хирургическое лечение или химиолучевую терапию (ХЛТ) [5, 6].
Стадия II
Стадия II сохранена без изменений. К IIA относят опухоли в пределах верхней и средней третей влагалища без параметриальной инфильтрации. Стадию IIA подразделяют на IIA1 (≤4 см) и IIA2 (>4 см) по наибольшему размеру первичной опухоли [7]. Параметриальная инвазия соответствует стадии IIB.
Стадия III
Стадия III представляет собой инвазию в нижнюю треть влагалища (IIIA), стенки таза и/или явления гидронефроза/нефункционирующей почки (IIIB), метастазы в ЛУ тазовой (IIIC1) и/или парааортальной групп (IIIC2) [3].
Учет состояния ЛУ (стадия IIIC) упрощает выбор тактики лечения [7, 8]. Индексы «r» или «p» уточняют способ оценки ЛУ. IIIC1r – при визуализации или IIIC1p в случае морфологического подтверждения. Метод исследования должен быть отражен в медицинской документации. Высокая информативность визуализации позволяет делать предварительные выводы о метастазах в ЛУ [7].
Стадия IV
Стадия IV классификации FIGO 2018 подразделяется на IVA и IVB. IVA – распространение опухоли на прилежащие органы, в т.ч. инвазия в слизистую оболочку мочевого пузыря и прямой кишки. IVB – отдаленные метастазы за пределы ЛУ тазовой и парааортальной групп [3]. Метастазы в яичник не влияют на выбор стадии.

Основные критерии обновленной оптимизированной системы стадирования РШМ FIGO 2018 представлены на рис. 1 [2].
Часть II: Руководство ESUR
Обновленное руководство ESUR определяет стандарт МР-исследования (табл. 2, 3). Показания к МРТ органов малого таза сохраняются без изменений. Подготовка к исследованиям освещена в ряде материалов ESUR.

Внутриполостное контрастирование с введением геля в полость влагалища повышает информативность в оценке инвазии стенок влагалища [3]. Т2-гиперинтенсивный гель для ультразвуковых исследований позволяет расправить своды и стенки влагалища, определив границы опухолевой инвазии.
Комбинация Т2-ВИ и ДВИ является основой современного протокола МР-исследования при РШМ. Для оценки опухоли используется двумерная (2D) и трехмерная (3D) Т2-визуализация. 2D режим более четко дифференцирует границы новообразования [9]. Параллельная интерпретация МР-изображений возможна при совпадении плоскости и толщины среза [10]. Стандартные ДВИ могут быть дополнены rFOV-ДВИ, обладающими высоким пространственным разрешением и меньшим количеством искажений, rFOV-ДВИ является дополнением ДВИ при визуализации ЛУ и опухолей области малого таза. ESUR рекомендует минимум два значения b-фактора при ДВИ (низкое b – 0–50 c/мм² и высокое – 800–1000 с/мм²) [11].
Часть III: Роль лучевой диагностики при первичном стадировании рака шейки матки
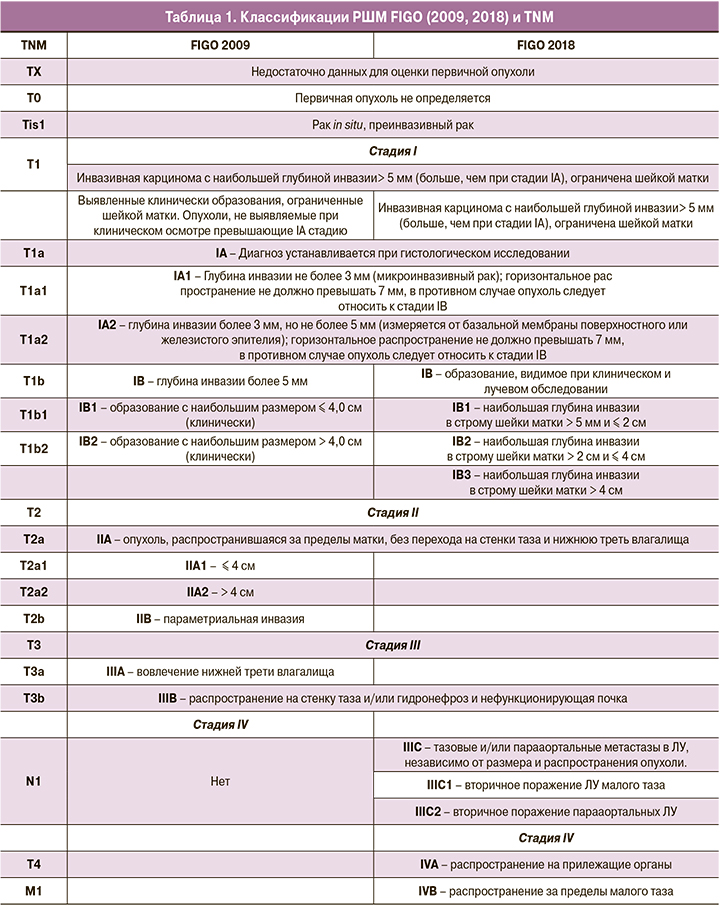
Высокое тканевое разрешение МРТ определяет информативность при оценке опухолевого распространения в области малого таза [12, 13]. Для опухолевой ткани характерна промежуточная интенсивность сигнала (ИС) на Т2-ВИ, повышенная на ДВИ и низкая на картах измеряемого коэффициента диффузии (ИКД) (рис. 2, 3) [3].
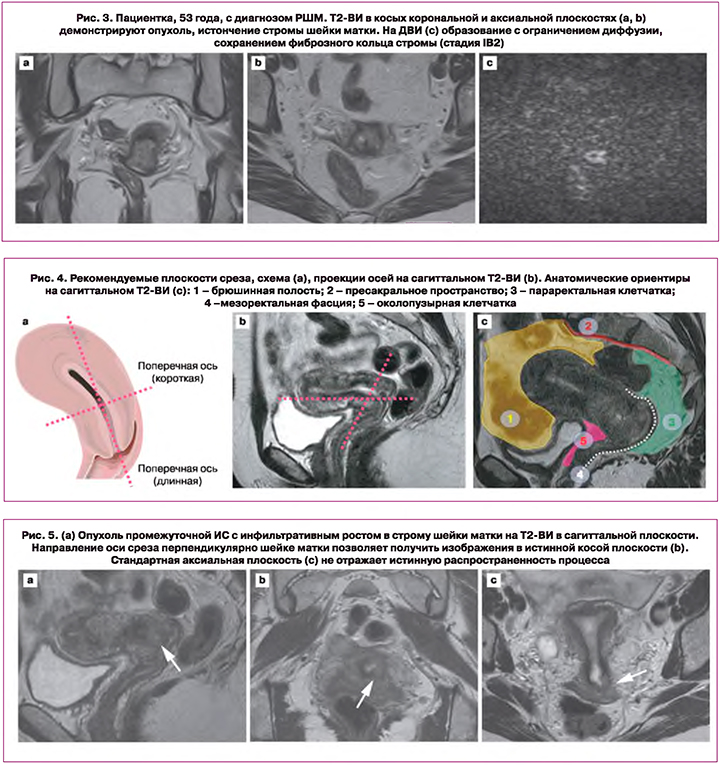
Первичное стадирование основано на Т2-ВИ в трех плоскостях. Срезы по короткой оси шейки матки локализуют опухолевый процесс и инвазию в параметрии (рис. 4, 5) [14]. Косые сагиттальные и аксиальные срезы позволяют оценить расстояние от опухоли до внутреннего маточного зева [15]. Параметриальная инвазия и расстояние до внутреннего маточного зева – критерии выбора органосохраняющего лечения [16, 17].
Сохранение слоя низкой ИС от фиброзного кольца стромы шейки матки на Т2-ВИ исключает инвазию в параметрии. Инвазивный рост опухоли в параметральную клетчатку подразумевает наличие узла или инфильтрата с вовлечением сосудов (рис. 6). [18] Применение МРТ с напряженностью магнитного поля 3 Тл, ДВИ, прием спазмолитических средств способствуют повышению информативности в оценке параметриальной инвазии; контрастное усиление не влияет на диагностическую ценность метода [3, 10].

Основным критерием, используемым для оценки состояния ЛУ при диагностике лимфогенного метастазирования при МРТ и компьютерной томографии (КТ) является увеличение размера ≥1,0 см по короткой оси. При ЛУ размерами менее 0,8 см, чувствительность метода может быть повышена за счет оценки структуры [13]. На ДВИ с высоким b-фактором для ЛУ характерна высокая ИС. Отсутствие различий в значениях ИКД интактных и метастатических ЛУ ограничивают возможности метода [19]. Согласно ряду исследований, ДКУ является необязательным при первичном стадировании РШМ [20].
Часть IV: Лучевая диагностика в оценке ответа на противоопухолевое лечение
Ответ на терапию определяет тактику дальнейшего лечения и прогноз заболевания. Дифференциальная диагностика изменений после ХЛТ, остаточной опухоли и рецидива имеет первостепенное значение. Лучевая диагностика на каждом этапе решает конкретные задачи.
МРТ до начала лечения определяет объемы лучевой терапии. Исследование в процессе ХЛТ позволяет оценить объем остаточной опухоли [3]. Высокая ИС может сохраняться до 6 месяцев после ХЛТ и соответствовать отеку, воспалению и некрозу, что затрудняет дифференцировку выявленных изменений с остаточной опухолью. Восстановление сигнала от стромы шейки матки до уровня низкой ИС на Т2-ВИ свидетельствует о полном ответе на терапию (рис. 7) [21].
Значения ИКД в ряде работ рассматриваются, как предикторы рецидива и выживаемости. Высокая ИС на ДВИ и низкая на картах ИКД характерна для остаточной опухоли [20, 22]. Низкие значения ИКД до и после противоопухолевой терапии (низкая ΔИКД) связаны с худшим прогнозом [23, 24]. Снижение перфузии по результатам ДКУ свидетельствует о низком ответе на ХЛТ [25–31].
Клиническое применение ДВИ и ДКУ является вспомогательным, однако МРТ позволяет влиять на выбор тактики противоопухолевого лечения с возможностью ее коррекции.
Часть V: Лучевая диагностика в выявлении рецидива рака шейки матки
Возможность возобновления опухолевого роста спустя 6 месяцев после регрессии первичного очага требует строгого динамического контроля с целью раннего выявления прогрессирования процесса. Возможности лечения рецидивов РШМ ограничены. На ранних этапах рецидивные опухоли могут не иметь патогномоничных клинических проявлений, поэтому с целью своевременной диагностики в алгоритм обследования больных РШМ, прошедших специализированное противоопухолевое лечения должны быть включены такие современные методы исследования как МРТ и позитронно-эмиссионная томография (ПЭТ)/КТ.
ESUR рекомендует проводить МРТ органов малого таза через 3–6 месяцев после ХЛТ. После органосохраняющего лечения МРТ проводится через 6 месяцев и далее по показаниям [3]. Изменения после ХЛТ усложняют интерпретацию МРТ. Рецидив опухоли отличается от изменений на фоне терапии и характеризуется промежуточной ИС на Т2-ВИ. Высокая ИС на ДВИ и низкая на картах ИКД позволяет заподозрить рецидив опухоли [22]. Остаточная опухоль при РШМ также ассоциирована с повышением ИС на ДКУ [3].
Часть VI: Перспективы лучевой диагностики рака шейки матки
Стандарты оказания онкологической помощи пациентам непрерывно развиваются. Совершенствование методов визуализации во многом способствует таким изменениям. В последнее время наибольшее внимание уделяется гибридным технологиям и радиомике, открывающим новые возможности визуализации в онкологии.
Функциональные методики имеют большое значение в диагностике онкопатологии. Анатомическая и функциональная визуализации в единой системе интерпретации дополняют друг друга, повышая информативность метода. Гибридные технологии, к которым относятся ПЭТ/КТ и ПЭТ/МРТ с 18-фтордезоксиглюкозой (ФДГ), успешно применяются в диагностике РШМ, несмотря на ограничение их доступности. ФДГ-ПЭТ/КТ применяется для первичного стадирования, оценки ЛУ, отдаленных метастазов, мониторинга ответа на терапию и выявления рецидива [32]. Гибридные методы позволяют индивидуализировать программу проведения лучевой терапии, в случае наличия показаний для ее продолжения [23].
Одним из перспективных направлений в онкологии является радиомика. Радиомный подход изменил взгляды на интерпретацию медицинских изображений. Анализ технических характеристик изображения позволяет применять их клинически. Радиомика реализует принципы прецизионной медицины через лучевую диагностику. Источником радиомных маркеров становятся также клинические, патоморфологические, генетические исследования [33]. Радиомный анализ РШМ исследуется по ряду направлений и модальностей – МРТ (T1-T2-ДВИ-ИКД), ПЭТ/КТ, УЗД.
Радиомные данные значимы для оценки ответа на противоопухолевое лечение. Появляются возможности прогноза эффективности до начала терапии [34]. Показана связь радиомных признаков с показателями безрецидивной и общей выживаемости [35]. Радиомика дифференцирует метастатические ЛУ от интактных [36]. Включение в прогностические модели нескольких модальностей и клинических данных повышает чувствительность и специфичность метода [37]. Оценка ЛУ на основе T2-ВИ и ДВИ превосходит модель только на основе T2-ВИ или ДВИ [38]. Количественный анализ изображения обеспечивает не только сегментацию опухоли, но и определяет гистологический тип РШМ [39]. Новизна направления объясняет исследования способов совершенствования методики. Проводится оценка возможностей 2D и 3D радиомного анализа опухоли [40].
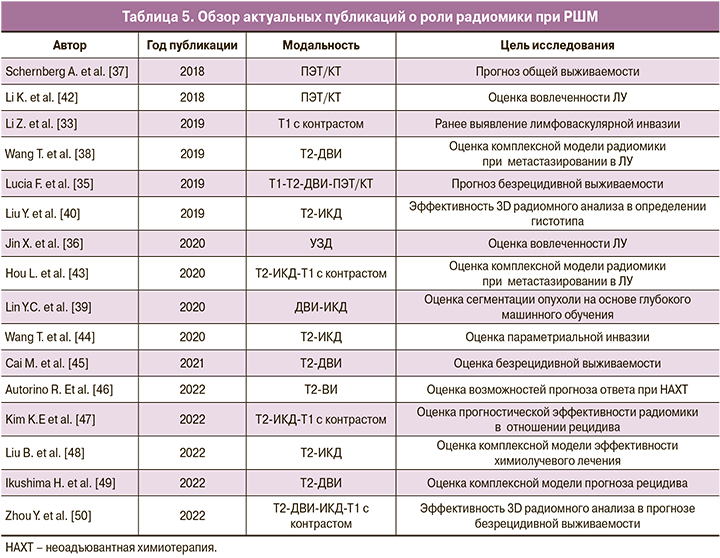
Перед лучевой диагностикой в онкологии стоит важная задача – поиск новых биомаркеров прогноза и эффективности лечения. Повышение информативности распространенных методов позволит преодолеть низкую доступность гибридной визуализации. Радиомика может стать методом дополнительной количественной оценки. Однако отсутствие стандартизации полученных данных затрудняет клиническое применение радиомики [41]. Для ее внедрения в рутинную практику необходимы многоцентровые исследования в больших группах пациентов и адаптация к клиническому использованию. Актуальные исследования по радиомике РШМ обобщены в таблице 5.
Заключение
В настоящее время предоперационное стадирование РШМ ориентировано на неинвазивные методы диагностики, в связи с чем, классификация FIGO 2018 дополнена методами визуализации. Возможности МРТ ведут к стандартизации исследования при РШМ. Система FIGO 2018 стала основой переработки рекомендаций ESUR. ESUR уточняет критерии первичного стадирования и планирования противоопухолевой терапии, а также возможности мониторинга ответа на лечение и выявления рецидива.
При возросшей роли МРТ в классификации FIGO 2018 патоморфологические данные по-прежнему имеют приоритет над данными визуализации. Лучевая диагностика дополняет гистологическое исследование. Наибольшие изменения в структуре классификации претерпели стадии I и III. В FIGO 2018 отсутствует стадия 0 (Tis) по TNM. Уточнение стадий IB1, IB2 и появление IB3 позволяет облегчить прогноз и оценить возможности проведения органосохраняющих операций в рамках обновленной классификации FIGO. Вовлеченность ЛУ в онкологический процесс также влияет на тактику ведения пациентки и исход заболевания. Введение стадии IIIC стало ключевым изменением новой классификации FIGO. Требуются дальнейшие исследования связи особенностей первичной опухоли и метастазирования в ЛУ. Дополнение критериев стадии IIIC сможет быть дальнейшим этапом развития классификации.
Развитие медицинской визуализации направлено на гибридные методы и радиомику. Все это позволит преодолеть существующие трудности при стадировании, мониторинге ответа на терапию и выявлении рецидива в каждом клиническом случае. Классификация FIGO 2018 и рекомендации ESUR способствуют объективизации стадирования РШМ.
- Каприн А.Д., Старинский В.В., Шахзадова А.О., ред. Состояние онкологической помощи населению России в 2020 году. М.: МНИОИ им. П.А. Герцена – филиал ФГБУ «НМИРЦ» Минздрава России; 2021. 239с. [Kaprin A.D., Starinsky V.V., Shakhzadova A.O., ed. The state of oncological care to the population of Russia in 2020. Moscow: P.A. Herzen Institute of Oncology – branch of the Federal State Budgetary Institution "NMIRC" of the Ministry of Health of Russia; 2021. (in Russian)].
- Bhatla N., Aoki D., Sharma D.N., Sankaranarayanan R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018; 143(Suppl. 2): 22-36. https://dx.doi.org/10.1002/ijgo.12611.
- Manganaro L., Lakhman Y., Bharwani N., Gui B., Gigli S., Vinci V. et al. Staging, recurrence and follow-up of uterine cervical cancer using MRI: updated Guidelines of the European Society of Urogenital Radiology after revised FIGO staging 2018. Eur. Radiol. 2021; 31(10): 7802-16. https://dx.doi.org/10.1007/s00330-020-07632-9.
- Bentivegna E., Maulard A., Pautier P., Chargari C., Gouy S., Morice P. Fertility results and pregnancy outcomes after conservative treatment of cervical cancer: a systematic review of the literature. Fertil. Steril. 2016; 106(5): 1195-211.e5. https://dx.doi.org/10.1016/j.fertnstert.2016.06.032.
- Koh W.J., Abu-Rustum N.R., Bean S., Bradley K., Campos S.M., Cho K.R. et al. Cervical Cancer, Version 3. 2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019; 17(1): 64-84. https://dx.doi.org/10.6004/jnccn.2019.0001.
- Marth C., Landoni F., Mahner S., McCormack M., Gonzalez-Martin A., Colombo N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017; 28(Suppl. 4): iv72-iv83.https://dx.doi.org/10.1093/annonc/mdx220.
- Bhatla N., Berek J.S., Cuello Fredes M., Denny L.A., Grenman S., Karunaratne K. et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019; 145(1): 129-35. https://dx.doi.org/10.1002/ijgo.12749.
- Lee S.I., Atri M. 2018 FIGO staging system for uterine cervical cancer: enter cross-sectional imaging. Radiology. 2019; 292(1): 15-24. https://dx.doi.org/10.1148/radiol.2019190088.
- Hori M., Kim T., Onishi H., Ueguchi T., Tatsumi M., Nakamoto A. et al. Uterine tumors: comparison of 3D versus 2D T2-weighted turbo spin-echo MR imaging at 3.0 T-initial experience. Radiology. 2011; 258(1): 154-63. https://dx.doi.org/10.1148/radiol.10100866.
- Park J.J., Kim C.K., Park S.Y., Park B.K. Parametrial invasion in cervical cancer: fused T2-weighted imaging and high-b-value diffusion-weighted imaging with background body signal suppression at 3 T. Radiology. 2015; 274(3): 734-41. https://dx.doi.org/10.1148/radiol.14140920.
- Moribata Y., Kido A., Fujimoto K., Himoto Y., Kurata Y., Shitano F. et al. Feasibility of computed diffusion weighted imaging and optimization of b-value in cervical cancer. Magn. Reson. Med. Sci. 2017; 16(1): 66-72.https://dx.doi.org/10.2463/mrms.mp.2015-0161.
- Hricak H., Gatsonis C., Chi D.S., Amendola M.A., Brandt K., Schwartz L.H. et al. Role of imaging in pretreatment evaluation of early invasive cervical cancer: results of the intergroup study American College of Radiology Imaging Network 6651-Gynecologic Oncology Group 183. J. Clin. Oncol. 2005; 23(36): 9329-37. https://dx.doi.org/10.1200/JCO.2005.02.0354.
- Liu B., Gao S., Li S. A comprehensive comparison of CT, MRI, positron emission tomography or positron emission tomography/CT, and diffusion weighted imaging-MRI for detecting the lymph nodes metastases in patients with cervical cancer: a meta-analysis based on 67 studies. Gynecol. Obstet. Invest. 2017; 82(3): 209-22. https://dx.doi.org/10.1159/000456006.
- Woo S., Moon M.H., Cho J.Y., Kim S.H., Kim S.Y. Diagnostic performance of MRI for assessing parametrial invasion in cervical cancer: a head-to-head comparison between oblique and true axial T2-weighted images. Korean J. Radiol. 2019; 20(3): 378-84. https://dx.doi.org/10.3348/kjr.2018.0248.
- Lakhman Y., Akin O., Park K.J., Sarasohn D.M., Zheng J., Goldman D.A. et al. Stage IB1 cervical cancer: role of preoperative MR imaging in selection of patients for fertility-sparing radical trachelectomy. Radiology. 2013; 269(1): 149-58. https://dx.doi.org/10.1148/radiol.13121746.
- Downey K., Attygalle A.D., Morgan V.A., Giles S.L., MacDonald A., Davis M. et al. Comparison of optimised endovaginal vs external array coil T2-weighted and diffusion-weighted imaging techniques for detecting suspected early stage (IA/IB1) uterine cervical cancer. Eur. Radiol. 2016; 26(4): 941-50.https://dx.doi.org/10.1007/s00330-015-3899-5.
- McEvoy S.H., Nougaret S., Abu-Rustum N.R., Vargas H.A., Sadowski E.A., Menias C.O. et al. Fertility sparing for young patients with gynecologic cancer: how MRI can guide patient selection prior to conservative management. Abdom. Radiol. (NY). 2017; 42(10): 2488-512. https://dx.doi.org/10.1007/s00261-017-1179-3.
- Raithatha A., Papadopoulou I., Stewart V., Barwick T.D., Rockall A.G., Bharwani N. Cervical cancer staging: a resident’s primer: women’s imaging. Radiographics. 2016; 36(3): 933-4. https://dx.doi.org/10.1148/rg.2016150173.
- Qi Y.F., He Y.L., Lin C.Y., Wang X.Q., Zhou H.L., Yuan L. et al. Diffusion-weighted imaging of cervical cancer: feasibility of ultra-high b-value at 3T. Eur. J. Radiol. 2020; 124: 108779. https://dx.doi.org/10.1016/j.ejrad.2019.108779.
- Jalaguier-Coudray A., Villard-Mahjoub R., Delouche A., Delarbre B., Lambaudie E., Houvenaeghel G. et al. Value of dynamic contrast-enhanced and diffusion-weighted MR imaging in the detection of pathologic complete response in cervical cancer after neoadjuvant therapy: a retrospective observational study. Radiology. 2017; 284(2): 432-42. https://dx.doi.org/10.1148/radiol.2017161299.
- Vincens E., Balleyguier C., Rey A., Uzan C., Zareski E., Gouy S. et al. Accuracy of magnetic resonance imaging in predicting residual disease in patients treated for stage IB2/II cervical carcinoma with chemoradiation therapy: correlation of radiologic findings with surgicopathologic results. Cancer. 2008; 113(8): 2158-65. https://dx.doi.org/10.1002/cncr.23817.
- Schreuder S.M., Lensing R., Stoker J., Bipat S. Monitoring treatment response in patients undergoing chemoradiotherapy for locally advanced uterine cervical cancer by additional diffusion-weighted imaging: a systematic review. J. Magn. Reson. Imaging. 2015; 42(3): 572-94. https://dx.doi.org/10.1002/jmri.24784.
- Onal C., Reyhan M., Guler O.C., Yapar A.F. Treatment outcomes of patients with cervical cancer with complete metabolic responses after definitive chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging. 2014; 41(7): 1336-42. https://dx.doi.org/10.1007/s00259-014-2719-5.
- Grigsby P.W., Siegel B.A., Dehdashti F., Rader J., Zoberi I. et al. Posttherapy [18F] fluorodeoxyglucose positron emission tomography in carcinoma of the cervix: response and outcome. J. Clin. Oncol. 2004; 22(11): 2167-71. https://dx.doi.org/10.1200/JCO.2004.09.035.
- Ho J.C., Allen P.K., Bhosale P.R., Rauch G.M., Fuller C.D., Mohamed A.S. et al. Diffusion-weighted magnetic resonance imaging as a predictor of outcome in cervical cancer after chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2017; 97(3): 546-53. https://dx.doi.org/10.1016/j.ijrobp.2016.11.015.
- Gladwish A., Milosevic M., Fyles A., Xie J., Halankar J., Metser U. et al. Association of apparent diffusion coefficient with disease recurrence in patients with locally advanced cervical cancer treated with radical chemotherapy and radiation therapy. Radiology. 2016; 279(1): 158-66. https://dx.doi.org/10.1148/radiol.2015150400.
- Onal C., Erbay G., Guler O.C. Treatment response evaluation using the mean apparent diffusion coefficient in cervical cancer patients treated with definitive chemoradiotherapy. J. Magn. Reson. Imaging. 2016; 44(4):1010-9.https://dx.doi.org/10.1002/jmri.25215.
- Zhou W., Yang X., Dai Y., Wu Q., He G., Yin G. Survey of cervical cancer survivors regarding quality of life and sexual function. J. Cancer Res. Ther. 2016; 12(2): 938-44. https://dx.doi.org/10.4103/0973-1482.175427.
- Himoto Y., Kido A., Fujimoto K., Daido S., Kiguchi K., Shitano F. et al. MR imaging-based evaluation of morphological changes in the uterus and ovaries of patients following neoadjuvant chemotherapy for cervical cancer. Magn. Reson. Med. Sci. 2015; 14(1): 65-72. https://dx.doi.org/10.2463/mrms.2014-0025.
- Heo S.H., Shin S.S., Kim J.W., Lim H.S., Jeong Y.Y., Kang W.D. et al. Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J. Radiol. 2013; 14(4): 616-25. https://dx.doi.org/10.3348/kjr.2013.14.4.616.
- Nakamura K., Kajitani S., Joja I., Haruma T., Fukushima C., Kusumoto T. et al. The posttreatment mean apparent diffusion coefficient of primary tumor is superior to pretreatment ADCmean of primary tumor as a predictor of prognosis with cervical cancer. Cancer Med. 2013; 2(4): 519-25. https://dx.doi.org/10.1002/cam4.100.
- Signorelli M., Guerra L., Montanelli L., Crivellaro C., Buda A., Dell'Anna T. et al. Preoperative staging of cervical cancer: is 18-FDG-PET/ CT really effective in patients with early stage disease? Gynecol. Oncol. 2011; 123(2): 236-40.https://dx.doi.org/10.1016/j.ygyno.2011.07.096.
- Li Z., Li H., Wang S., Dong D., Yin F., Chen A. et al. MR-based radiomics nomogram of cervical cancer in prediction of the lymph-vascular space invasion preoperatively. J. Magn. Reson. Imaging. 2019; 49(5): 1420-6. https://dx.doi.org/10.1002/jmri.26531.
- Tian X., Sun C., Liu Z., Li W., Duan H., Wang L. et al. Prediction of response to preoperative neoadjuvant chemotherapy in locally advanced cervical cancer using multicenter CT-based radiomic analysis. Front. Oncol. 2020; 10: 77. https://dx.doi.org/10.3389/fonc.2020.00077.
- Lucia F., Visvikis D., Vallières M., Desseroit M.C., Miranda O., Robin P. et al. External validation of a combined PET and MRI radiomics model for prediction of recurrence in cervical cancer patients treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging. 2019; 46(4): 864-77. https://dx.doi.org/10.1007/s00259-018-4231-9.
- Jin X., Ai Y., Zhang J., Zhu H., Jin J., Teng Y. et al. Noninvasive prediction of lymph node status for patients with early-stage cervical cancer based on radiomics features from ultrasound images. Eur. Radiol. 2020; 30(7): 4117-24. https://dx.doi.org/10.1007/s00330-020-06692-1.
- Schernberg A., Reuze S., Orlhac F., Buvat I., Dercle L., Sun R. et al. A score combining baseline neutrophilia and primary tumor SUVpeak measured from FDG PET is associated with outcome in locally advanced cervical cancer. Eur. J. Nucl. Med. Mol. Imaging. 2018; 45(2): 187-95. https://dx.doi.org/10.1007/s00259-017-3824-z.
- Wang T., Gao T., Yang J., Yan X.., Wang Y, Zhou X. et al. Preoperative prediction of pelvic lymph nodes metastasis in early-stage cervical cancer using radiomics nomogram developed based on T2-weighted MRI and diffusion-weighted imaging. Eur. J. Radiol. 2019; 114: 128-35. https://dx.doi.org/10.1016/j.ejrad.2019.01.003.
- Lin Y.C., Lin C.H., Lu H.Y., Chiang H.J., Wang H.K., Huang Y.T. et al. Deep learning for fully automated tumor segmentation and extraction of magnetic resonance radiomics features in cervical cancer. Eur. Radiol. 2020; 30(3):1297-305. https://dx.doi.org/10.1007/s00330-019-06467-3.
- Liu Y., Zhang Y., Cheng R., Liu S., Qu F., Yin X. et al. Radiomics analysis of apparent diffusion coefficient in cervical cancer: a preliminary study on histological grade evaluation. J. Magn. Reson. Imaging. 2019; 49(1): 280-90. https://dx.doi.org/10.1002/jmri.26192.
- Zwanenburg A., Vallières M., Abdalah M.A., Aerts H.J.W.L., Andrearczyk V., Apte A. et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020; 295(2): 328-38. https://dx.doi.org/10.1148/radiol.2020191145.
- Li K., Sun H., Lu Z., Xin J., Zhang L., Guo Y. et al. Value of [18F]FDG PET radiomic features and VEGF expression in predicting pelvic lymphatic metastasis and their potential relationship in early-stage cervical squamous cell carcinoma. Eur. J. Radiol. 2018; 106: 160-6. https://dx.doi.org/10.1016/j.ejrad.2018.07.024.
- Hou L., Zhou W., Ren J., Du X., Xin L., Zhao X. et al. Radiomics analysis of multiparametric MRI for the preoperative prediction of lymph node metastasis in cervical cancer. Front. Oncol. 2020; 10: 1393. https://dx.doi.org/10.3389/fonc.2020.01393.
- Wang T., Gao T., Guo H., Wang Y., Zhou X., Tian J. et al. Preoperative prediction of parametrial invasion in early-stage cervical cancer with MRI-based radiomics nomogram. Eur. Radiol. 2020; 30(6): 3585-93. https://dx.doi.org/10.1007/s00330-019-06655-1.
- Cai M., Yao F., Ding J., Zheng R., Huang X., Yang Y. et al. MRI radiomic features: a potential biomarker for progression-free survival prediction of patients with locally advanced cervical cancer undergoing surgery. Front. Oncol. 2021; 11: 749114. https://dx.doi.org/10.3389/fonc.2021.749114.
- Autorino R., Gui B., Panza G., Boldrini L., Cusumano D., Russo L. et al. Radiomics-based prediction of two-year clinical outcome in locally advanced cervical cancer patients undergoing neoadjuvant chemoradiotherapy. Radiol. Med. 2022; 127(5): 498-506. https://dx.doi.org/10.1007/s11547-022-01482-9.
- Kim K.E., Kim C.K. Magnetic resonance imaging-based texture analysis for the prediction of postoperative clinical outcome in uterine cervical cancer. Abdom. Radiol. (NY). 2022; 47(1): 352-61. https://dx.doi.org/10.1007/s00261-021-03288-1.
- Liu B., Sun Z., Xu Z.L., Zhao H.L., Wen D.D., Li Y.A. et al. Predicting disease-free survival with multiparametric MRI-derived radiomic signature in cervical cancer patients underwent CCRT. Front. Oncol. 2022; 11: 812993.https://dx.doi.org/10.3389/fonc.2021.812993.
- Ikushima H., Haga A., Ando K., Kato S., Kaneyasu Y., Uno T. et al. Prediction of out-of-field recurrence after chemoradiotherapy for cervical cancer using a combination model of clinical parameters and magnetic resonance imaging radiomics: a multi-institutional study of the Japanese Radiation Oncology Study Group. J. Radiat. Res. 2022; 63(1): 98-106. https://dx.doi.org/10.1093/jrr/rrab104.
- Zhou Y., Gu H.L., Zhang X.L., Tian ZF., Xu X.Q., Tang W.W. Multiparametric magnetic resonance imaging-derived radiomics for the prediction of disease-free survival in early-stage squamous cervical cancer. Eur. Radiol. 2022; 32(4): 2540-51. https://dx.doi.org/10.1007/s00330-021-08326-6.
Received 13.07.2022
Accepted 08.08.2022
Alina E. Solopova, MD, PhD, Associate Professor, Leading Researcher, Department of Radiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology,
a_solopova@oparina4.ru, https://orcid.org/0000-0003-4768-115X, 117997, Russia, Moscow, Academician Oparin str., 4.
Nikita I. Ukraintsev, MD, resident doctor, Loginov Moscow Clinical Scientific Center, Moscow Healthcare Department,
ukraincev.nikita@mail.ru,
111123, Russia, Moscow, Shosse Entuziastov, 86.
Natalia A. Rubtsova, Dr. Med. Sci., Professor, Head of the Department of Radiology, P.A. Hertsen Moscow Oncological Center,
rna17@yandex.ru,
125284, Russia, Moscow, 2nd Botkinsky pr., 3.
Authors' contributions: Solopova A.E. – analysis of sources, writing the article, selection of images; Ukraintsev N.I. – analysis of current literature, writing the article, selection of images; Rubtsova N.A. – peer-reviewing and editing the text of the article, assistance in adapting the recommendations.
Conflicts of interest: The authors declare that there are no conflicts of interest regarding this publication.
Funding: The investigation has not been sponsored.
For citation: Solopova A.E., Ukraintsev N.I., Rubtsova N.A. Magnetic resonance imaging in the initial staging of cervical cancer: updating the 2021 ESUR guidelines.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 8: 36-46 (in Russian)
https://dx.doi.org/10.18565/aig.2022.8.36-46









