Modern principles of pregnancy management in patients with lymphomas
Objective. To study the characteristics of pregnancy course and analyze maternal and perinatal outcomes in patients with lymphomas, based on anticancer treatment during pregnancy.Shmakov R.G., Akhmedova A.I., Polushkina E.S., Demina E.A., Mangasarova Ya.K., Tumyan G.S., Vinogradova M.A., Kravchenko S.K., Zubkov V.V.
Materials and methods. An analysis of the evolution of approaches to pregnancy management resulting from a 31-year observation experience of 70 women with lymphomas has been carried out. There were 46 women with Hodgkin lymphoma (LH) and 24 women with non-Hodgkin lymphoma (NHL). Patients were divided into three groups based on the time and tactics of their treatment: group 1 - without lymphoma treatment during pregnancy (n=7); group 2 – chemotherapy during pregnancy and mostly preterm delivery at 34-35 weeks gestation (n=26); group 3 – chemotherapy during pregnancy and mostly term delivery (n=37).
Results. Chemotherapy was performed in 35 pregnant women (50%), and 27 patients (38.6%) were not treated. The most common complication of pregnancy in patients who received treatment was anemia (71.4% vs. 44.4%, p=0.03). Six treated patients (37.5%) with NHL and one patient with LH (5.3%) had a pregnancy complicated by catheter-associated thrombosis. In patients with NHL, the frequency of preterm labor was statistically significantly higher than in patients with HL (twofold, p=0.03). In 80% of cases, the cause of preterm labor was iatrogenic (the need to start or continue the treatment). The comparative analysis of the frequency of preterm birth showed a significant decrease of it over the past five years (from 57.7% to 27.8%, p=0.02). Premature delivery significantly affected the health of newborns and in 2/3 of cases, premature-born children needed treatment under the ICU conditions.
Conclusion. There was no correlation between the health status of the newborn and the chemotherapy received by mother, the morbidity of children at birth was mainly due to their prematurity. The radical change in the management of patients over the past five years has reduced the frequency of premature births by two times (p=0.02), which accordingly contributed to the reduction in the morbidity of newborns.
Keywords
The choice of effective tactics for managing pregnancy in the presence of the oncohematological disease is the subject of close attention for both obstetrician-gynecologists and hematologists.
Lymphomas are the most common type of hematologic neoplasms encountered during pregnancy (incidence of 1 in 1000-6000 pregnancies) [1]. In young women Hodgkin lymphoma (HL) is more frequently detected than non-Hodgkin lymphoma (NHL). HL is diagnosed mainly in young women of reproductive age (15–34 years), and with timely started treatment, the disease has a favorable prognosis [2]. NHL usually occurs in women at a later age (after 30 years), it is characterized by the aggressive course, and in most cases requires timely initiation of antitumor therapy [2]. According to the data published by International Network on Cancer, Infertility and Pregnancy [3], the modern programs of treatment can significantly increase the number of saved pregnancies among the patients with oncological diseases and increase the frequency of performing chemotherapy (CT) during pregnancy by 20%.
This paper analyzes the evolution of approaches to managing pregnancy based on a 31-year observation experience of 70 women with various types of lymphomas diagnosed during pregnancy. The features of pregnancy course were studied and the maternal and perinatal outcomes in patients with lymphomas were assessed, depending on the antitumor treatment during pregnancy. The main factors affecting the health of infants born to mothers with lymphomas were identified. The results of long-term observation of the maternal and child health were analyzed.
Materials and Methods
The study evaluated the tactics of managing pregnancy, the health of newborns, and a prognosis of the disease detected in 70 women during pregnancy was made. HL was diagnosed in 46 women, and NHL was revealed in 24 patients. Three groups of pregnant women were identified depending on the time and tactics of their observation: group 1 – retrospective (from 1987 to 2000); the treatment of lymphomas during pregnancy was not carried out (n = 7, including 7 patients with HL, no patients with NHL); group 2 – prospective (from 2001 to 2013); anticancer treatment was carried out during pregnancy, and preterm delivery was at 34–35 weeks (n = 26, including 15 patients with LH, 11 patients with NHL); group 3 – prospective (from 2014 to September 2018); the patients underwent CT during pregnancy, and parturition was preferably at term (n = 37, including 24 patients with HL, 13 patients with NHL).
The clinical diagnosis was established on the basis of standard diagnostic methods for lymphomas. Histological and immunohistochemical examination of a lymph node bioptate was performed in all patients. The disease stage was established in accordance with the Ann-Arbor classification (1971) and with the supplements proposed in Cotswolds (1989). The staging of the disease was defined on the basis of ultrasound and radiographic studies. In radiography of the lungs, a special lead apron was used to protect the fetus. If necessary, bilateral iliac trepanobiopsy was performed to clarify the stage of the disease. Magnetic resonance imaging (MRI) was performed, if a patient had superior vena cava syndrome or the signs of compression of the airways and the esophagus. All pregnant women underwent clinical blood test and biochemical blood test, and the status of the hemostatic system was assessed.
Throughout pregnancy, dynamic monitoring of the fetal health was performed using ultrasound examination, dopplerometry and cardiotocography.
In order to determine the overall and relapse-free survival of women with oncohematological diseases and assess the long-term results of their children’s health, we conducted a single-time survey of patients (the survey range was from 6 months to 16 years after delivery).
Statistical analysis. Statistical data processing was performed with the use of the IBM SPSS Statistics 21 software package. Quantitative data, subject to a normal distribution of the attributes, were presented as mean values with a standard deviation (t-test), while an abnormal distribution of the attributes was presented as a median (Mann – Whitney test). Qualitative indicators were presented both in absolute and in relative values. To compare categorical data in two groups, as well as to evaluate significant differences between them, the Fisher’s exact test for contingency tables was used.
Results
The average age of patients with the diagnosis of malignant disease in pregnant women with HL was 26.2 years (4.1), and with NHL – 30.9 years (4.2). There were 39 primigravida women, 31 multigravida women; among them there were 47 primiparas and 23 multiparas. Multiparous women included 21 patients who had second births, and two patients who had third and fourth births.
In the first trimester of pregnancy, the disease regardless of the type of lymphoma, was diagnosed in 8 patients (11.5%), in the second trimester – in 53 patients (75.7%), and in the third trimester – in 7 patients (10%). The average gestational age was 19.3 weeks (6.4), when the lymphoma was diagnosed (19.7 weeks (6.6) and 18.5 weeks (6.0) for HL and NHL, respectively, p = 0.44). In the group of patients with HL, one pregnancy occurred in a woman with radiation therapy (1.4%) and one pregnancy – in a woman with chemotherapy (1.4%). In four patients recurrence of HL was detected during pregnancy, in one of them – 10 years after the end of chemotherapy, in the others – after 2-3 years.
It should be noted that in patients with HL there were less aggressive histological variants than with NHL. In pregnant women with HL, the histological version of the tumor prevailed – nodular sclerosis type I and II (86.9%). In pregnant women with NHL, the most common lymphomas were diffuse large B-cell lymphoma (41.6%) and primary mediastinal large B-cell lymphoma (33.3%). A relatively small group of patients with NHL were pregnant women with follicular lymphoma (8.3%), B-cell lymphoma from cells of the marginal zone of the spleen (4.2%), anaplastic large cell lymphoma (ALK-positive) (4.2%), Burkitt’s lymphoma (4.2%) and hairy cell leukemia (4.2%).
In group 1 (n = 7), termination of pregnancy due to medical reasons (conclusion of oncologist/hematologist) was performed in the first and second trimesters of pregnancy in four patients (57.1%) with HL.
In group 2 (n = 26), termination of pregnancy was performed in two cases (7.7%): in the first case, termination of pregnancy in the first trimester was performed at the patient’s request; in the second case, termination of pregnancy was at 16 weeks in the patient with NHL due to the development of severe respiratory failure (mother of many children: three live children). In one case (3.8%), in a patient with HL and high-risk congenital thrombophilia (heterozygous mutations of Leiden and prothrombin) who did not undergo CT, a late miscarriage occurred at 24 weeks (until 2012, miscarriages up to 28 weeks were considered as late miscarriages).
In group 3 (n = 37), one woman (2.7%) with NHL, who received a course of R-CHOP chemotherapy, interruption of pregnancy was at 6 weeks due to the known teratogenicity of CT in the first trimester.
In the observation groups, CT was performed in 35 pregnant women (50%). In group 1, only one patient with HL (14.3%) received treatment. In group 2, the treatment was administered in 10 patients (38.5%). In group 3, CT was performed in 24 pregnant women (64.9%).
The treatment was postponed until the postpartum period in 27 pregnant women (38.6%) in three groups: in 21patients with HL (45.6% of the overall number of patients with HL), and less almost twice in patients with NHL – 6 women (25% of the overall number of patients with NHL).
Thus, over a 31-year period, there was a significant decrease in the frequency of artificial termination of pregnancy from 57.1 to 2.7%, and an increase in the frequency of the use of chemotherapy from 14.3 to 64.9%.
In 35 patients, the average gestational age at the start of performing chemotherapy was 24 weeks (5.4) (from 15 to 35 weeks). Tumor treatment regimens were selected by oncologists-hematologists depending on the histological type of tumor, the stage and degree of disease aggressiveness. Doses of drugs were calculated on the basis of the actual body weight of the pregnant patient. In pregnant women with HL, the ABVD hemotherapy regimen was administered to nine patients (25.7%); BEACOPP-14 – in three patients (8.6%); СOPP – in one patient and ABVD + vinblastine in one patient (2.8%); five patients (14.3%) underwent monotherapy (vinblastine). In patients with NHL, more aggressive treatment regimens were prescribed: VACOP-B in three patients (8.6%), R-CHOP in four (11.5%), R-EPOCH and EPOCH/DA-EPOCH in three (8.6 %) and in five patients (14.3%), respectively; continuous interferon-alpha therapy was administered to one patient (2.8%).
The main complication of pregnancy in women with lymphomas was anemia of various degrees of severity. Differences in the frequency of anemia in the groups of patients who received and did not receive chemotherapy were statistically significant: anemia was observed in 71.4% of women in the group of patients with chemotherapy, and among the patients without chemotherapy it was in 44.4% of cases (p = 0.03). The threat of termination of pregnancy (threatening early/late miscarriage; threatened preterm labor) was often observed both in patients, who received CT (37.1%) and in patients who did not receive it (44.4%), p = 0.6. Placental insufficiency with fetal growth retardation in pregnant women who received chemotherapy was in 8.6% of patients, and in pregnant women who did not receive treatment – in 3.7% (p = 0.62). Late moderate preeclampsia developed in one patient (3.7%) who did not receive CT during pregnancy. Pregnancy was complicated by pneumocystic pneumonia during CT in two women (5.7%), and by the development of gram-negative sepsis in one woman (2.8%). It should be noted that in six women (37.5%) with NHL and in one woman (5.3%) with HL during CT, pregnancy was complicated by venous thrombosis of various localization (jugular vein, inferior vena cava, femoral and iliac veins) at the site where intravenous catheter was installed during CT, despite the anticoagulant therapy during antitumor treatment.
Thus, the main complication of chemotherapy during pregnancy was anemia of various degrees of severity and catheter-associated venous thrombosis (in most cases in patients with NHL who received CT). The frequency of other pregnancy complications did not depend on CT.
The average period of delivery in 62 patients was 36.5 weeks (2.7) (from 26 to 40 weeks). Pregnancies resulted in full term delivery in 59.7% of cases. Preterm births occurred in 40.3% of cases. Delivery at 26 – 34 weeks was in 8 women (12.9%), and at 34 – 37 weeks in 17 women (27.4%). Preterm birth was observed in 30% of women with HL. Statistically, preterm birth was significantly more frequent in women with NHL, it was in 59.1% of cases (p = 0.03). Caesarean section was performed in 58.1% of patients, 21 women were with HL (52.5% of the number of pregnant women with HL) and 15 women with NHL (68.2% of the number of pregnant women with NHL), p = 0.28. The mode of delivery did not depend on whether antitumor treatment was or was not performed during pregnancy (p = 0.79). In 80% of cases early surgical delivery was associated with the need to start/continue the antitumor treatment. It should be noted that a comparative analysis of the frequency of preterm birth in groups 1 and 2 in contrast to group 3 showed a statistically significant decrease from 57.7 to 27.8% in the observation groups (p = 0.02), that was due to the review of management tactics for patients.
During the observation period, 64 live babies were born (including two cases of twins). The average weight of infants at birth was 2751.2 g (780.4) (ranging from 490 g to 4450 g). The weight of newborns, whose mothers received CT during pregnancy did not differ from the weight of babies born to mothers without treatment (2623.8 g (833.9) versus 2950.8 g (656.3), respectively, p = 0.11).
In the study group, the women, who received CT gave birth to 36 children (including one woman who gave birth to twins); 44.4% of them were premature infants. The women who did not receive treatment, gave birth to 28 children (including one delivery of twins). In this case, 39.3% of infants were premature. It should be noted that in groups 1 and 2 the number of premature babies is almost twice higher than in group 3.
Among 64 newborns, 13 babies (50%) from observation groups 1 and 2, and 8 babies (21.1%) from group 3 underwent treatment in neonatal intensive care unit (NICU) due to prematurity. The average length of stay there was 6.8 days (3.6) (from 1 to 13 days). The frequency of stay of the newborns in NICU, whose mothers received CT during pregnancy did not differ from frequency of stay of the babies born to mothers without treatment (36.1% versus 28.6%, p = 0.59). However, when comparing the groups of women with HL and NHL, statistically significant difference between the stay of newborns in the NICU was found. Therefore, babies born to patients with NHL were almost three times more likely to require treatment in NICU than babies born to patients with HL (56.5 and 19.5%, respectively, p = 0.01). At the second stage of nursing, the infants were transferred to the neonatal pathology department. The morbidity pattern among the newborns is shown in Table 1.

Early neonatal mortality occurred in one case. The course of pregnancy was complicated by the development of placental insufficiency, fetal growth retardation, severe oligohydramnios and severe anemia (erythrocyte mass transfusion was performed) in a patient with a history of recurrent miscarriage (two miscarriages) and with HL and involved hilar lymph nodes, lungs, pulmonary trunk (ECOG performance status 2), who underwent five courses of chemotherapy (from 18 to 26 weeks) according to the BEACOPP-14 regimen. Due to fetal deterioration at 26 weeks, emergency cesarean section was performed. A boy weighing 490 g was born with congenital pneumonia, disseminated intravascular coagulation, gastrointestinal bleeding, pulmonary hemorrhage, and sepsis. The baby died on the second day of life. The boy’s death was due to deep prematurity, developmental delay, respiratory distress and infectious complications.
Among 36 infants born to mothers who received CT during pregnancy, minor developmental anomalies were detected in 11.1% of infants: one infant had capitate hypospadias, one – bilateral pyeloectasia, one – congenital stridor and one – congenital muscular clubfoot, two infants (5.5%) had congenital heart disease – ventricular septal defect. In the group of 28 infants whose mothers did not receive CT, pyeloectasia was detected in two infants (7.1%).
We conducted a prospective observation of 43 children who were born to mothers with cancer during pregnancy. The observation period was from 6 months to 16 years; the main questions were previous diseases, vaccinations, the assessment of physical and mental development, the use of antibacterial and immunotherapy, etc. By the end of the first year of life, physical and mental development of premature infants was in conformity to their peers; no deviations were observed in their cardiovascular systems.
Among 70 patients, it was possible to follow-up the patient’s history of 43 women, who were diagnosed with lymphoma during pregnancy (the observation period was from 6 months to 16 years); no cases of relapse were noted, and at the time of writing this paper, the patients are in stable remission.
Discussion
The existing information on tactics of managing pregnancy and treatment of women with cancer, which has been published in the modern medical literature, is scarce. The actual experience allows us to define three options for managing pregnancy in patients with lymphoproliferative diseases: termination of pregnancy, prolongation of pregnancy with treatment in the second and third trimesters, prolongation of pregnancy with treatment delay until the postpartum period.
In 2013, the European Journal of Obstetrics and Gynecology published the results of a survey of practicing obstetrician-gynecologists and gynecological oncologists about their attitude to treatment of patients with cancer during pregnancy [4]. According to the survey, a fairly large group of doctors (37%) totally refused to recommend chemo- or radiation therapy during pregnancy. If cancer was revealed in the first or at the beginning of the second trimesters, 44% of specialists supported the termination of pregnancy. In case of detecting cancer in the second and third trimesters, 58% of respondents preferred the induction of preterm birth with subsequent treatment in the postpartum period. According to another European study, 88% of preterm birth was due to the requirement of obstetrician-gynecologists and oncologists for delivering and then treating the patient [3]. Therefore, there is still no consensus among the practitioners regarding the choice of options for pregnancy management and delivery period.
The choice of the CT regimen for treatment of pregnant patients should meet at least two conditions: to effectively kill tumor cells and not have a teratogenic effect on the fetus. According to some researchers [5, 6], the most preferred treatment regimen of patients with local lesions of HL is the ABVD chemotherapy (doxorubicin, bleomycin, vinblastine and dacarbazine). A number of authors recommend monotherapy with vinblastine (“bridge therapy”) [7, 8] as a reasonable alternative to the use of ABVD in the first trimester or, if necessary, to reverse the symptoms of the disease detected in late pregnancy and create favorable conditions for delivery. The most studies have shown more than 75% of tumor response to treatment with vinblastine and minimal fetal toxicity [7]. However, after delivery and a full-scale examination of the mother, adequate therapy should be performed, providing the woman with the highest chances for recovery.
There are no publications in the contemporary scientific literature on the study of the effect of chemotherapy on the fetuses of pregnant women exposed to chemotherapy with BEACOPP-14 regimen (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone). Some authors [1, 9] note that a high dose of procarbazine, which belongs to the group of alkylating compounds, is used in this regimen, and therefore, the use of BEACOPP-14 is undesirable during pregnancy. However, if the effect of the ABVD regimen in the second and third trimesters of pregnancy is insufficient, it is possible to intensify the therapy with BEACORP-14 with the replacement of procarbazine for dacarbazine.
In pregnant patients with B-cell NHL, more aggressive regimens of treatment (CHOP and EPOCH) are used in combination with monoclonal anti-CD20 antibodies (rituximab) [1, 10]. Aviles et al. [5] studied the long-term health outcomes for babies born to mothers, who received treatment with the use of CHOP regimen during pregnancy (doxorubicin, cyclophosphamide, vincristine, and prednisone), and did not reveal neurological abnormalities or fertility disorders.
Rituximab may cross the placenta and have a toxic effect on the B-cells immunity of the fetus [11, 12]. The literature describes the use of the drug for the treatment of pregnant patients (including in the first trimester of pregnancy). The study by E.F. Chakravarty et al. [12] detected a high frequency of reproductive losses (up to 40%) when the drug was used for the treatment of pregnant women, and two cases of congenital malformations in fetuses. E.F. Chakravarty et al. [12] found that when using rituximab, the risk of depletion of the B-cell pool in newborns increases, and it leads to immunological disorders. Out of the 90 live births, 11 babies had hematological disorders: 5 had depletion of the B-cell pool, 3 had thrombocytopenia, and 3 had leukopenia. In our study, when rituximab was used in treatment regimens (in 6 patients), hematological disorders and malformations were not observed in newborn children.
According to most researchers, the use of antimetabolites (methotrexate, etc.) should be avoided in treatment of lymphomas during pregnancy, as they penetrate the placental barrier resulting in the development of malformations (fetal aminopterin syndrome) [1].
Recently, F. Amant et al. [3] published the data on the health status of 895 children born to mothers who had cancer during pregnancy. Two groups of newborns were monitored: group 1 – babies born to mothers who received antitumor therapy during pregnancy, group 2 – babies born to mothers who did not receive treatment during pregnancy. The authors did not find any differences in neurocognitive functions and the activity of the cardiovascular system in newborn children. F. Amant et al. emphasized that the disease of infants in both groups is mainly due to their prematurity, but not to antitumor treatment. According to the authors [3], 167 babies (21%) out of 796 had low birth weight. Moreover, the birth rate of low birth weight babies in group 1 was higher than rate in group 2. The frequency of developmental anomalies in newborns in both groups did not differ from that in children in the general population.
According to our study, over the past five years there has been an increase in the number of patients (by 24.7%), who received chemotherapy during pregnancy, and it corresponds to the global trend. In this study, pregnant women received CT in the second and third trimesters. In two patients with the BEACOPP-14 regimen, placental insufficiency developed with fetal growth retardation. The BEACOPP-14 was used in treatment of three patients with large tumor volumes, severe tumor intoxication and anemia, which were extremely unfavorable for fetuses. Therefore, one cannot assert with confidence that there is a cause and effective relationship between the development of placental insufficiency in a patient and the chemotherapy regimen used for tumor treatment.
No relationship was found between a specific regimen of antitumor therapy and a number of treatment courses, and the health condition of newborn children. The frequency of congenital malformations (5.5%) in the observation group did not differ from the frequency in the general population (4–6%) [13].
Pregnancy and the postpartum period are hypercoagulable conditions with an increased risk of venous thromboembolic complications (VTEC) development [14, 15]. The risk of VTEC in pregnant women is five times higher than in non-pregnant women. After the birth, the risk of VTEC development immediately increases by 50 times. The oncological disease increases the risk of VTEC by four times, and chemotherapy – by seven times [16, 17]. At present, there are no reliable data on the incidence of VTEC in combination with the above factors.
A hypercoagulable state in combination with various risk factors for VTEC under certain conditions may cause placental thrombosis, fetal growth retardation, an increased risk of deep vein thrombosis and pulmonary embolism [18]. The increased activity of the blood coagulation system in patients with cancer during gestation is associated with formation of inflammatory cytokines, the adhesion of tumor cells to endothelial cells, and pregnancy-induced coagulant activity of phagocytes. Recent studies have shown that high-level expression of heparinase by placenta and tumor cells also leads to a hypercoagulative state [19].
To prevent VTEC in pregnant women, low molecular weight heparins (LMWH) are used, which are safe drugs during pregnancy and they do not pass through the placenta [20]. All patients from the observation group received prophylactic or high prophylactic doses of LMWH.
In our study, 37.5% of women with NHL who received CT during pregnancy were diagnosed with venous thrombosis of various localization. Only in one patient (5.3%) with HL during CT, thrombosis of the internal jugular vein at the site of the intravenous catheter was detected. It is known that in non-pregnant patients with NHL, VTEC is as common as in solid tumors, but thrombosis is extremely rare in all situations of HL. Thus, the frequency of thrombosis is more related to the nature of the disease than to the type of treatment. Therefore, we recommend that high prophylactic doses of LMWH should be prescribed to all pregnant women with NHL in the presence of superior vena cava compression syndrome, additional thrombophilia factors, during chemotherapy containing glucocorticoids, under the control of hemostasis and platelet count.
In 2010, Van Calsteren et al. found that 54% of pregnant women with cancer had preterm births [21]. The likelihood of premature birth is increased by 11.8% in the case of anticancer treatment in women.
In our study, the frequency of preterm births among 62 patients was 40.3%. There were no significant differences between the frequency of preterm births in pregnant women who received chemotherapy, and those who did not receive the treatment (42.8% versus 37%, p = 0.79). It should be noted that the frequency of preterm births in patients with NHL was twice higher than in patients with HL. In our opinion, this is because NHL is a more aggressive disease than HL, and more often the course of the disease predetermines the preterm delivery due to the need for treatment with teratogenic drugs. So, in our study, 80% of patients had preterm delivery particularly because of this treatment.
It should be noted that over the past five years, delivery tactics (the timing and mode) have changed dramatically. So, if in the past the delivery at 34–35 weeks was a preferred strategy, and the mode of delivery was caesarean section, now, if the clinical situation allows, vaginal delivery at term is preferred. This approach made it possible to reduce significantly the rate of preterm delivery (from 57.7 to 27.8%, p = 0.02), and contributed to reduction of neonatal morbidity.
The results of our study indicate that preterm delivery has a significant impact on newborn children’s health. Morbidity of preterm infants in the study was mainly due to respiratory distress, intracranial hemorrhage and asphyxia (see Table 1), resulting from preterm delivery. At the first stage, almost two-thirds of preterm infants (77.8%) were in the NICU after birth. Respiratory support was required for every second baby.
It was found in the studies [22, 23] that impairment of cognitive functions and physical development in preterm babies is mainly observed up to the end of the first year of life. The results of our study show the absence of the CT effect on the distant (after the first year of life) results of physical, neuropsychic development and activity of the cardiovascular system in children.
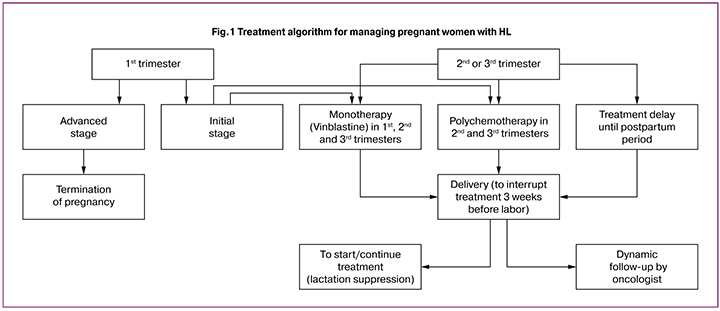
Conclusion
The results of the analysis of pregnancy in women with lymphomas show that CT may be safely used during pregnancy, and it does not lead to an increase in the number of adverse outcomes and thereby helps to prolong pregnancy to full term.
It was found that anemia is the most common pregnancy complication in women with lymphomas, especially during CT (p = 0.03). The main challenge in NHL therapy is high frequency of catheter-associated venous thrombosis in patients (37.5%); for this reason high prophylactic doses of LMWH are recommended to women throughout pregnancy and at least 6 weeks after delivery.
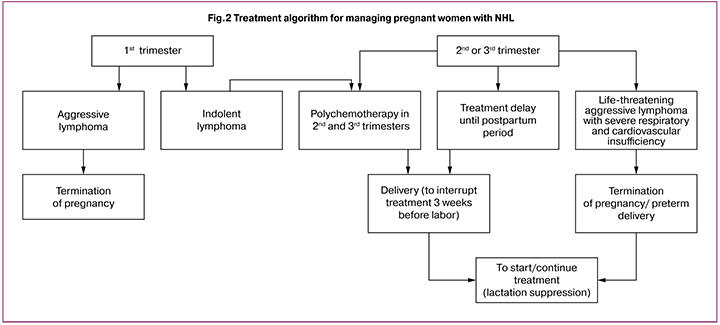
In this study, no relationship was found between the condition of the newborn’s health and CT; morbidity was mainly due to preterm births. Preterm delivery in 80% of cases is associated with the need to start/continue antitumor treatment immediately after birth. Over the past five years a dramatic change in patient management tactics has reduced the rate of preterm births by two times (p = 0.02), and it led to an improvement in perinatal outcomes.
Long-term observation and further analysis made it possible to optimize the algorithm (Fig. 1-2) of managing pregnancy in patients with lymphomas, and on the one hand, to enable women to perform their reproductive functions, and on the other hand, not to worsen the prognosis of cancer.
References
- Lavi N., Horowitz N.A., Brenner B. An update on the management of hematologic malignancies in pregnancy. Womens Health (Lond). 2014; 10(3): 255-66.
- Cohen J.B., Blum K.A. Evaluation and management of lymphoma and leukemia in pregnancy. Clin. Obstet. Gynecol. 2011; 54(4): 556-66.
- de Haan J., Verheecke M., Van Calsteren K., Van Calster B., Shmakov R.G., Mhallem Gziri M. et al.; International Network on Cancer and Infertility Pregnancy (INCIP). Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018; 19(3): 337-46.
- Han S.N., Kesic V.I., Van Calsteren K., Petkovic S., Amant F.; ESGO ‘Cancer in Pregnancy’ Task Force. Cancer in pregnancy: a survey of current clinical practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013; 167(1):18-23.
- Avilés A., Neri N., Nambo M.J. Hematological malignancies and pregnancy: treat or no treat during first trimester. Int. J. Cancer. 2012; 131(11): 2678-83.
- Azim H.A. Jr., Pavlidis N., Peccatori F.A. Treatment of the pregnant mother with cancer: a systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part II: hematological tumors. Cancer Treat. Rev. 2010; 36(2): 110-21.
- Bachanova V., Connors J.M. Hodgkin lymphoma in pregnancy. Curr. Hematol. Malig. Rep. 2013; 8(3): 211-17.
- Connors J.M. Challenging problems: coincident pregnancy, HIV infection, and older age. Hematol. Am. Soc. Hematol. Educ. Program. 2008: 334-9.
- Mahmoud H.K., Samra M.A., Fathy G.M. Hematologic malignancies during pregnancy: a review. J. Adv. Res. 2016; 7(4): 589-96.
- Peccatori F.A., Azim H.A. Jr., Orecchia R., Hoekstra H.J., Pavlidis N., Kesic V., Pentheroudakis G.; ESMO Guidelines Working Group. Сancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013; 24(Suppl. 6): vi160-70.
- Mandal P.K., Dolai T.K., Bagchi B., Ghosh M.K., Bose S., Bhattacharyya M. B cell suppression in newborn following treatment of pregnant diffuse large B-cell lymphoma patient with rituximab containing regimen. Indian J. Pediatr. 2014; 81(10): 1092-4.
- Chakravarty E.F., Murray E.R., Kelman A., Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011; 117(5): 1499-506.
- Boere I., Lok C., Vandenbroucke T., Amant F. Cancer in pregnancy: safety and efficacy of systemic therapies. Curr. Opin. Oncol. 2017; 29(5): 328-34.
- Horowitz N.A., Lavi N., Nadir Y., Brenner B. Haematological malignancies in pregnancy: An overview with an emphasis on thrombotic risks. Thromb. Haemost. 2016; 116(4): 613-7.
- Eyre T.A., Lau I.J., Mackillop L., Collins G.P. Management and controversies of classical Hodgkin lymphomain pregnancy. Br. J. Haematol. 2015; 169(5): 613-30.
- Heit J.A., Kobbervig C.E., James A.H., Petterson T.M., Bailey K.R., Melton L.J. 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann. Intern. Med. 2005; 143(10): 697-706.
- Mandalà M., Barni S., Prins M., Labianca R., Tondini C., Russo L. et al. Acquired and inherited risk factors for developing venous thromboembolism in cancer patients receiving adjuvant chemotherapy: a prospective trial. Ann. Oncol. 2010; 21(4): 871-6.
- El-Messidi A., Patenaude V., Abenhaim H.A. Incidence and outcomes of women with Hodgkin’s lymphoma in pregnancy: a population-based study on 7.9 million births. J. Obstet. Gynaecol. Res. 2015; 41(4): 582-9.
- Nadir Y., Kenig Y., Drugan A., Zcharia E., Brenner B. Involvement of heparanase in vaginal and cesarean section deliveries. Thromb. Res. 2010; 126(6): e444-50.
- The Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green-top Guideline No. 37a. April 2015.
- Van Calsteren K., Heyns L., De Smet F., Van Eycken L., Gziri M.M., Van Gemert W. et al. Cancer during pregnancy: An analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J. Clin. Oncol. 2010; 28(4): 683-9.
- Amant F., Van Calsteren K., Halaska M.J., Gziri M.M., Hui W., Lagae L. et al. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: an observational study. Lancet Oncol. 2012; 13(3): 256-64.
- Amant F., Vandenbroucke T., Verheecke M., Fumagalli M., Halaska M.J., Boere I. et al. Pediatric outcome after maternal cancer diagnosed during pregnancy. N. Engl. J. Med. 2015; 373(19): 1824-34.
Received 21.01.2019
Accepted 22.02.2019
About the Authors
Roman G. Shmakov, MD, professor, Director of the Institute of Obstetrics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Address: 117997, Russia, Moscow, Oparina st., 4. E-mail: r_shmakov@oparina4.ruAminat I. Akhmedova, postgraduate student, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov.
Address: 117997, Russia, Moscow, Oparina st., 4. E-mail: akhmedovag@yandex.ru
Evgeniya S. Polushkina, PhD, senior researcher, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Address: 117997, Russia, Moscow, Oparina st., 4 E-mail: e_polushkina@oparina4.ru
Elena A. Demina, MD, professor, N.I. Pirogov National Medical and Surgical Center.
Address: 105203, Russia, Moscow, Nizhnyaya Pervomayskaya str., 70. E-mail: drdemina@yandex.ru
Yana K. Mangasarova, PhD, senior researcher, National Medical Research Center for Hematology, Ministry of Health of Russia.
Address: 125167, Russia, Moscow, Novaya Zukovskaya str. 8. E-mail: V.k.jana@mail.ru
Gayane S. Tumyan, MD, professor, leading researcher, N. N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia.
Address: 115478, Russia, Moscow, Kashirskoye Highway, 23. E-mail: gaytum@mail.ru
Maria A. Vinogradova, PhD, head of the Department of Reproductive Hematology and Clinical Hemostasiology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Address: 117997, Russia, Moscow, Oparina st., 4 E-mail: mary-grape@yandex.ru
Sergey K. Kravchenko, PhD, associate professor, head of the Department of Intensive High-dose Chemotherapy for Hemoblastosis with a Hospital and Outpatient Facility, National Medical Research Center for Hematology, Ministry of Health of Russia. Address: 125167, Russia, Moscow, Novaya Zukovskaya str. 8. E-mail: skkrav@mail.ru
Viсtor V. Zubkov, MD, head of the Department of Neonatology and Pediatrics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Address: 117997, Russia, Moscow, Oparina st., 4 E-mail: v_zubkov@oparina4.ru
For citations: Shmakov R.G., Akhmedova A.I., Polushkina E.S., Demina E.A., Mangasarova Ya.K., Tumyan G.S., Vinogradova M.A., Kravchenko S.K., Zubkov V.V. Modern principles of pregnancy management in patients with lymphomas. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 7: 40-8 (in Russian).
http://dx.doi.org/10.18565/aig.2019.7.40-48



